Abstract
Objective
Since it is unclear whether clinical parameters can independently predict the subsequent treatment response following high intensity focused ultrasound (HIFU) ablation of benign thyroid nodules, we aimed to examine clinical factors that may independently predict 12-month efficacy after HIFU treatment.
Methods
One hundred and forty patients who had single ablation were categorized into two groups, those with 12-month nodule shrinkage above the median (Group I, n = 70) and with shrinkage below or equal to the median (Group II, n = 70). Baseline characteristics, treatment parameters, percentage change in serum TSH, Free thyroxine (FT4) and thyroglobulin (Tg) from baseline to Day 4 and appearance of microbubbles (hyperechoic marks (HEMs)) during treatment were compared between groups. To determine independent factors, a multivariate analysis was done by logistic regression analysis.
Results
Baseline characteristics and treatment parameters were comparable between groups. However, on Day-4, group I had significantly lower serum TSH (0.49mIU/L vs. 0.84mIU/L, p = 0.011) and higher FT4 (22.11 pmol/L vs. 18.47 pmol/L, p = 0.008) than group II. The percentage change in TSH, FT4 and Tg were significantly greater in group I (p = 0.002, p = 0.009 and p = 0.001 respectively). The proportion of HEMs observed during treatment was also significantly higher in group I (42.69% vs. 31.72%, p = 0.030). Among the significant factors, the percentage change in FT4 was the only independent factor for 12-month shrinkage (OR = 1.018, 95%CI =1.003–1.032, p = 0.017).
Conclusions
Percentage change in serum FT4 on post-treatment Day-4 was an independent blood parameter for the subsequent nodule shrinkage at 12 months. This finding could potentially facilitate the decision for earlier retreatment of treated nodules.
Introduction
Thyroid nodules are common. Although most are benign and will remain relatively unchanged over time, some do cause symptoms necessitating surgical resection [Citation1–3]. However, surgery is not without risks and requires a general anesthesia and hospitalization. As a result, there has been a growing interest in developing less invasive, non-surgical techniques in treating benign thyroid nodules [Citation4–6]. For predominantly-solid or solid nodules, several thermal ablation techniques such as laser, microwave and radiofrequency ablation (RFA) have been shown to be effective [Citation4–6]. High intensity focused ultrasound (HIFU) is one of the newer ablation techniques that is effective in inducing significant nodule shrinkage and in alleviating nodule-related symptoms [Citation7–12].
However, it is clear that not every nodule treated by HIFU ablation responds in the same way. In our experience, up to 20% of the treated nodules shrink less satisfactorily and may require a second ablation 6 months later [Citation13]. At present, there is no way of predicting whether the target nodule would shrink satisfactorily after ablation. Although factors such as smaller nodule volume, greater post-ablative rise in serum thyroglobulin (Tg) and presence of microbubbles within the target nodule during treatment (also known as hyperechoic marks (HEMs)) do favor greater nodule shrinkage after ablation [Citation14–17], it is unclear whether these parameters are independent predictors (i.e., when other significant factors have been considered). We hypothesized that the initial change in serum Tg and free thyroxine (FT4) in the early treatment period may correlate with the extent of heat-induced damage and subsequent nodule shrinkage. Identification of factors can lead to more-timely reintervention and better treatment outcomes. To our knowledge, no studies have examined whether any clinical and/or early blood parameters could independently predict the subsequent nodule shrinkage after HIFU ablation. Therefore, the present study aimed to identify clinical or early blood factors/parameters that may independently predict nodule shrinkage 12 months after single HIFU ablation.
Methods
This retrospective analysis was approved by local institutional review board. All relevant clinical and treatment data were recorded prospectively after obtaining informed consent from patients. Standard terminology was applied in the present study [Citation18]. Consecutive patients who underwent HIFU ablation for a symptomatic, solid (≤10% cystic area) or predominantly solid (<30% cystic areas) benign thyroid nodule from January 2016 to May 2018 were analyzed. At our institution, only patients who required but refused surgery were considered for HIFU ablation. To be eligible, first, patients had to receive only one single application of HIFU to either a single thyroid nodule or a dominant nodule in a multinodular goiter. Second, patients had to have at least a 12 months follow-up after treatment. Third, patients had to have a complete set of blood tests including serum thyroid stimulating hormone (TSH), FT4, Tg and serum anti-thyroid auto-antibodies taken before treatment (on the day of ablation or Day-0) and 4 days after treatment (Day-4). Fourth, patients had to have an independent person who carefully examined the B-mode ultrasonography screen for the appearance of HEMs after each treatment pulse. Any patients who underwent two or more sequential ablations within the same session or previous ablation were excluded. Also patients with follow-up of <12 months or with missing blood results or HEMs recording were excluded.
To evaluate possible association between clinical parameters and nodule shrinkage, patient and nodule characteristics, pain scores, treatment parameters, percentage change in serum FT4 and Tg from Day-0 to Day 4, number of HEMs and proportion of HEMs and the extent of nodule shrinkage (%) at 3-, 6- and 12-month were compared between those whose nodule shrinkage (12-month VRR) were above the median (Group I) and those whose nodule shrinkage (12-month VRR) were equal to or below the median after ablation (Group II).
Pretreatment clinical evaluation
All thyroid swellings were clinically graded according to the World Health Organization (WHO) grading system [Citation19] while the severity of pressure/local symptoms were rated on a visual analog scale (VAS, 0-10cm).
HIFU treatment
All patients were asked to fast overnight and admit in the morning. Serum thyroid function tests (FT4 and TSH levels), Tg and anti-thyroid autoantibodies were checked. All ablations were performed one person (B.H.L.) using a commercially available US-guided HIFU device. Maximal power was used according to the patient’s tolerability [Citation20]. For each treatment, the objective was to ablate the entire nodule in one single session.
Before treatment, all patients were placed in a supine position with neck slightly extended and were given a bolus of intravenous Diazepam (Actavis) (10–20mg) and Pethidine (Martindale Pharmaceuticals) (50–100mg)). Patients’ heart rate, blood pressure, respiration rate and peripheral oxygenation were monitored throughout. Patients were asked to give a hand sign in case of pain. In such situation, either the energy was lowered or more medications were administered. For solid nodules with a cystic component, the cystic portion was emptied with a 19 G needle under US guidance before the commencement of the ablation.
The US-guided HIFU device comprised an energy generator, a treatment head, a skin cooling device, and a touch-screen interface for planning (). The treatment head incorporated an image transducer (7.5 MHz, 128 elements, linear array) and HIFU transducer (3 MHz, single element, 60 mm in diameter). The device computer (Beamotion version no. TUS 3.2.2, Theraclion) automatically divided the nodule into multiple ablation voxels. Each voxel which measured 9 × 7 mm received a continuous 8-s (8-s) pulse of HIFU energy followed by 20-30 s of cooling time before the beam was moved to the adjacent voxel. This continued until all the planned voxels had received the treatment pulse. To be objective in measuring the number of 8-s HIFU pulses that resulted in the appearance of HEMs afterwards, an independent person was assigned to look carefully at the treatment screen and decide whether HEMs appeared following each 8-s pulse. Since a larger-volume nodule had a higher chance of HEMs because of more treatment pulses, the proportion of HEMs (%) was calculated based on the formula: [(Number of 8-s pulses that resulted in HEMs)/(Total number of 8-s pulses given per treatment)] × 100. For example, if a nodule received a total of 50 8-s pulses in the entire treatment cycle and 17 of these pulses resulted in HEMs in the target, the proportion of HEMs was 34.0%.
Figure 1. The ultrasound-guided high intensity focused ultrasound device for treatment of thyroid nodules.
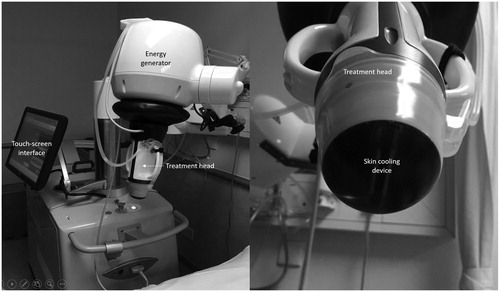
To ensure safety, nearby structures like the carotid artery, trachea, and skin were marked out on the treatment screen before the start of treatment by the operator. To avoid inadvertent heat injury to important surrounding structures, the device automatically selected the safety margins for the skin, the trachea and recurrent laryngeal nerve and from the ipsilateral carotid artery. A laser-based movement detector enabled immediate power interruption when the patient moved or swallowed during ablation. To avoid skin burn, the skin was cooled by a balloon (filled with 10 °C liquids) at the tip of the treatment head. Both the total amount of energy delivered to the nodule (in KJ) and the ‘on-beam’ (sonification) time taken (in mins) were automatically recorded by the device’s computer. The ‘on-beam’ treatment time was the duration between the first to the last pulse (in minutes). Upon completion of treatment, patients were asked to rate their overall (global) pain experience during treatment (T1), 2 h after treatment (T2) and the following morning (T3). The pain level was scored on a visual analog scale (VAS) from 0 to 100 (0 = no pain and 100 = worse possible pain) by one dedicated person not directly involved with the study. Oral diet was resumed immediately afterwards and patients were discharged home a few hours after treatment. Afterwards, a transcutaneous laryngeal US was done to assess the mobility of both vocal cords as this was found to be a highly accurate alternative to laryngoscopy [Citation21]. Vocal cord palsy (VCP) was defined as having an impaired or absent movement in one of the vocal cords corresponding to the ablated side. All patients were seen on Day-4 at the clinic where serum TSH, FT4, Tg and anti-thyroid auto-antibodies were checked again.
Treatment efficacy
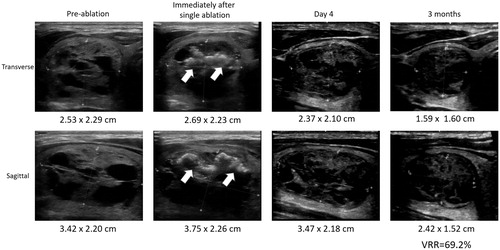
The extent of shrinkage of a treated nodule over time was taken as the treatment efficacy. Each nodule was measured by US on the day of treatment (baseline), 3-, 6- and 12-month. Nodule dimensions were measured using the LOGIQ e (GE Healthcare) scanner equipped with a 10-14 MHz linear matrix transducer. Three orthogonal diameters of the index nodule (its longest diameter and two other perpendicular diameters) were measured. In general, the longest diameter was the cranio-caudal dimension (length) of the nodule while the other two perpendicular diameters were the medio-lateral (width) and antero-posterior (depth) dimensions of the nodule. All measurements were made to the nearest 0.1 mm. To estimate nodule volume, we used the formula: volume (mL) = (width (in cm) × length (in cm) × depth (in cm)) × (π/6) where π was taken as 3.14159. The volume reduction ratio (VRR) was calculated based on the formula: [Baseline volume – volume at visit]/[Baseline volume] * 100. shows the echogenic change and nodule dimensions following treatment. Treatment success was defined as the VRR ≥ 50% from baseline.
Statistical analysis
Continuous variables were expressed as mean ± SD and/or median and interquartile range (IQR) when appropriate. For comparison of continuous variables, t-test was used when the data followed a normal distribution. Otherwise, the Mann-Whitney U test was used. Chi-square or Fisher’s Exact test was used to compare categorical variables. To determine independent factors affecting 12-month VRR, multivariate analysis were done by binary logistic regression analysis. In multivariate analysis, only parameters significantly associated with 12-month VRR > the median were entered together using the step down procedure. All statistical analyses were performed using SPSS version 20.0 (SPSS, Inc.). All significance tests were two-tailed and those with a p-value less than 0.05 were considered.
Results
During the study period, 202 consecutive patients completed their treatment for a benign thyroid nodule. Of these, 39 (19.3%) underwent two sequential treatment in one session, 14 (6.9%) patients had incomplete blood test results and 9 (4.5%) had a missed follow-up visit or follow-up period < 12 months. As a result, 140 (87.7%) were analyzed. Of these, 34 patients had their 6-month treatment efficacy reported previously [Citation22]. All patients completed their respective treatment successfully within the same session and were able to be discharged home on the same day. shows the baseline patient and nodule characteristics. In terms of treatment efficacy, the mean nodule volume at 3-month was 11.05 ± 13.42 ml and the mean VRR was 50.50 ± 22.96% (median = 53.27%; IQR = 27.16%). Ninety (64.3%) patients achieved treatment success. At 6 months, the mean VRR increased to 65.32 ± 19.80% (median = 68.32%; IQR = 30.10%). One hundred and seven (76.4%) patients achieved treatment success. At 12 months, the mean VRR increased to 68.72 ± 18.67% (median = 74.11%; IQR = 31.63%). Four (2.9%) patients suffered from a vocal cord paresis shortly after treatment but they all had a full recovery within 2 months.
Table 1. Baseline patient characteristics.
Patient characteristics and treatment parameters were compared between those whose 12-month VRR were > the median of 74% (group I) and between those whose 12-month VRR were ≤ the median of 74% (group II) (). Patient age, sex ratio, body weight, body height, body mass index and WHO nodule grade were comparable between the two groups. Side and location of nodule, the three nodule dimensions, baseline nodule volume and distance from skin to center of nodule were also comparable between the two groups. Baseline thyroid function, Tg and anti-thyroid autoantibody levels were not significantly different between the two groups (p > 0.05).
Table 2. A comparison of patient and treatment variables between those with 12-month volume reduction ratio above the median (Group I) and those with 12-month VRR equal to or below the median (group II).
However, on day 4 after treatment, group I had significantly lower serum TSH level than group II (0.49 mIU/L vs. 0.84 mIU/L, p = 0.011) and significantly higher FT4 level than group II (22.11 pmol/L vs. 18.47 pmol/L, p = 0.008). Both the percentage drop in TSH and rise in FT4 on day-4 were significantly greater for group I than group II (p = 0.002 and p = 0.009, respectively). For Tg, in group I, the mean level significantly rose from 170.32 ± 154.68 ng/mL to 1937.75 ± 1705.79 ng/mL (p < 0.001) while in group II, the mean level rose from 336.74 ± 715.22 ng/mL to 1909.31 ± 1959.24 ng/mL. The percentage rise in Tg (%) was significantly greater in group I than group II (6897.32% vs. 3183.65 ± 7936.95 p = 0.001).
Treatment parameters were comparable between the two groups. However, the number of HEMs observed tended to be higher in group I (21.75 vs. 17.44, p = 0.078) and the proportion of HEMs observed during treatment was significantly higher in group I than group II (42.69% vs. 31.72%, p = 0.030).
Given that the change in serum TSH and FT4 were inter-correlated, both factors were entered first into regression model and of the two co-variates but only the change in FT4 was significant (OR = 1.104, 95%CI = 1.011 − 1.029, p = 0.040). Therefore, the change in serum FT4 was entered as a co-variate (instead of change in TSH) into the final model. When all three significant factors (i.e., percentage change in FT4, in Tg and proportion of HEMs observed) were entered into the final model (), only the percentage change in FT4 was an independent, significant factor for 12-month VRR above the median of 74% (OR = 1.018, 95%CI = 1.003 − 1.032, p = 0.017). If percentage change in FT4 was categorized, it was found that those with a change in serum FT4 of >20.0% had a significantly greater 12-month VRR than those with a change in serum FT4 of ≤20.0% (74.82% vs. 65.16%, p = 0.021).
Table 3. Binary logistic regression analysis of clinical variables predictive of nodule shrinkage (or volume reduction ratio) above the median 12 months after single treatment.
Discussion
Although surgical excision i.e., thyroidectomy remains the treatment of choice for the majority of patients with a symptomatic benign thyroid swelling, image-guided thermal ablation has rapidly become an acceptable primary treatment (i.e., not only confined to patients unwilling or unfit for surgery or general anesthesia) [Citation23,Citation24]. Apart from the benefits of avoiding a general anesthesia and hospitalization, thermal ablation can preserve and maintain the underlying normal thyroid function [Citation22–24]. As a result, there has been a growing interest in developing minimally invasive, non-surgical techniques. Among all thermal ablation options including HIFU, RFA and laser ablation remain to be the most established and used techniques in the treatment of benign thyroid nodules [Citation25]. In comparison, the adoption for HIFU ablation for both toxic and nontoxic thyroid diseases has been relatively slow as the longer-term efficacy remains unclear [Citation26,Citation27].
Our results showed that the extent or percentage of serum FT4 change on Day-4 after single HIFU treatment was not only significantly associated with 12-month nodule shrinkage but was also an independent parameter for the subsequent nodule shrinkage at 12 months. After taking into account of the change in serum Tg on Day-4 and proportion of HEMs observed during ablation, the rise in FT4 level was the only significant factor for 12-month nodule shrinkage (OR = 1.018, 95%CI = 1.003 − 1.032, p = 0.017). It is worth noting that the extent of nodule shrinkage after single HIFU treatment was significant greater among those whose serum FT4 level on Day-4 rose by more than 20% from baseline level as compared to those whose serum FT4 did not rise by the similar amount (74.82% vs. 65.16%, p = 0.021). Although studies previously reported similar hemodynamic changes in serum TSH, FT4 and Tg 24 h after ablation, no correlation between the post-ablative changes and subsequent nodule shrinkage was reported [Citation28,Citation29]. Given the fact that HIFU is only one form of thermal ablation, we believe our findings could potentially be applicable to other forms of thermal ablation to the thyroid gland like RFA and laser ablation.
Although, as said, similar changes in TSH, FT4 and Tg shortly after ablation were described previously [Citation28,Citation29], the exact mechanism remains unclear. The most popular and likely mechanism is due to the release of intra-glandular store of thyroid hormones after heat damage to the follicles inside the target nodule. This could also explain why there appeared to be a direct association between the percentage rise in FT4 level on Day-4 and the extent of nodule shrinkage at 12-month. A more extensive nodule damage by HIFU would result in a greater amount of thyroid hormones and Tg being released into the systemic circulation and that in turn lead to a higher rise in serum levels. Therefore, we believe the sharp rise in FT4 and Tg levels were a result of the release of intra-glandular stores from thermal damage while the drop in TSH level on Day-4 was a normal physiological secondary response from the pituitary following the sharp rise in FT4 on Day-4.
Even though one previous study did find the change in serum Tg on Day-4 significantly correlated with nodule shrinkage [Citation30], we think the major advantage of using the change in serum FT4 over serum Tg is the fact that Tg level measurement is often interfered by the presence of anti-Tg auto-antibody [Citation31]. Therefore, it is clinically more convenient and reliable to measure the change of FT4 on Day-4 relative to baseline as a biomarker over serum Tg to determine subsequent treatment efficacy. If this could be further confirmed in future studies, perhaps, measuring the post-ablative FT4 level could facilitate the decision for earlier second ablation for less satisfactory treated nodules, instead of the ‘wait and watch’ approach [Citation13].
Despite these findings, we would like to acknowledge several shortcomings. First, our study was only a moderate-sized study and so, some of the non-significant findings might simply be due to inadequate power of the study. Second, since the analysis was based on retrospective data, it would be important to prospectively evaluate and confirm the role of FT4 percentage change as a biochemical predictor for treatment efficacy.
Conclusion
Our study showed that there was a significant association between the percentage FT4 level rise on day-4 and the extent of nodule shrinkage 12 months after single HIFU treatment. Also, the percentage serum FT4 change on Day-4 relative to baseline after single HIFU treatment was an independent blood parameter for the subsequent nodule shrinkage at 12 months. These findings could potentially facilitate clinicians to decide on a more-timely reintervention of target treated nodules.
Author contributions
BHH Lang/YC Woo/KWH Chiu were involved in the review of literature, acquisition of data and drafting and completing the manuscript. BHH Lang/YC Woo/KWH Chiu were also involved in the review of literature and drafting the manuscript. BHH Lang/YC Woo/KWH Chiu conceived the study, participated in the co-ordination and the acquisition of data and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgment
The authors thank Mr. Hill Yu for conducting all the pain assessments during and after HIFU treatment.
Disclosure statement
All authors had nothing to disclose. No competing financial interests exist.
References
- Gharib H, Papini E, Garber JR, et al. AACE/ACE/AME task force on thyroid nodules. American association of clinical endocrinologists. American college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules – 2016 update. Endocr Pract. 2016;22(5):622–639.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. Jama. 2015;313(9):926–935.
- Korkusuz Y, Gröner D, Raczynski N, et al. Thermal ablation of thyroid nodules: are radiofrequency ablation, microwave ablation and high intensity focused ultrasound equally safe and effective methods?. Eur Radiol. 2018;28(3):929–935.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33(8):1–919.
- Korkusuz H, Fehre N, Sennert M, et al. Volume reduction of benign thyroid nodules 3 months after a single treatment with high-intensity focused ultrasound (HIFU). J Ther Ultrasound. 2015;3(1):44.
- Kovatcheva RD, Vlahov JD, Stoinov JI, et al. Benign solid thyroid nodules: US-guided high-intensity focused ultrasound ablation-initial clinical outcomes. Radiology. 2015;276(2):597–605.
- Lang BH, Woo YC, Wong C. High-intensity focused ultrasound for treatment of symptomatic benign thyroid nodules: a prospective study. Radiology. 2017;284(3):897–906.
- Trimboli P, Bini F, Marinozzi F, et al. Giovanella L High-intensity focused ultrasound (HIFU) therapy for benign thyroid nodules without anesthesia or sedation. Endocrine. 2018;61(2):210–215.
- Trimboli P, Pelloni F, Bini F, et al. Giovanella L High-intensity focused ultrasound (HIFU) for benign thyroid nodules: 2-year follow-up results. Endocrine. 2019;65(2):312–317.
- Prakash PS, Oh HB, Tan WB, et al. The efficacy and safety of high-intensity focused ultrasound (HIFU) therapy for benign thyroid nodules-a single center experience from Singapore. World J Surg. 2019;43(8):1957–1963.
- Lang BH, Woo YC, Chiu KW. Role of second high-intensity focused ultrasound (HIFU) treatment for unsatisfactory benign thyroid nodules after first treatment. Eur Radiol. 2019;29(3):1469–1478.
- Lang BH, Woo YC, Chiu KW. Single-session high-intensity focused ultrasound treatment in large-sized benign thyroid nodules. Thyroid. 2017;27(5):714–721.
- Sennert M, Happel C, Korkusuz Y, et al. Gröner D further investigation on high-intensity focused ultrasound (HIFU) treatment for thyroid nodules: effectiveness related to baseline volumes. Acad Radiol. 2017;pii(17):30335–30335, S1076-6332.
- Lang BHH, Woo YC, Chiu KWH. The percentage of serum thyroglobulin rise in the first-week did not predict the eventual success of high-intensity focussed ablation (HIFU) for benign thyroid nodules. Int J Hyperthermia. 2017;33(8):1–887.
- Lang BHH, Woo YC, Chiu KW. Significance of hyperechoic marks observed during high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Eur Radiol. 2018;28(6):2675–2681.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Zimmermann M, Saad A, Hess S, et al. Thyroid ultrasound compared with World Health Organization 1960 and 1994 palpation criteria for determination of goiter prevalence in regions of mild and severe iodine deficiency. Eur J Endocrinol. 2000;143(6):727–731.
- Bini F, Trimboli P, Marinozzi F, et al. Treatment of benign thyroid nodules by high intensity focused ultrasound (HIFU) at different acoustic powers: a study on in-silico phantom. Endocrine. 2018;59(3):506–509.
- Wong KP, Lang BH, Ng SH, et al. A prospective, assessor-blind evaluation of surgeon-performed transcutaneous laryngeal ultrasonography in vocal cord examination before and after thyroidectomy. Surgery. 2013;154(6):1158–1164.
- Lang BHH, Wong CKH, Ma EPM, et al. A propensity-matched analysis of clinical outcomes between open thyroid lobectomy and high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Surgery. 2019;165(1):85–91.
- Papini E, Pacella CM, Solbiati LA, et al. G. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Kim JH, Baek JH, Lim HK, et al.; Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. 2017. Korean J Radiol. 2018;19(4):632–655.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: A systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Lang BH, Woo YC, Chiu KW. Two-year outcomes of single-session high-intensity focused ultrasound (HIFU) treatment in persistent or relapsed Graves’ disease. Eur Radiol. 2019;29(12):6690–6698.
- Giovanella L, Piccardo A, Pezzoli C, et al. Comparison of high intensity focused ultrasound and radioiodine for treating toxic thyroid nodules. Clin Endocrinol. 2018;89(2):219–225.
- Heck K, Happel C, Grünwald F, et al. Percutaneous microwave ablation of thyroid nodules: effects on thyroid function and antibodies. Int J Hyperthermia. 2015;31(5):560–567.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20(11):1253–1261.
- Lang BHH, Woo YC, Chiu KW. Changes in serum thyroglobulin and antithyroglobulin shortly following high-intensity focused ablation of benign thyroid nodules in patients with positive antithyroglobulin status. Int J Hyperthermia. 2018;35(1):637–643.
- Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83(4):1121–1127.