Abstract
Objective
To determine the clinical efficacy of laser ablation for the tredatment of primary hyperparathyroidism (pHPT).
Materials and methods
Twelve patients with pHPT were treated with laser ablation. Energy was administered by means of 1.5 m optical fibers percutaneously placed into the target via 21 G needles. A laser ablation unit (EchoLaser X4, Esaote) applied 3 W power for 400–600 s/fiber/insertion to a total 3600–9000 Joules of energy. Patient serum parathyroid hormone (PTH) and calcium levels were checked at baseline and thereafter every 6 months. Patients were followed-up for 2 years with serologic and contrast-enhanced ultrasound. Therapeutic success was defined as normal PTH and calcium levels together with disappearance of nodule-related symptoms.
Results
All procedures were performed in single session. Immediately following ablation, contrast enhanced ultrasound confirmed that all but one target had become avascular (technical success rate 11/12; 92%), remaining avascular at all follow-up ultrasound examinations, thereafter. The mean volume of parathyroid nodules decreased from 0.54 cc to 0.36 cc (72.0%). Serum PTH and calcium levels were significantly lower at 1, 12 and 24 m compared to baseline (p < 0.01). By 6 m, PTH and calcium returned to normal and were stable until 24 m in all successfully treated patients. All cases of hyperparathyroid-related symptoms resolved by 6 m (ostealgia [n = 5], repeated renal colic [n = 5], vomiting [n = 3]). Only one patient (8%) reported transient dysphonia as a minor complication.
Conclusion
Laser ablation of enlarged, symptomatic parathyroid glands is safe and well-tolerated and can produce long-term, sustained reduction of serum PTH and calcium levels.
Introduction
Primary hyperparathyroidism (pHPT) is the most common cause of chronic hypercalcemia, with its incidence increasing with age [Citation1]. Single-gland adenoma is the most common cause (75–85%), and in most patients, it can be clearly identified by ultrasound [Citation2]. Surgery is the recommended treatment for patients with symptomatic pHPT due to its high long-term cure rate. However, potentially both radical neck exploration and associated procedural general anesthesia can be associated with morbidity. This is even further increased in elderly patients and/or patients with comorbidities that are contraindications to surgery under general anesthesia [Citation1,Citation3–5]. Furthermore, pHPT patients with few or no symptoms, most often detected during screening programs, may decline surgery. For these reasons, it is often difficult to advise individual patients about the correct balance between long-term advantages and disadvantages of surgery. This explains the considerable interest in identifying therapeutic alternatives to surgery for pHPT.
Initially, minimally invasive image-guided therapy for pHPT was performed with chemical ablation, using ethanol as the sclerosing agent [Citation6,Citation7]. However, over time this approach aroused progressively reduced enthusiasm, particularly as the success rate of percutaneous ethanol instillation was found to be inversely correlated with the size of the parathyroid tumor and duration of follow-up [Citation8–13]. Moreover, side effects were not uncommon, and included pain, vocal cord affection and extra-parathyroid fibrosis, which could hamper subsequent surgery [Citation10], if needed. Some of these issues have been directly attributed to poor control of the diffusion of the chemical agent. Thus, the use of thermal ablation sources could potentially overcome these challenges – if adequate precision and safety could be achieved. One potential solution is the use of laser ablation whose energy distribution pattern may be more focal and controlled than radiofrequency (RF) or microwave ablation (MW) [Citation14–17]. Specifically, light energy has a potential advantage in that it may be delivered interstitially by implanting extremely thin lasers fiber directly into body tissues under real-time imaging guidance, potentially further reducing issues of cosmesis that may accompany larger applicator sizes [Citation17]. Laser thermal ablation has been applied experimentally and clinically [Citation14–17] for palliative therapy in a variety of primary and secondary malignant neoplasms and also for the ablation of benign lesions. Thus, the aim of the present study was to evaluate the feasibility, safety and efficacy of laser ablation for the treatment of pHPT in patients who were ineligible for surgery or refused surgery.
Materials and methods
This retrospective study was approved by our local ethical committee, and all patients signed informed consent prior to each procedure.
Study cohort
From 3/2011 to 3/2014, a total of 12 patients (three men and nine women; mean age 67 y ± 8.2; age range, 43–81 y) were treated with US-guided laser ablation in our department. All patients had a single target nodule located in one side of the neck. Patients were enrolled if they fulfilled the following criteria: age ≥18 years, diagnosis of hyperparathyroidism on the basis of recommendations proposed by the International Workshop on pHPT, i.e., lesion diameter ≤30 mm, lack of suitability or willingness to undergo surgery and follow-up for at least 2 years after ablation [Citation7]. The baseline size of the parathyroid nodules (largest diameter measured at ultrasound), and patient demographic details, baseline serum parathyroid hormone (PTH) and calcium levels are summarized in . All had classic sonographic features of a hypervascular, hypoechoic, capsulated solid nodule, in 8/12 (66.7%) cases lying in close proximity with the laryngeal recurrent nerve.
Table 1. patient demographic and laboratory details.
Imaging and ablation equipment
The ablation unit (EchoLaser X4, Esaote, Genova, Italy) used combines both an ultrasound scanner and a 4–13 MHz linear transducer with a laser system. This system has four independent laser channels and is based upon a Diode laser of 1064 nm wavelength that produces a beam with diameter of 0.3 mm and an output power level ranging from 1 to 7 W. Optical fibers of 1.5 m with a 300 µm core can be percutaneously placed into the target via 21 G needles. A needle guide with different angles enables one or two simultaneous insertions. Three watts power was applied for 400–600 s/fiber/insertion with 1600–7200 Joules total energy applied. A pull-back technique was employed by treating the deepest portion of the nodule until hyperechogenicity surrounded the fiber at which point the fiber was manually retracted to enable treatment of more superficial portions of the lesion [Citation17]. The system has a touch screen laser display, allowing to independently select the energy delivered by each fiber. The procedure was technically facilitated by the use of dedicated software enabling to precisely plan the location of needle insertion and to predict final ablation volume (Elesta Echolaser, Calenzano, FI, Italy).
In all patients, contrast-enhanced ultrasound (CEUS) was performed immediately before and 5–7 min after the end of ablation. A single dose of 4.8 ml of sulfur hexafluoride microbubbles (SonoVue, Bracco, Italy) was intravenously administered both before and after ablation to monitor and assess the therapeutic result before terminating the ablation session. Contrast-specific software (Contrast-tuned Imaging – CnTI, Esaote, Italy) in continuous mode, with very low mechanical index (0.01–0.1) was employed. The aim of CEUS was to demonstrate the complete post-ablation avascularity of all the parathyroid adenomas, in comparison with their pre-ablation diffuse hypervascularity (mostly in arterial phase).
Procedure
All treatments were performed as outpatient procedures by a team of two experienced radiologists working in tandem, each have been performing ultrasound-guided biopsies and ablations for clinical care for over 10 and 25 years, respectively. Patients were placed in a supine position with their neck extended. Local anesthesia was achieved by subcutaneous (up to the anterior surface of the parathyroid adenoma) injection of 1–2 ml of 2% lidocaine (AstraZeneca, Basiglio, MI, Italy) and 3 ml of Ropivacaine (Aspen Pharma, Dublin, Ireland). Next, the needle insertion path and the ablation volume to be achieved was planned using the dedicated planning software system. The adenoma was then targeted under direct ultrasound guidance along its longest axis, using either one or two simultaneous insertions ( and ). Once adequate positioning of the fiber was ascertained by ultrasound, laser energy was delivered and controlled independently for each fiber. Pull-back of the fibers commenced once the echogenicity reached the margin of the gland. The fiber tip was initially positioned in the deepest portion of the nodule, and once an echogenic spot of approximately 10-mm diameter and length surrounded it, the fiber was gradually withdrawn in millimeter increments more proximally toward the superficial untreated portion of the tumor. Throughout the entire procedure, the patients were intermittently interrogated, with the intent of assessing their status of phonation. Upon completing the treatment, we attempted to minimize bleeding complications with light compression on the neck for 15 min and subsequently evaluated for any complications while keeping patients under direct observation for 2–4 h. Patients were discharged if there was no complication that required hospitalization.
Figure 1. Single-fiber laser ablation for primary hyperparathyroidism. (A) A 69-year-old female presented with severe osteoporosis, nephrocalcinosis and elevated parathormone levels. A 1.6 × 0.6 cm adenoma was identified behind the lower pole of the thyroid. (B) Contrast enhanced ultrasound confirms marked homogeneous vascularity. (C) Gray scale ultrasonography demonstrates insertion of a single fiber (central white line) along the longitudinal axis of the gland. Normalization of parathormone was noted at 6 months. (D) Two-year follow-up shows a marked reduction in the gland size (0.7 × 0.4 cm) with elimination of vascularity at contrast enhanced ultrasound (yellow circle).
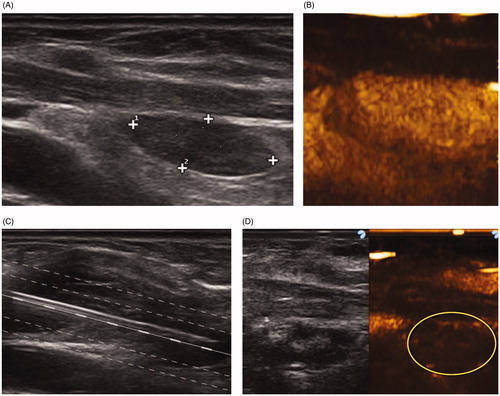
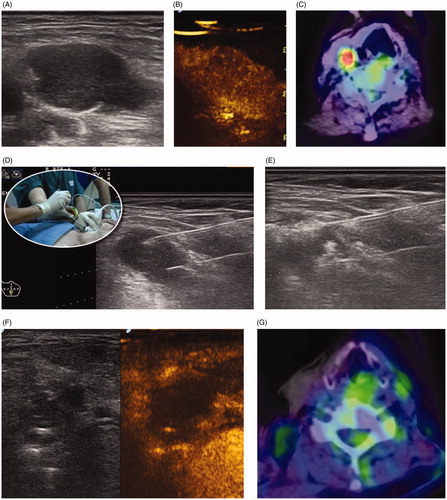
Figure 2. Dual-fiber laser ablation for primary hyperparathyroidism. (A) A 65-year-old female presented with increasing bone pain and arthralgia, increased parathormone levels and hypercalcemia. A 2.0 × 1.3 cm adenoma was identified at gray scale ultrasound caudal to the thyroid. (B) Contrast enhanced ultrasound demonstrated a heterogeneously enhancing nodule. (C) Baseline Tc-99 Sestamibi is highly positive (red color). (D) Intraprocedural gray scale ultrasound demonstrated the placement of two laser fibers spaced 1 cm apart inserted into the gland. (E) Increased echogenicity is identified surrounding the fibers during the active application of laser energy. Normalization of parathormone and calcium levels occurred by 6 months. (F) Two-year follow-up gray scale and contrast enhanced ultrasound demonstrate a non-enhancing 0.9 × 0.7 cm gland. (G) Tc-99 Sestamibi shows no evidence for increased uptake.
Pre-ablation assessment
All patients were evaluated by ultrasound examination, 99mTc sestamibi scintigraphy, laboratory examinations and clinical symptoms assessment. The ultrasound assessment included size, location and volume documentation (V = 0.5 × height × depth × width). Laboratory examinations included measurements of serum PTH (normal range, 20–80 pg/mL, Intact PTH (Beckman Coulter S.p.A., Cassina de’ Pecchi, Milan, Italy), and serum Calcium, (Arsenazo- Beckman Coulter S.p.A., Cassina de’ Pecchi, Milan, Italy, normal ranges from 8.6 to 10.3 mg/dl), in addition to platelet count and blood coagulation tests. Given that a large portion (up to 45%) of serum calcium bound to proteins, mainly albumin, in all our patients the reported serum calcium was corrected for albumin using the following calculation: corrected calcium = measured calcium (mg/dl) + 0.8 × [4 – serum Albumin (g/dl)].
Follow-up evaluation
All patients underwent a medical visit, ultrasound and blood tests for serum PTH and calcium measurements prior to the procedure, and at 1, 6, 12 and 24 months after the procedure. During these visits, potential nodule-related symptoms such as bone pain and renal colic, and/or other complications (hematomas, skin burns, fever, voice change, brachial plexus injury and Horner syndrome) were recorded. Therapeutic success was defined by normal PTH and calcium levels at 24-month follow-up together with disappearance of nodule-related symptoms.
Statistical analysis
Values for quantitative variables are expressed as means ± standard deviation and range. Wilcoxon signed-rank tests were used to compare changes in largest nodule diameter, volume, vascularity and serum PTH and calcium levels from before ablation to follow-up. Differences were considered statistically significant when the p value was less than 0.05. All analyses were conducted using SPSS for Windows (version16.0; IBM, Armonk, New York).
Results
All procedures were performed in a single session and all except one were successful (technical success rate of 11/12 = 92%). Three patients (25%) had two fibers activated, with the remainder of the procedures performed using a single fiber. The single refractory patient had an exceptionally high PTH level (2000) that did not decrease significantly post-ablation, and thus, after the 6-month follow-up he was referred to surgical parathyroidectomy. The pathological specimen clearly documented a central focus of ablated tissue in the center of this gland. All treatments were performed administering 3 W power for a duration ranging from 400 to 600 s/fiber/insertion. The mean total amount of energy delivered was 3533 ± 1893 Joules. The mean duration of the energy delivery was 10.2 min ± 1.2 (range 8.0–14 min).
Treatment response and clinical outcome
Characteristics of the parathyroid nodules were measured before laser ablation and at each follow-up period. Largest diameter, volume and vascularity of the nodules were significantly lower at last follow-up than before treatment. The largest diameter decreased from 3.0 to 2.1 cm. The mean volume of parathyroid nodules decreased from 0.54 to 0.36 cc (72.0 ± 15.9%). Following ablation, contrast enhanced ultrasound confirmed that all targets had become avascular, and with the exception of the single case of recurrence remained avascular at all follow-up ultrasound examinations.
The changes in serum PTH and calcium levels before ablation and at each follow-up period are summarized in . Serum PTH and calcium levels were significantly lower at 1, 12 and 24 months compared to before treatment (p < 0.01 for all comparisons), with stable reductions seen when comparing 12 to 24 m (p > 0.50). In 11/12 (91.7%) patients, PTH and calcium levels returned to normal, and 99mTc sestamibi scintigraphy confirmed success of the ablation ( and ). These, together with disappearance of nodule-related symptoms including ostealgia (in all five cases where present), and vomiting (three patients) by 6 months post-ablation revealed the effectiveness of ablation. Likewise, none of the five patients with repeated bouts of renal colic reported further episodes over the 2-year follow-up.
Table 2. The changes in serum PTH and albumin- corrected calcium levels before ablation and at each follow-up period.
Side effects and complications
The treatment was well tolerated. A mild sensation of heat in the neck was experienced by most patients, but no patient requested termination of the procedure. Only one patient (8.3%) reported a transient voice change, and laryngoscopic evaluation demonstrated vocal-cord palsy, but this resolved without any treatment within 3 weeks. No cases of local infection, skin burning or damage to the vital structures were observed.
Discussion
Surgical parathyroidectomy is the gold-standard treatment for pHPT. However, not all patients are ideal candidates or even eligible for surgery. Thus, early interventional oncologists proposed US-guided percutaneous ethanol injection to parathyroid adenomas and proved its utility in treating pHPT in highly selected patients [Citation6,Citation7]. Nevertheless, because of the need for repeated ethanol injections, based in part upon the significant incidence of relapse, and reported side effects, the use of percutaneous ethanol injection for pHPT is less than ideal [Citation8–13]. Accordingly, in a manner similar to the ultimate migration from chemical ablation to thermal based methods for the treatment of hepatocellular carcinoma [Citation18], RF and microwave have been proposed as possible ablation techniques for PTA ablation. Thus, several series such as by Liu et al. [Citation19], have shown MW ablation to be feasible. However, the very high temperatures of microwave have raised a substantial concern of safety, particularly issues of overtreatment leading to damage of adjacent structures by high energy sources [Citation20].
US-guided laser ablation has been proposed as a potentially better match for PTA ablation. Prior reports have noted extremely well-defined, well-demarcated zones of ablation, based upon limited energy penetration. Thus, although these attributes of laser ablation may not be ideal for the treatment of liver metastases and have led to greater adoption of other energy sources in the liver [Citation21–24], these very properties are likely to be more suited for parathyroid pathology.
Several series of US-guided laser ablation for parathyroid adenomas have been published, all based either upon few patients or short post treatment follow-up period. Andrioli et al. [Citation16], published a series of six patients, with a mean duration of follow-up of 54 ± 34 months. Laser ablation therapy was safe and without permanent side effects. Similar to our series, one patient reported transient dysphonia. Two months after laser ablation, serum PTH and calcium levels decreased in six and five patients, respectively. At the last follow-up examination, serum PTH and calcium levels were above the normal range in six and three patients, respectively. Nevertheless, three patients (i.e., half the population) underwent surgery for persistent pHPT. Overall, they concluded that laser ablation produces a transient reduction of serum PTH and calcium levels but not a lasting resolution of hyperparathyroidism. On the other hand, Jiang et al. [Citation25], treated 21 patients, with only 1-year follow-up. They used contrast-enhanced sonography guidance for the procedure. Normalization of PTH and serum calcium was achieved in 81% of the patients at 1 year follow-up. No serious complications were observed. They concluded that ultrasound-guided PTA laser ablation with CEUS is a viable alternative to surgery for primary parathyroid adenoma.
In our study, we report upon 12 patients with a full 2-year follow-up. All procedures were performed in a single session and all except one were successful (technical success rate of 11/12 = 92%). This single refractory patient had an exceptionally high PTH level that did not decrease significantly post-ablation, and thus, after the 6-month follow-up he was referred to surgical treatment. The treatment was well tolerated by all patients. Vocal-cord palsy in one patient resolved without any treatment within 3 weeks. On the other hand, resolution of the clinical symptoms of bone pain and repeat renal colic associated with hyperparathyroidism were noted in all 10 patients where present by 6-months follow-up.
Interestingly, for the majority of patients (and all successfully treated patients) we noted a slow, but progressive return to normal serological levels. Trends to normalization of calcium and PTH were seen at 1 m, but only truly normal values were achieved at 6 months. Most notably these remained stable and within the normal range in all cases by the 2-year follow-up.
Although we demonstrate excellent 2 years outcomes including resolution of the major clinical symptoms and complaints in the majority of our patients, it must be noted that several earlier series reported only transient reduction of serum PTH and calcium levels, with recurrence of hyperparathyroidism, for example the study by Andrioli mentioned earlier [Citation16]. Analysis of these suggest that their population had much greater and/or aggressive disease. Indeed, we note the relatively small volumes of the parathyroid glands in our study. Accordingly, similar to most other ablation procedures, judicious patient selection is likely critical for ensuring optimal results, and further study is needed to better define and categorize the different patient sub-populations. Alternatively, differences in technique may be responsible for the differences in results achieved. For example, it is well-known that parathyroid cells have the capacity to grow and replicate [Citation26,Citation27]. Persistence of even a few adenomatous parathyroid cells after ablation might result in suboptimal efficacy of ablation and require more treatment sessions. Therefore, complete ablation of the periphery of the parathyroid nodule is important to prevent marginal regrowth. Indeed, we note greater amounts of energy per target volume deposited in our study compared to previous series [Citation16].
Overall, we found that laser ablation creates a sharp demarcation line between the treated-necrotic tissue, and the untreated surrounding structures [Citation26]. We further note that contrast enhanced ultrasound enables to clearly depict this demarcation line and to add ablation session if necessary. This ensures complete ablation with minimal surrounding damage and minimizes the risk of recurrence. Moreover, one potential benefit of laser is the ability to split the energy delivery via multiple fibers in an array [Citation28]. Likewise, we note that the thin pliant laser fibers were extremely easy to use. Given that they are less cumbersome than larger MW and RF probes, they may cause less damage and hence hold the potential for better cosmesis than other methods of thermal ablation.
Clearly, our work is subject to many of the same limitations facing many retrospective reports of interventional oncologic procedures performed for relatively rare disease states or conditions. Specifically, we note the limited number of patients and limited follow-up interval. Indeed, we acknowledge that additional recruitment and further study may enable us to gain further insights such as whether or not we can identify which patients present with findings that make therapy unlikely to be successful. A case in point is the unsuccessful treatment of our patient with the highest PTH level. This suggests that there may be a predictive cutoff above which the disease is too aggressive to realistically anticipate success. Yet, a single case is clearly insufficient to draw definitive conclusions on this issue.
Conclusion
Our results using laser ablation to treat patients with functional parathyroid adenomas demonstrate high levels of sustained complete response at the clinically relevant follow-up period of 24 months compared. Hyperparathyroid clinical symptoms disappeared by 6 months and sustained serologic normalization of parathormone and calcium were seen by 6 months as well. Although these findings support the use of laser ablation as an effective minimally invasive therapy for primary hyperthyroidism, future work including larger series with more patients and longer follow-up periods is likely warranted. Defining the true range of optimal patients and the upper limit in size and number for a single or multiple fiber approach should be further investigated as well.
Medical relevance
Our results using laser ablation to treat patients with functional parathyroid adenomas demonstrate high levels of sustained complete response at the clinically relevant follow-up period of 24 months compared.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Khan AA, Hanley DA, Rizzoli R, et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos Int. 2017;28(1):1–19.
- Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism. Lancet. 2018;391(10116):168–178.
- Kovatcheva RD, Vlahov JD, Shinkov AD, et al. High-intensity focused ultrasound to treat primary hyperparathyroidism: a feasibility study in four patients. AJR Am J Roentgenol. 2010;195(4):830–835.
- Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98(3):1122–1129.
- Bilezikian JP, Silverberg SJ. Clinical practice. Asymptomatic primary hyperparathyroidism. N Engl J Med. 2004;350(17):1746–1751.
- Rubin MR, Bilezikian JP, McMahon DJ, et al. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab. 2008;93(9):3462–3470.
- Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3561–3569.
- Marcocci C, Cianferotti L, Cetani F. Bone disease in primary hyperparathyroidism. Ther Adv Musculoskelet Dis. 2012;4(5):357–368.
- Campbell MJ. The definitive management of primary hyperparathyroidism: who needs an operation? JAMA. 2017;317(11):1167–1168.
- Udelsman R. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg. 2002;235:665–670.
- Solbiati L, Giangrande A, De Pra L, et al. Percutaneous ethanol injection of parathyroid tumors under US guidance: treatment for secondary hyperparathyroidism. Radiology. 1985;155(3):607–610.
- Bennedbaek FN, Karstrup S, Hegedüs L. Percutaneous ethanol injection therapy in the treatment of thyroid and parathyroid disease. Eur J Endocrinol. 1997;136(3):240–250.
- Karstrup S, Holm HH, Glenthoj A, et al. Nonsurgical treatment of primary hyperparathyroidism with sonographically guided percutaneous injection of ethanol: results in a selected series of patients. AJR. 1990;154(5):1087–1090.
- Bennedbaek FN, Karstrup S, Hegedüs L. Ultrasound guided laser ablation of a parathyroid adenoma. BJR. 2001;74(886):905–907.
- Adda G, Scillitani A, Epaminonda P, et al. Ultrasound-guided laser thermal ablation for parathyroid adenomas: analysis of three cases with a three-year follow-up. Horm Res Paediatr. 2006;65(5):231–234.
- Andrioli M, Riganti F, Pacella CM, et al. Long-term effectiveness of ultrasound-guided laser ablation of hyperfunctioning parathyroid adenomas: present and future perspectives. AJR Am J Roentgenol. 2012;199(5):1164–1168.
- Pacella CM, Bizzarri G, Guglielmi R, et al. Thyroid tissue: US-guided percutaneous interstitial laser ablation—a feasibility study. Radiology. 2000;217(3):673–677.
- Livraghi T, Goldberg SN, Lazzaroni S, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210(3):655–661.
- Liu C, Wu B, Huang P, et al. US-guided percutaneous microwave ablation for primary hyperparathyroidism with parathyroid nodules: feasibility and safety study. J Vasc Interv Radiol. 2016;27(6):867–875.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. 2009;251(3):933–940.
- Isbert C, Roggan A, Ritz JP, et al. Laser-induced thermotherapy: intra- and extralesionary recurrence after incomplete destruction of experimental liver metastasis. Surg Endosc. 2001;15(11):1320–1326.
- Giorgio A, Tarantino L, de Stefano G, et al. Interstitial laser photocoagulation under ultrasound guidance of liver tumors: results in 104 treated patients. Eur J Ultrasound. 2000;11(3):181–188.
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–968.
- Meijerink MR, Puijk RS, van Tilborg A, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41(8):1189–1204.
- Jiang T, Chen F, Zhou X, et al. Percutaneous ultrasound-guided laser ablation with contrast-enhanced ultrasonography for hyperfunctioning parathyroid adenoma: a preliminary case series. Int J Endocrinol. 2015;2015:1–6.
- Mauri G, Nicosia L, Della Vigna P, et al. Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography. 2019;38(1):25–36.
- Guerin C, Paladino NC, Lowery A, et al. Persistent and recurrent hyperparathyroidism. Updates Surg. 2017;69(2):161–169.
- Stafford RJ, Fuentes D, Elliott AA, et al. Laser-induced thermal therapy for tumor ablation. Crit Rev Biomed Eng. 2010;38(1):79–100.
