Abstract
Purpose
To investigate technical success, technique efficacy, safety and outcome of MR-guided microwave ablation (MWA) in hepatic malignancies.
Material and methods
In this prospective IRB-approved study, patients scheduled for percutaneous treatment of hepatic malignancies underwent MR-guided MWA in a closed-bore 1.5 T MR system. Technical success was assessed on post-procedural MR control imaging. Technique efficacy was evaluated 4 weeks after the procedure on multi-parametric MRI. Assessment of safety followed the Society of Interventional Radiology grading system. Kaplan–Meier survival estimates were calculated to evaluate overall survival (OS), time to local tumor progression (TLTP), and time to non-target progression (TNTP).
Results
Between 2015 and 2019, 47 patients (60.5 ± 12.2 years; 39 male) underwent 50 procedures for 58 hepatic tumors (21 hepatocellular carcinomas; 37 metastases). Mean target tumor size was 16 ± 7mm (range: 6–39 mm). Technical success and technique efficacy were 100% and 98%, respectively. Lesions were treated using 2.6 applicator positions (range: 1–6). Mean energy, ablation duration per tumor, and procedure duration were 43.2 ± 23.5 kJ, 26.7 ± 13.1 min and 211.2 ± 68.7 min, respectively. 10 minor (20%) and 3 major (6%) complications were observed. Median post-interventional hospital admission was 1 day (range: 1–19 days). Median OS was 41.6 (IQR: 26.4–) months. Local recurrence occurred after 4 procedures (8%) with TLTP ranging between 3.1 and 41.9 months. Non-target recurrence was observed in 64% of patients after a median TNTP of 13.8 (IQR 2.3–) months.
Conclusion
MR-guided MWA allows for safe and successful treatment of hepatic malignancies with a high technique efficacy however with relatively long procedure durations.
Introduction
Minimally invasive, percutaneous ablation has become an integral part in modern treatment regimes of local and metastatic neoplastic diseases [Citation1,Citation2]. This is particularly true for the management of primary and secondary hepatic malignancies, for which local tumor ablation procedures are embedded in current guidelines [Citation3–5].
While radiofrequency ablation (RFA) has been the most commonly used approach for the last decades, several new techniques have been developed with the aim to improve treatment efficiency and safety [Citation6,Citation7]. The most widely used alternative approach to RFA is microwave ablation (MWA) given the more beneficial physical features such as independence against increasing tissue impedance during the coagulation process and reduced susceptibility to target tissue conductivity (i.e. heat sink effect) [Citation8]. This results in higher intra-target lesion temperatures, which essentially allows for generating larger ablation zones in a shorter time making it the more preferable technique [Citation9–11]. In addition to the device, the imaging modality used for guidance is key for a successful and safe ablation procedure to ensure precise positioning of the applicator and reliable therapy monitoring to avoid damage of adjacent anatomical structures [Citation12]. Given their widespread availability and relatively low cost, ultrasound and computed tomography are the most commonly used imaging modalities [Citation13,Citation14]. However, their obvious benefits come at the cost of potentially limited visualization of the target tumor during planning (e.g. patient´s body composition), monitoring (e.g. formation of gas bubbles, poor image contrast) and controlling (e.g. limited differentiation between residual tumor and ablation zone) with the risk for insufficient treatment or damage of critical anatomical structures [Citation15–17]. MR image guidance has been shown to be a possible solution to overcome the aforementioned drawbacks as it provides high soft tissue contrast even for small lesion, near real-time imaging in arbitrary planes for accurate applicator positioning and precise monitoring of changes in tissue properties during the ablation procedure [Citation18–20]. This makes MRI the most desirable modality for image guidance although its routine use is limited to specialized centers given the higher costs and the special expertise and equipment needed.
The purpose of this study was to prospectively investigate technical success, technique efficacy, safety and outcome of MR-guided microwave ablation of hepatic malignancies as a treatment concept in clinical practice over a time period of 5 years.
Material and methods
This prospective study was approved by the local ethics committee and all patients gave written informed consent. A subset of 15 patients included in the investigated cohort was previously analyzed in a pilot study investigating initial experiences in a clinical setting [Citation21].
Patient populations
Between 2015 and 2019, 47 patients were prospectively included in this study and underwent MR-guided microwave ablation of primary or secondary hepatic malignancies based on the decision of an interdisciplinary tumor board. Inclusion criteria were (1) know hepatic malignancies based on pre-interventional imaging, (2) no contraindications for MR imaging, (3) ≤3 target lesions, (4) target lesion size ≤5 cm (all hepatocellular carcinomas had a diameter of ≥10 mm), and (5) appropriate location of the target tumor, (6) adequate lab works (Quick test ≥50%; platelet count ≥50,000/μL).
Ablation procedure protocol
Ablations were performed using a tissue permittivity feedback control microwave ablation system with a maximum antenna power of 36 W (Medwaves Avecure, Medwaves, San Diego, CA, USA). Active tip length (2 cm, 3 cm or 4 cm) of the MR-compatible microwave-applicator was chosen based on target lesion size and location. Ablations were conducted under temperature control mode with the pulsed output-power, and the generator frequency is automatically adjusted.
All procedures were performed by an alternating team of two trained radiologists with 4–12 years of experience in MR-guided ablation procedures following a recently published protocol [Citation21]. In brief, a 1.5 T closed-bore MR system (Magnetom ESPREE, Siemens Healthineers, Germany) was used for planning, targeting, monitoring and assessment of immediate treatment response. Planning imaging and targeting (T1-weighted and T2-weighted sequences) was performed without contrast agent administration when possible. If necessary, additional body-weighed adapted contrast-enhanced (Ga-EOB-DTPA; Bayer Healthcare, Germany) or diffusion-weighted sequences were acquired. Prior to targeting, the cutaneous puncture site was disinfected and local anesthesia was applied (Xylocaine 1%, AstraZeneca, Germany). Targeting was performed under near real-time fluoroscopy using a T1-weighted gradient-echo sequence, which enables a continuous visualization of the applicator and target lesion in arbitrary imaging planes for safe and precise applicator placement. Coagulation time and generator settings were chosen according to vendor recommendations. Temperature feedback and repeated control imaging (T1-weighted sequences) were used for monitoring. When imaging revealed an adequate ablation zone the applicator was retracted. Subsequent post-procedural control imaging was performed to rule out complications and assess technical success with T2-weigted and contrast-enhanced (Gadobutrol; Bayer Healthcare, Germany) T1-weighted sequences. Detailed acquisition parameters are provided in .
Table 1. Summary of acquisition parameters for planning, targeting, monitoring and controlling.
For pain relief, all procedures were either performed in i.v. analgosedation (Midazolam and/or Piritramid) or under general anesthesia if the target lesion was in close proximity to the liver capsule.
Per protocol, all patients were admitted for one night after the procedure and discharged on the following day if physical condition, lab works and ultrasound of the abdomen were unremarkable. The first routine post-procedural control imaging was scheduled 4 weeks after the procedure with multiparametric MR imaging followed by subsequent follow-up visits every 3 months for one year, thereafter every 6 months. Detailed information on the examination protocol has been described in detail earlier [Citation21].
Technical success, technique efficacy and safety
Technical success was assessed on immediate post-procedural contrast-enhanced control imaging and was defined as successful, if the ablation procedure followed the protocol as intended including a complete coverage of the target tumor by the ablation zone.
Following measurements were conducted on post-procedural control imaging: Short-axis diameter (SAD) and long-axis diameter (LAD) diameter were evaluated using dedicated post-processing software (syngo.via, Siemens Healthineers, Erlangen, Germany). Furthermore, the safety margin was determined as the minimum distance of the target tumor and the ablation zone margin as depicted in . The shape of the ablation zone was determined by calculating the sphericity index: SI = SAD/LAD.
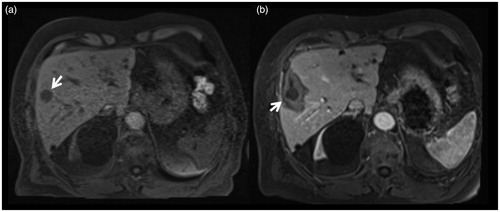
Figure 1. 79-years-old male patient treated for a singular hepatic metastasis of a melanoma. Unenhanced T1-weighted planning imaging (a) depicts the target tumor in segment V (arrow) measuring 18 mm. Contrast-enhanced control imaging in the portal-venous phase shows the ablation zone with the contour of the treated tumor (b). Die smallest safety margin was measured with 5 mm at the lateral portion of the ablation zone (arrow).
Technique efficacy was evaluated 4 weeks after the ablation on multi-parametric contrast-enhanced MRI and defined as successful if no macroscopic tumor was detectable in or adjacent to the ablation zone.
Assessment of safety followed the SIR grading system [Citation22].
Overall survival and recurrence
Overall survival, local recurrence free survival and non-target recurrence free survival were assessed during routine follow-up for all patients
Statistical analysis
All statistical analyses were performed in Stata, version 15.1 (StataCorp, College Station, TX, USA). Binary variables are given as absolute/relative frequencies. For ordinal and continuous variables median and range or mean and standard deviation are provided. To investigate the association between the number of procedures performed and the overall procedure time, a linear regression model was constructed. Initial size of tumors that locally recurred versus tumors that did not recur as well as minimal safety margin between HCC and liver metastasis were compared with the Wilcoxon-rank sum test. Kaplan–Meier survival estimates were computed for overall survival, local recurrence free survival and non-target recurrence free survival. A 2-sided p value below 0.05 was considered as statically significant.
Results
Patient and target tumor baseline characteristics are provided in .
Table 2. Baseline patient characteristics and ablation procedure related parameters.
Technical success, technique efficacy and safety
Technical success
Technical success was achieved in all 50 procedures following the protocol as initially intended. In 79.3% (46/58) no contrast agent was necessary for planning and targeting (). The mean lesion size was 1.6 ± 0.7 cm (range: 0.6–3.9 cm)., the mean size of the ablation zone 2.8 ± 0.8 cm and the mean safety margin 0.45 ± 0.25 cm, which was not significantly different between HCCs and liver metastasis; 0.47 ± 0.27 vs. 0.46 ± 0.25 cm; p = .85. 2.6 ± 1.2 (range: 1–6) applicator positions were necessary to achieve a satisfactory ablation zone requiring a mean energy of 43.2 ± 23.5 kJ. On average, the ablation time per lesion was 26.7 ± 13.1 min while the total time of MR suite occupancy per procedure was 211.2 ± 68.7 min, resulting in a per-tumor procedure duration of 180 ± 54 min. When evaluating whether there is an association between the number of procedures performed and the total duration time, linear regression revealed a significant result (β = −1.7 (95% CI −3.1 to −0.3); p = .01) indicating that with every new case treated, procedure duration decreased by 1.7 min. In other words, across all procedures in the investigated study cohort the overall procedure time decreased by 85 min. A summary of all procedure related parameters is given in .
Figure 2. Images of a 56-year-old male patient with metastasis of a mixed adenoneuroendocrine carcinoma in segment VIII. Metastasis is clearly depictable in planning imaging without administration of intravenous contrast agent (a; arrows). During first targeting with MR fluoroscopy (b) the applicator was positioned toward the cranial part of the metastasis (arrow). After ablation for 10 min with 18 kJ there is no coagulated tissue visible on the caudal part in monitoring (c; circle). Therefore, the applicator was repositioned (d) for an additional ablation for 15 min with 27 kJ toward the caudal part of the metastasis (e; circle). On final postprocedural imaging (f) there is a well-defined, T1-weighted hyterintense, oval shaped ablation zone with a 4 mm safety margin. No local recurrence was observed during follow-up.
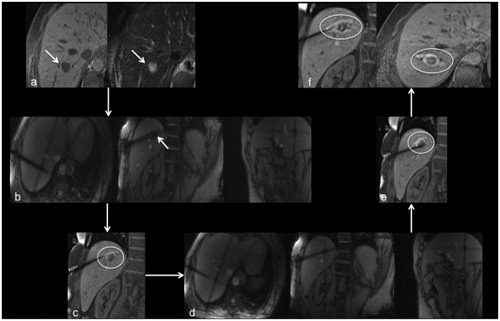
Table 3. Ablation procedure related parameters.
Technique efficacy
Follow-up imaging after 4 weeks revealed residual tumor at the ablation site in one patient with a 15 mm colorectal liver metastasis in segment VIII, resulting in a technique efficacy of 98%. In this case, further liver metastases were detected in the right liver at control imaging, so that the patient underwent right-sided hemi-hepatectomy.
Safety
Following the SIR reporting system, a total of 13 complications (26%) were observed in this study cohort including 10 minor (20%) and 3 major (6%) complications (). The median hospital admission after the procedure was 1 day ranging from 1 to 19 days.
Table 4. Summary of complications according to the SIR reporting system.
Minor complications comprised 3 SIR-category A complications including two patients with subcutaneous necrosis of the extrahepatic applicator tract without local discomfort at the puncture site and one patient with a minor post-procedural subcapsular hematoma which did not require any treatment. Seven patients developed post-ablation syndrome, which was treated symptomatically (SIR category B).
Major complications were one patient with a pneumothorax treated with a chest tube (SIR category C) and one patient with pleural effusion with dyspnea and a new, partial thrombosis of the portal vein causing hospital admission and treatment for 11 days (SIR category D). An additional category D complication occurred in one patient who underwent MR-guided MWA of a liver metastasis in segment III and a coil marking (for stereotactic radiation therapy) of another centrally located metastasis in segment IVa during the same procedure. After releasing the coil, the patient reported strong abdominal pain. During the following hospitalization period of 19 days, a biliary peritonitis was diagnosed, which was most likely caused by a bile duct injury during the coil marking.
Overall survival and recurrence
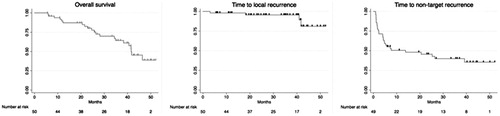
Median overall survival in the investigated cohort was 41.6 (IQR: 26.4–) months. In 4 patients (8%) local recurrence was diagnosed () after a minimum of 3.1 and a maximum of 41.9 months. Target lesions that recurred were significantly larger (3.0 ± 1.0 cm) than the ones that did not recur (1.5 ± 0.6 cm; p = .006). In 64% (30/47) of patients non-target recurrence was observed after a median time of 13.8 (IQR 2.3–) months. Kaplan–Meier survival curves are presented in .
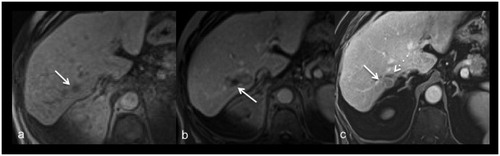
Figure 3. Images of a 77-year-old female with metastasis of a melanoma. Planning imaging (a) depicts the small metastasis (17 mm) in segment VII (arrow). Post-procedural imaging (b) depicts an ablation zone with an almost non-visible safety margin dorso-medial (arrow). After 40 months local recurrence (arrow) was diagnosed on routine contrast-enhanced follow-up imaging (c) dorsal to the shrunken ablation zone (dash arrow).
Discussion
In this study, we prospectively investigated technical success, technique efficacy and safety of MR-guided microwave ablation in patients with primary and secondary hepatic malignancies. Our results indicate that this approach allows for an effective treatment option in clinical routine as we observed a high primary efficacy rate of 98% and a local control rate of 90% in one session.
From an efficacy perspective, these are encouraging results as it has been reported that overall survival after tumor ablation is independently associated with the number of treatment sessions per case and successful initial complete response [Citation23,Citation24]. To achieve this, the ablation device as well as the modality used for image guidance play a crucial role [Citation25]. The ablation device is important for an efficient and reliable coagulation process and complete destruction of the target tissue. While MWA generally exhibits a higher and more homogeneous temperature distribution as compared to RFA, prolonged or insufficient ablations are less likely [Citation26,Citation27]. Regardless of the device used, accurate and precise applicator placement within the target lesion is key for treatment success. Therefore, reliable and continuous visualization of the applicator and the target lesion during targeting and monitoring is crucial [Citation12]. MR image guidance provides the required conditions due to its high soft tissue contrast and the possibilities for acquiring different sequences in arbitrary planes helpful to distinguish alterations in tissue properties [Citation28]. Moreover, in the majority of procedures (74%) in our study target lesions could be reliably detected without additional contrast agent administration. If, in questionable situations, contrast agent was used, the timeframe of tissue enhancement is longer as compared to computed tomography or ultrasound – in particular, for hepatocyte-specific agents, which is a distinct advantage of MR image guidance, especially, if target lesions are small or cannot be reliably depicted with a different image guidance modality such as CT or ultrasound [Citation29–31].
Local control rates in the investigated cohort were convincing although the mean safety margin was below the recommended minimum of at least ≥5 mm. This suggests that intraprocedural MR monitoring with a reliable depiction of the target tumor and the formation of the ablation zone enables accurate and effective treatment despite safety margins below the recommended dimensions for CT-guided procedures [Citation32,Citation33].
Even though procedure time significantly shortened with the number of cases treated, overall durations were still in the range as previously reported for MR-guided RFA [Citation19]. Regardless of the advantages and performance, these long procedure times remain a limiting factor for the widespread and routine use of MRI as guidance modality as costs are about twice as high as for CT-guided procedures [Citation34]. The main reasons for this are the still relatively long ablation times with the low-power system and the high number of applicator readjustments needed to achieve a satisfactory ablation zone. As reported in earlier studies, this could be overcome with perfusion-cooled high-power systems that allow for larger and more spherical ablation zones in a shorter time [Citation35], therefore, more research and development is desirable to address demands and limitations of MR-guided tumor ablation.
Overall, we found a relatively high complication rate as compared to the current literature with major complication rates about 2–3% for CT- or ultrasound guided procedures [Citation36–38] In part they can be attributed to the MWA system and/or MRI as a guidance modality. During the first procedures, a mild coagulation of the extrahepatic applicator tract was observed in two patients [Citation21]. This was most likely caused by increased heating of the applicator shaft during the coagulation process as the device lacks an internal cooling mechanism. To reduce risk for such complications, the shaft was manually cooled during the following procedures with sterile water and a short intrahepatic applicator tract was avoided, which successfully prevented further complications of this type. Nevertheless, internally cooled devices are a potential option to reduce these complications and enable applicator tracts with shorter intrahepatic forerun. In a different patient, a post-procedural pneumothorax was detected. Although this is a known complication of percutaneous liver interventions the relatively poorly visible applicator artifact might limit accurate depiction. Thus, future developments should focus on making applicator visibility more precise.
The following limitations need to be addressed. Due to the internal institutional workup no histological sampling was performed prior to the ablation to confirm the diagnosis. Moreover, this was a prospective study to evaluate treatment efficacy and safety of a low-power, non-perfusion-cooled MWA system for application in clinical routine. Therefore, future modifications of the generator technique enabling a higher ablation power might cause different results concerning clinical results and procedure durations. Lastly, combining primary and secondary hepatic malignancies into a combined survival analysis might be clinically questionable, however, given the sample size a meaningful sub-analysis was not feasibly and beyond the scope of this study.
In conclusion, MR-guided MWA allows for safe and successful treatment of hepatic malignancies with a high technique efficacy however with relatively long procedure durations at this stage. Future studies are necessary to thoroughly investigate the clinical benefits of MR image guidance in comparison to more established guidance modalities like CT or ultrasound.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sucandy I, Cheek S, Golas BJ, et al. Longterm survival outcomes of patients undergoing treatment with radiofrequency ablation for hepatocellular carcinoma and metastatic colorectal cancer liver tumors. HPB (Oxford). 2016;18(9):756–763.
- Kim YS, Lim HK, Rhim H, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58(1):89–97.
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750.
- EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422.
- Ricke J, Thormann M, Ludewig M, et al. MR-guided liver tumor ablation employing open high-field 1.0T MRI for image-guided brachytherapy. Eur Radiol. 2010;20(8):1985–1993.
- Giorgio A, Amendola F, Calvanese A, et al. Ultrasound-guided percutaneous irreversible electroporation of hepatic and abdominal tumors not eligible for surgery or thermal ablation: a western report on safety and efficacy. J Ultrasound. 2019;22(1):53–58.
- Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng. 2010;38(1):65–78.
- Andreano A, Brace CL. A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovasc Intervent Radiol. 2013;36(2):505–511.
- Fan W, Li X, Zhang L, et al. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am J Roentgenol. 2012;198(1):W46–50.
- Potretzke TA, Ziemlewicz TJ, Hinshaw JL, et al. Microwave versus radiofrequency ablation treatment for hepatocellular carcinoma: a comparison of efficacy at a single center. J Vasc Interv Radiol. 2016;27(5):631–638.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–260.
- Du J, Li HL, Zhai B, et al. Radiofrequency ablation for hepatocellular carcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in guiding and assessing early therapeutic response and short-term follow-up results. Ultrasound Med Biol. 2015;41(9):2400–2411.
- Koh YH, Choi JI, Kim HB, et al. Computed tomographic-guided radiofrequency ablation of recurrent or residual hepatocellular carcinomas around retained iodized oil after transarterial chemoembolization. Korean J Radiol. 2013;14(5):733–742.
- Kim JE, Kim YS, Rhim H, et al. Outcomes of patients with hepatocellular carcinoma referred for percutaneous radiofrequency ablation at a tertiary center: analysis focused on the feasibility with the use of ultrasonography guidance. Eur J Radiol. 2011;79(2):e80–e84.
- Park MH, Rhim H, Kim YS, et al. Spectrum of CT findings after radiofrequency ablation of hepatic tumors. Radiographics. 2008;28(2):379–390; discussion 390–372.
- Carberry GA, Smolock AR, Cristescu M, et al. Safety and efficacy of percutaneous microwave hepatic ablation near the heart. J Vasc Interv Radiol. 2017;28(4):490–497.
- Weiss J, Hoffmann R, Rempp H, et al. Feasibility, efficacy, and safety of percutaneous MR-guided ablation of small (</=12 mm) hepatic malignancies. J Magn Reson Imaging. 2019;49(2):374–381.
- Rempp H, Waibel L, Hoffmann R, et al. MR-guided radiofrequency ablation using a wide-bore 1.5-T MR system: clinical results of 213 treated liver lesions. Eur Radiol. 2012;22(9):1972–1982.
- van Oostenbrugge TJ, Langenhuijsen JF, Overduin CG, et al. Percutaneous MR imaging-guided cryoablation of small renal masses in a 3-T closed-bore MR imaging environment: initial experience. J Vasc Interv Radiol. 2017;28(8):1098–1107. e1091.
- Hoffmann R, Rempp H, Keßler D-E, et al. MR-guided microwave ablation in hepatic tumours: initial results in clinical routine. Eur Radiol. 2017;27(4):1467–1476.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9):S199–S202.
- Ding J, Jing X, Wang Y, et al. Thermal ablation for hepatocellular carcinoma: a large-scale analysis of long-term outcome and prognostic factors. Clin Radiol. 2016;71(12):1270–1276.
- Takahashi S, Kudo M, Chung H, et al. Initial treatment response is essential to improve survival in patients with hepatocellular carcinoma who underwent curative radiofrequency ablation therapy. Oncology. 2007;72 (1):98–103.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. J Vasc Interv Radiol. 2014;25(11):1691–1705.e1694.
- Izzo F, Granata V, Grassi R, et al. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 2019;24(10):e990–e1005.
- Ziemlewicz TJ, Hinshaw JL, Lubner MG, et al. Percutaneous microwave ablation of hepatocellular carcinoma with a gas-cooled system: initial clinical results with 107 tumors. J Vasc Interv Radiol. 2015;26(1):62–68.
- Clasen S, Pereira PL. Magnetic resonance guidance for radiofrequency ablation of liver tumors. J Magn Reson Imaging. 2008;27(2):421–433.
- Silverman PM, O'Malley J, Tefft MC, et al. Conspicuity of hepatic metastases on helical CT: effect of different time delays between contrast administration and scanning. AJR Am J Roentgenol. 1995;164(3):619–623.
- Kim YJ, Lee MW, Park HS. Small hepatocellular carcinomas: ultrasonography guided percutaneous radiofrequency ablation. Abdom Imaging. 2013;38(1):98–111.
- Joo I, Lee JM. Recent advances in the imaging diagnosis of hepatocellular carcinoma: value of gadoxetic acid-enhanced MRI. Liver Cancer. 2015;5(1):67–87.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–175.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: Ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275. e261.
- Maurer MH, Schreiter N, de Bucourt M, et al. Cost comparison of nerve root infiltration of the lumbar spine under MRI and CT guidance. Eur Radiol. 2013;23(6):1487–1494.
- Hoffmann R, Rempp H, Erhard L, et al. Comparison of four microwave ablation devices: an experimental study in ex vivo bovine liver. Radiology. 2013;268(1):89–97.
- Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3(5):317–325.
- Livraghi T, Meloni F, Solbiati L, et al. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35(4):868–874.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. 2009;251(3):933–940.