 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
To compared the benefits of sorafenib with microwave ablation (MWA) in intermediate-stage hepatocellular carcinoma (HCC) patients with tumor size ≤7 cm and tumor number ≤5 after Transcatheter Arterial Chemoembolization (TACE) failure.
Methods
A retrospective, single-center study was conducted using a one-to-one propensity score matching (PSM) analysis and involved 52 intermediate-stage HCC patients with absence of evidence of intrahepatic vascular invasion and extrahepatic metastasis after TACE failure and underwent treatment with MWA or sorafenib between 2007 and 2019. The overall survival (OS) and progression-free survival (PFS) were evaluated by the Kaplan-Meier method. The factors with OS and PFS were determined by Cox regression.
Results
Of the 52 patients included in our study, 30 (57.7%) underwent MWA and 22 (42.3%) received sorafenib. After PSM, 22 pairs were enrolled into different groups for further analysis. Patients in the MWA-group had a significantly longer median PFS than patients in the sorafenib-group on both before (median, 9.3 vs. 2.8 months, p = .001) and after PSM (median, 9.0 vs. 2.8 months, p = .006). They also had a significantly longer median OS than patients in the sorafenib-group on before (median, 48.8 vs. 16.6 months, p = .001) and after PSM (median, Not reached vs. 16.6 months, p = .001). Besides, Cox regression analysis showed that the treatment and age were the independent prognostic factors of OS and PFS (p<0.05).
Conclusions
MWA was superior to sorafenib in improving survival for intermediate-stage hepatocellular carcinoma (HCC) patients with tumor size ≤7 cm and tumor number ≤5 after TACE failure.
Compared with sorafenib, microwave ablation may be a more reasonable alternative treatment for intermediate-stage hepatocellular carcinoma (HCC) patients with tumor size ≤7 cm and tumor number ≤5 after TACE refractoriness.
The treatment (MWA vs sorafenib) and the age of patients were the independent prognostic factors of OS and PFS.
Key Points
Introduction
Liver cancer, estimated by Global Cancer Statistics 2018, was the sixth most common cancer and the fourth leading cause of cancer-related death worldwide in 2018 [Citation1]. Hepatocellular carcinoma (HCC) was the most common liver tumor, accounting for 70% to 90% [Citation2].
Transarterial Chemoembolization (TACE) is the standard treatment recommended for patients with HCC diagnosed as Barcelona Clinic Liver Cancer stage B (BCLC B) [Citation3–6]. But in fact, it may not be the most appropriate treatment for all patients. The guidelines estimate that the target population for TACE accounts for less than 20% of all HCC patients, and partial responses in 15–55% of patients are achieved by TACE [Citation3]. Therefore, a promising treatment strategy for BCLC B HCC patients with TACE failure should be established.
However, there is no standardized definition for failure of TACE treatment currently. JSH-LCSGJ defined TACE failure/refractoriness Criteria in 2014 [Citation7]: Two or more consecutive ineffective responses of the treated tumor (viable lesion >50% or tumor number increased after TACE) seen on response evaluation CT/MRI at 1–3 months after having fully performed selective TACE, even after reselecting chemotherapies or reanalyzing artery. Arizumi et al. thought that patients with TACE refractoriness without extrahepatic spread or vascular invasion who received ≤2 consecutive ineffective TACE procedures before sorafenib administration had a longer OS than those who received ≥3 consecutive ineffective TACE [Citation8]. Therefore, one ineffective TACE procedure (necrotic lesion <25% or tumor number increased) should also be included in the definition of TACE failure. Therefore, in our study, TACE refractoriness was defined as ≥2 consecutive ineffective responses of treated tumors (necrotic lesion <50%) or one ineffective response of treated tumors (necrotic lesion <25%) or tumor number increased.
The current treatment methods for advanced HCC patients with TACE refractoriness are mainly based on targeted drugs alone or in combination and systemic chemotherapy, such as apatinib [Citation9], TACE combined sorafenib [Citation10], sorafenib and hepatic arterial infusion chemotherapy (HAIC) [Citation11]. Sorafenib, an oral multikinase inhibitor with anti-proliferative and anti-angiogenic effects, is recommended as the standard first-line therapy for patients with advanced HCC [Citation12]. Recently, some studies have shown that sorafenib may also a promising option for intermediate-HCC stage patients with TACE refractoriness [Citation13,Citation14]. However, whether sorafenib is suitable for all intermediate-HCC patients with TACE failure is worth further study.
Microwave ablation (MWA), a local thermal ablation using electromagnetic waves, is recommended as a treatment method for those who are not suitable for surgical curative treatments [Citation15]. Several studies have shown that TACE combined with MWA have greater advantages in prolonging the progression-free survival (PFS) and overall survival (OS) of HCC patients with BCLC A or B stage than TACE monotherapy [Citation16–19]. Besides, a prospective randomized trial showed that TACE combined with radiofrequency ablation (RFA) was significantly superior to RFA-alone in improving survival for patients with HCC less than 7 cm [Citation20]. However, none of studies make comparison of the efficacy of MWA versus sorafenib for intermediate-stage hepatocellular carcinoma with TACE refractoriness. Therefore, we conducted a retrospective study to compare the efficacy of microwave ablation (MWA) with sorafenib in intermediate-stage hepatocellular carcinoma (HCC) patients with tumor size ≤7 cm and tumor number ≤5 after TACE refractoriness through propensity score matching.
Materials and methods
Patients
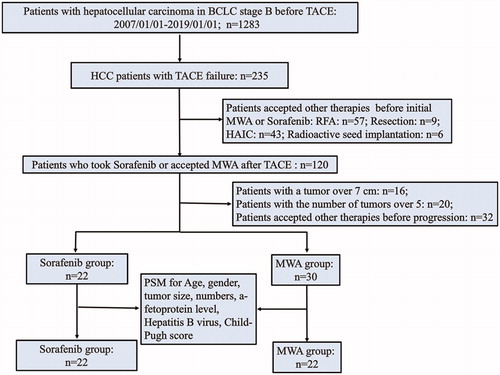
Our study was a single-center, retrospective study that data included a total of 235 intermediate-stage HCC patients with TACE failure in our hospital from January 2007 to January 2019. The inclusion criteria were as follows: (1) patients with BCLC stage B diagnosed based on histological or radiological findings using contrast-enhanced Computed Tomography (CE-CT) and/or dynamic Magnetic resonance imaging (MRI) before initial TACE; (2) patients with TACE refractoriness that was defined as ≥2 consecutive ineffective responses of treated tumors (necrotic lesion <50%) or one ineffective response of treated tumors (necrotic lesion <25%) or tumor number increased; (3) Child-Pugh class A or B; (4) patients with a tumor no more than 7 cm and the number of tumors no more than 5 after TACE refractoriness; (5) patients with absence of evidence of intrahepatic vascular invasion and extrahepatic metastasis. The exclusion criteria included: (1) severe disorders of coagulation function; (2) HIV infection or immune deficiency; (3) second primary malignancy; (4) any other therapies undertaken such as RFA, resection, hepatic artery infusion chemotherapy (HAIC) and radioactive implantation during the period extending from ineffective TACE to disease progression. A total of 52 patients with TACE refractoriness were included, including 22 patients received sorafenib (sorafenib-group), and 30 patients received MWA (MWA-group). A flowchart of the patient selection process was shown in . Within a week before the start of treatment, the patients’ datum of imaging, biochemistry, tumor markers, and blood routine were obtained.
Figure 1. Patient selection flowchart. BCLC: Barcelona Clinic Liver Cancer; TACE: transarterial chemoembolization; MWA: microwave ablation; HAIC: hepatic artery infusion chemotherapy.
The study was conducted in accordance with the Helsinki Declaration. The written informed consent was waived because of its retrospective nature. This study was approved by our Hospital Ethics Committee in China.
TACE procedure
TACE was based on the injection of lipiodol, lobaplatin, and (or) pirarubicin (i.e., lipiodol: pirarubicin: lobaplatin =5 ∼ 15ml: 20 ∼ 40mg: 20 ∼ 40mg, the dose depending on whether the tumor was embolized completely). And those drugs could be delivered directly into the tumors, thus played the dual role of embolism and chemotherapy. The endpoint of the TACE was defined as the stagnation of blood flow in the feeding arteries and no stained tumor angiographically after procedure completed. TACE was repeated by interventional radiologist with more than 5 year experience until refractoriness ().
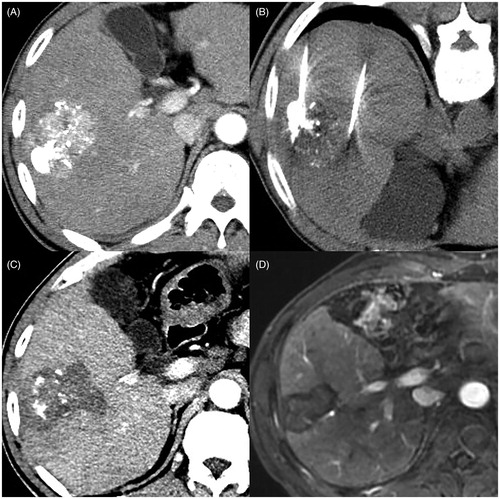
Figure 2. Images in a 64 year-old male patient with BCLC stage B hepatocellular carcinoma (HCC) before TACE, who has two lesions measuring approximately 6.1 cm and 1.0 cm (no shown), show microwave ablation (MWA) as first-line treatment after transarterial chemoembolization (TACE) refractoriness. (A) Arterial-phase CT image obtained about one month after first TACE shows that necrotic volume is less than 20% of large lesion, and small lesion disappears (no shown). (B) He underwent CT-guided multiple-position MWA for non-necrotic areas of large lesion with the output of 60 W for 69 min in total. (C) Arterial-phase CT image obtained about 1 month after MWA shows that complete ablation was achieved. (D) Arterial-phase MRI image obtained about 34 months after MWA (5 December 2019) shows no tumor recurrence.
Therapeutic methods after TACE refractoriness
Patients with TACE refractoriness could choose whether to accept the advice (Sorafenib or MWA) from multidisciplinary teams (MDTs) in our hospital according to their physical condition, personal willingness and economic and/or social status.
Sorafenib management
For patients in the sorafenib-group, sorafenib was administered orally after TACE refractoriness at a dosage of 400 mg, twice per day until unacceptable and unrelieved toxicity or disease progression. Dose reductions (i.e., 400 mg once daily, or 400 mg every other day) and drug interruptions were allowed and adjusted based on package instruction.
MWA procedure
In order to completely eradicate the tumor, the number of needles, the power (range, 40–80w) and corresponding time (range, 5–25 min per position), and the adjustable position of needles were chosen by physicians with at least 5 years of experience in percutaneous MWA. For example, for tumors smaller than 5.0 cm in the greatest long diameter, complete ablation was achieved with an ablation needle. However, for tumors larger than 5.0 cm, complete ablation was achieved with two or more microwave ablation needles (). For multiple tumors, complete ablation was achieved with one operation by two or more microwave ablation needles. For tumors that were capable of complete ablation, the endpoint of microwave ablation was defined as a safety margin of at least 5 mm beyond the tumor boundary [Citation21]. For tumors that were unable to ablate completely, the endpoint of microwave ablation was defined as treating more than 95% of the entire lesions. All patients were routinely hospitalized for 2–3 days after MWA unless complications occurred.
MWA equipment: A microwave delivery system (FORSEA; Qinghai Microwave Electronic Institute, Nanjing, China) was used during MWA therapy. This system consisted of a MTC-3 microwave generator (FORSEA) with a frequency of 2450 MHz, a power output of 10–150 W, a flexible low-loss cable, and a 16-gauge cooled-shaft antenna.
Follow-up, tumor response assessment, and the end point
In both groups, tumor response was assessed every 1–2 months based on contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) by at least two diagnostic radiologists with more than 5 year experience according to the modified Response Evaluation Criteria in Solid Tumors 1.1 (mRECIST 1.1) ().
The primary endpoint, overall survival (OS), was defined as the interval time from initial sorafenib or MWA treatment to death because of any cause. The secondary endpoint, progression-free survival (PFS), was defined as interval time from initial sorafenib or MWA treatment to progression disease (PD) or death.
Statistical analysis
Given the differences in baseline characteristics between eligible participants in the two groups (), the propensity score matching (PSM) was used to identify a group of patients with similar baseline characteristics. Possible variables associated with treatment selection, including age, gender, Child-Pugh score, hepatitis B virus, tumor size, numbers and alpha-fetoprotein (AFP) levels, were all included in propensity score generation. The match was made using a 1:1 matching scheme, and the caliper width was equal to 0.2 of the logit standard deviation of the propensity score.
Table 1. Baseline Characteristics of patients before and after Propensity Score Matching (PSM).
Continuous data were compared by using the t test. Categorical data were compared by using the χ2 test or the Fisher exact test. Survival curves were estimated by the Kaplan-Meier method and compared by the log-rank test. Prognostic factors associated with survival and disease progression were assessed with univariable and multivariable analysis, which were performed for all patients before PSM through a Cox proportional hazards model. A two-sided P value less than 0.05 indicated statistically significant differences. Statistical analysis was carried out by using IBM SPSS statistics software for Windows version 25.0 (IBM, New York, United States of America) and GraphPad Prism 8.0.1(244) (USA, GraphPad Software).
Results
Baseline characteristics of sorafenib-group and MWA-group before and after PSM
The clinical characteristics of the patients, who were administrated sorafenib and MWA with hepatocellular carcinoma (HCC) patients with tumor size ≤7 cm and tumor number ≤5 after TACE refractoriness from January 2007 to January 2019, were displayed in . Before PSM, a total of 52 patients were enrolled in this study, 22(42.3%) of whom were in the sorafenib-group and 30(57.7%) in the MWA-group. After PSM, a total of 44 patients were enrolled, 22(50%) of whom were in the sorafenib-group and 22(50%) in the MWA-group. There were no significant differences in all of variables (i.e., a-fetoprotein level, tumors size, numbers, HBV infection, Child-Pugh class, gender, age) before and after PSM.
Overall survival (OS)
The median OS of the entire cohort was 26.8 months (95% CI: 18.3–35.3 months). And the median OS was 48.8 months (95% CI: not available) in the MWA-group and 16.6 months (95% CI: 13.4–19.8 months) in the sorafenib-group before PSM (p = .001) (). After one-to-one propensity score matching analysis, the median OS of the entire cohort was 26.8 months (95% CI: 19.3–34.3 months). And the median OS was Not reached in the MWA-group and 16.6 months (95% CI: 13.4–19.8 months) in the sorafenib-group (p = .001) (). Those variables (i.e., gender, age, a-fetoprotein level, tumors size, numbers, HBV infection, Child-Pugh class, the treatment) were enrolled into Cox proportional hazard for univariate analysis, and multivariable analysis showed that the treatment (MWA vs. sorafenib) and age were the independent prognostic factors of the OS ().
Figure 3. Kaplan-Meier curves of overall-all survival (OS) and progression-free survival (PFS) in patents who took Sorafenib or accepted microwave ablation (MWA) before one-to-one PSM (Sorafenib-group: n = 22; the median OS, 16.6 months; 95% CI: 13.4–19.8 months). MWA-group: n = 30; the median OS, 48.8 months; 95% CI: not available. p = .001) (A) and (Sorafenib-group: n = 22; the median PFS, 2.8 months; 95% CI: 1.2–4.4 months. MWA group: n = 30; the median PFS, 9.3 months; 95% CI: 0.0–27.2 months. p = .001) (C), respectively. After one-to-one PSM, Kaplan-Meier curves of overall-all survival (OS) and progression-free survival (PFS) in patents who took Sorafenib or accepted microwave ablation (MWA) (Sorafenib-group: n = 22; the median OS, 16.6 months; 95% CI: 13.4–19.8 months. MWA-group: n = 22; the median OS, Not reached; 95% CI: not available. p = .001) (B) and (Sorafenib group: n = 22; the median PFS, 2.8 months; 95% CI: 1.2–4.4 months. MWA group: n = 22; the median PFS, 9.0 months; 95% CI: 6.6–11.4 months. p = .006) (D), respectively.
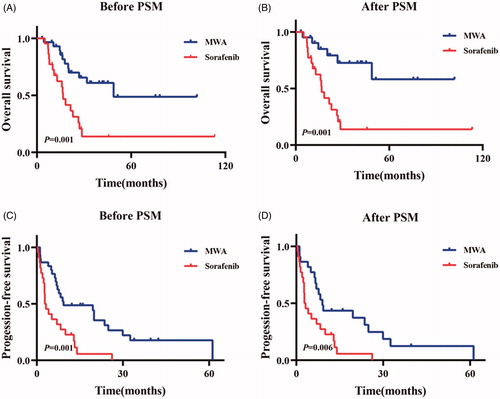
Table 2. Univariate and Multivariate analysis of OS in the enrolled cohort.
Progression-free survival (PFS)
The median PFS of the entire cohort was 7.2 months (95% CI: 5.2–9.2 months). And the median PFS was 9.3 months (95% CI: 0.0–27.2 months) in the MWA-group and 2.8 months (95% CI: 1.2–4.4 months) in the sorafenib-group before PSM (p = .001) (). After one-to-one propensity score matching analysis, the median PFS of the entire cohort was 6.9 months (95% CI: 3.8–10.0 months). And the median PFS was 9.0 months (95% CI: 6.6–11.4 months) in the MWA-group and 2.8 months (95% CI: 1.2–4.4 months) in the sorafenib-group (p = .006) (). Multivariable analysis showed that the treatment and age were the independent prognostic factors of PFS ().
Table 3. Univariate and Multivariate analysis of PFS in the enrolled cohort.
Discussion
In our study, we evaluated MWA vs. sorafenib for intermediate-stage hepatocellular carcinoma (HCC) patients with tumor size ≤7 cm and tumor number ≤5 after TACE failure. Our study showed compared with sorafenib, MWA provided a longer median OS (48.8 vs. 16.6 months) and PFS (9.3 vs. 2.8 months) for those patients. This result was further proved by PSM and Cox proportional hazard.
As we all know, TACE is the standard therapy for patients with intermediate-stage HCC [Citation3,Citation4]. It is reported that the objective response rate of HCC patients treated with TACE is only 15%–61%, while CR is only observed in 20%–35% of patients [Citation10]. That is to say, 39%-85% patients will develop resistance to TACE after one or more TACE. In those TACE-refractory HCC patients, continued TACE will not continue to increase TACE efficacy, but will further worsen non-cancerous liver tissue, thus shortening survival time. Due to the limitations, subsequent treatments of TACE refractoriness deserves further study.
Sorafenib may be regarded as the effective treatment for TACE-refractory HCC [Citation7,Citation10,Citation13,Citation22]. Ikeda et al. had reported the outcome of 48 HCC patients refractory to TACE receiving sorafenib with the median time to progression (TTP) of 3.9 months and the median overall survival (OS) of 16.4 months [Citation23]. Arizumi et al. showed that sorafenib could improve the median OS of intermediate-stage patients with BCLC B stage HCC refractory to TACE compared with repeated TACE (24.7 vs 13.6 months) [Citation13]. However, the efficacy of sorafenib in those patients with TACE refractoriness was still relatively moderate, and all of those studies are retrospective. Besides, The SPACE trial showed that sorafenib plus DEB-TACE did not improve TTP of patients with intermediate stage HCC in a clinically meaningful manner compared with DEB-TACE alone [Citation24]. Therefore, a more promising strategy for intermediate stage HCC patients with TACE failure should be established.
MWA was recommended as a treatment method for those who were not suitable for surgical curative treatments. More and more studies have shown that TACE combined with MWA can benefit patients with BCLC A or B stage hepatocellular carcinoma [Citation25–27]. For example, Ni et al. had reported the outcome of 64 HCC patients with BCLC stage A or B receiving TACE combined with MWA, who had the median OS times of 36 and 25 months, respectively [Citation25]. Zheng et al. reported that compared with TACE-alone, TACE combined with MWA have more advantages in prolonging OS (26.6 vs. 17.1 months) [Citation27]. Besides, Peng et al. conducted a prospective randomized trial which showed that patients with BCLC stage A or B HCC less than 7 cm treated with RFA plus TACE had better 4 year overall survival rate than those treated with RFA-alone (61.8% vs. 45%) Citation20. However, at present, none of studies make comparison of the efficacy of MWA versus sorafenib for intermediate-stage hepatocellular carcinoma (HCC) patients with tumor size ≤7 cm and tumor number ≤5 after TACE resistance. Therefore, we conducted this retrospective study, and our study suggested that sorafenib was not well suitable for all patients with intermediate-stage HCC with TACE failure, and MWA could be a better option for intermediate-stage hepatocellular carcinoma (HCC) patients with tumor size ≤7 cm and tumor number ≤5 after TACE resistance. Besides, our study also showed that the prognosis of younger patients was significantly worse than that of older patients. Therefore, younger intermediate-stage hepatocellular carcinoma (HCC) patients with tumor size ≤7 cm and tumor number ≤5 after TACE resistance should use more optimized treatments, such as MWA combined with sorafenib, or other combined treatment options. Therefore, those results provided a new perspective on the treatment of certain intermediate-stage HCC patients with TACE failure, not just sorafenib.
There are still some limitations. Firstly, our research is single-centered and retrospective and can easily lead to selection bias, but we used propensity score matching analysis to reduce selection bias to some extent. Secondly, the second limitation is that the sample size of the entire queue is relatively small, which limited the robustness of our analysis. For these reasons, some prospective and multicenter randomized controlled trials will be conducted in the future.
Conclusions
Our study first revealed that compared with sorafenib, MWA showed a more promising outcomes in intermediate-stage hepatocellular carcinoma (HCC) patients with tumor size ≤7 cm and tumor number ≤5 after TACE refractoriness.
Acknowledgements
Here, we would like to especially thank Shuanggang Chen’s girlfriend, Ms Binyan Shen, for her great support for him when he has been pursuing the dream of conquering HCC.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The datasets used and analyzed in the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Lee DW, Lee KH, Kim HJ, et al. A phase ii trial of s-1 and oxaliplatin in patients with advanced hepatocellular carcinoma. Bmc Cancer. 2018;18(1):252.
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. Easl-eortc clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236.
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. HEPATOLOGY. 2011;53(3):1020–1022.
- Arii S, Sata M, Sakamoto M, et al. Management of hepatocellular carcinoma: report of consensus meeting in the 45th annual meeting of the japan society of hepatology (2009). Hepatol Res. 2010;40(7):667–685.
- Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: jsh-lcsgj criteria 2014 update. Oncology. 2014;87(Suppl 1):22–31.
- Arizumi T, Ueshima K, Chishina H, et al. Validation of the criteria of transcatheter arterial chemoembolization failure or refractoriness in patients with advanced hepatocellular carcinoma proposed by the lcsgj. Oncology. 2014;87(Suppl 1):32–36.
- Qiu Z, Shen L, Chen S, et al. Efficacy of apatinib in transcatheter arterial chemoembolization (tace) refractory intermediate and advanced-stage hepatocellular carcinomaa propensity score matching analysis.Cancer Manag Res. 2019;11:9321–9330.
- Wu J, Li A, Yang J, et al. Efficacy and safety of tace in combination with sorafenib for the treatment of tace-refractory advanced hepatocellular carcinoma in chinese patients: a retrospective study. Onco Targets Ther. 2017;10:2761–2768.
- Kodama K, Kawaoka T, Aikata H, et al. Comparison of clinical outcome of hepatic arterial infusion chemotherapy and sorafenib for advanced hepatocellular carcinoma according to macrovascular invasion and transcatheter arterial chemoembolization refractory status. J Gastroenterol Hepatol. 2018;33(10):1780–1786.
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390.
- Arizumi T, Ueshima K, Minami T, et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (tace) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4(4):253–262.
- Raoul JL, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a european perspective. Liver Cancer. 2014;3(2):119–124.
- Benson AB, D’Angelica MI, Abbott DE, et al. Nccn guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15(5):563–573.
- Zhang R, Shen L, Zhao L, et al. Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in bclc stage b hepatocellular carcinoma. Diagn Interv Radiol. 2018;24(4):219–224.
- Xu LF, Sun HL, Chen YT, et al. Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J Gastroenterol Hepatol. 2013;28(3):456–463.
- Hu H, Chen GF, Yuan W, et al. Microwave ablation with chemoembolization for large hepatocellular carcinoma in patients with cirrhosis. Int J Hyperthermia. 2018;34(8):1351–1358.
- Liu C, Liang P, Liu F, et al. Mwa combined with tace as a combined therapy for unresectable large-sized hepotocellular carcinoma. Int J Hyperthermia. 2011;27(7):654–662.
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31(4):426–432.
- Kudo M. Local ablation therapy for hepatocellular carcinoma: current status and future perspectives. J Gastroenterol. 2004;39(3):205–214.
- Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in japan: consensus-based clinical practice guidelines proposed by the japan society of hepatology (jsh) 2010 updated version. Dig Dis. 2011;29(3):339–364.
- Ikeda M, Mitsunaga S, Shimizu S, et al. Efficacy of sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. J Gastroenterol. 2014;49(5):932–940.
- Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus tace with doxorubicin-eluting beads for intermediate stage hcc: the space trial. J Hepatol. 2016;64(5):1090–1098.
- Ni JY, Sun HL, Chen YT, et al. Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma. World J Gastroenterol. 2014;20(46):17483–17490.
- Zhang TQ, Huang ZM, Shen JX, et al. Safety and effectiveness of multi-antenna microwave ablation-oriented combined therapy for large hepatocellular carcinoma. Therap Adv Gastroenterol. 2019;12:175628481986296–175628321915466.
- Zheng L, Li HL, Guo CY, et al. Comparison of the efficacy and prognostic factors of transarterial chemoembolization plus microwave ablation versus transarterial chemoembolization alone in patients with a large solitary or multinodular hepatocellular carcinomas. Korean J Radiol. 2018;19(2):237–246.
