Abstract
Purpose
To compare acute kidney injury (AKI) incidence between nephron sparing surgery (NSS) and microwave ablation (MWA) for T1a RCC patients, reveal the effect of AKI on survival prognosis, construct AKI nomogram and use Law of Total Probability for survival probability (SP) prediction.
Materials and methods
Patients were studied retrospectively after NSS (n = 1267) or MWA (n = 210) from January 1, 2011 to June 30, 2017. Using one to one Propensity Score Matching (PSM), 158 pairs of patients were identified for the cohort study. AKI incidence, risk factors and impact on survival outcomes were analyzed using Chi-square test, logistic and cox regression analysis. AKI risk and SP were predicted by nomogram and Law of Total Probability. The performance of the nomogram was assessed with respect to its discrimination, calibration, and clinical usefulness.
Results
AKI occurred more commonly in NSS (27.85%) cohort, when compared to MWA (17.72%) cohort (p = 0.032), but treatment modality was not independently predictive of AKI occurrence (odds ratio [OR]: 0.598; 95% confidence interval [CI]: 0.282–1.265; p = 0.178). The 5-yr overall survival (OS) was lower in AKI patients (73.5%) compared with non-AKI patients (94.8%; p < 0.001). AKI was an independent risk factor for all-cause mortality in RCC patients (hazard ratio [HR]: 2.820; 95% confidence interval [CI]: 1.110–7.165; p = 0.029). Predictors for both NSS- and MWA-related AKI included tumor diameter, baseline eGFR and CCI score. RENAL score and tumor blood supply can predict AKI after NSS and MWA, respectively. The AKI normograms demonstrated good discrimination, with AUCs >0.86, excellent calibration and net benefits at the decision curve analysis with probabilities ≥5%. SP predicted by Law of Total Probability was comparable to actual OS.
Conclusion
AKI was an early indicator for poor overall survival in RCC patients. It can be predicted by several oncological parameters. Nomogram and Law of Total Probability can accurately predict AKI risk and SP.
1. Introduction
Acute kidney injury (AKI) is a major complication following surgical resection or local radical ablation for renal cell carcinoma (RCC) [Citation1]. Numerous studies demonstrated that AKI was independently associated with increased risk of all-cause patients’ mortality after surgery, sepsis or nephrotoxic drug administration [Citation2–4]. However, for RCC treatment, the effects of AKI on prognosis of RCC patients were only studied in nephrectomy. AKI following PN or RN is associated with increased mortality, new-onset CKD, worsening of preexisting CKD, and prolonged hospitalization [Citation5]. The role of AKI in TA for treating RCC has not been revealed.Given the favorable oncologic efficacy across T1a RCC management strategies, a better renal function preservation and lower non-cancer causes of death are often of paramount concerns [Citation6–9]. The top four causes of mortality for T1a RCC patients are cardiovascular disease, pulmonary disease, renal events and other malignancies [Citation10]. AKI identified as a vital predictor for all causes of death in other fields was only studied on incidence, impact on in-hospital mortality and promoting effect on chronic kidney disease in RCC [Citation5]. No study directly investigated the relationship between AKI and long-term survival prognosis in RCC patients.The least invasive methods, such as nephron sparing surgery (NSS) and thermal ablation (TA), are recommended by the American Urological Association (AUA), the European Association of Urology (EAU) for T1a RCC treatment [Citation11,Citation12]. NSS was reported to have 30.3%–55.7% AKI incidence [Citation13] and there was no data reporting on AKI after MWA. Thus, the differences in AKI incidence, impact of AKI on survival prognosis and AKI risk factors for the two techniques, are unknown. Based on these, we performed a cohort study to compare AKI incidence between NSS and MWA and to explore AKI impact on survival prognosis in T1a RCC patients. We further constructed nomogram that assess AKI risk and used Law of Total Probability to predict survival probability (SP) based on AKI risk. This study comprehensively evaluates the role of AKI in RCC patients.
2. Patients and methods
2.1. Patient selection
A total of 1554 consecutive (>18 years of age) RCC patients who underwent elective NSS (n = 1330) and MWA (n = 224) from the 1st of January 2011 to the 30th of June 2017, were reviewed in electronic medical records. The choice of NSS or MWA was based on a result of multidisciplinary discussion after a review of clinical, imaging and functional studies. Indications for MWA of 210 renal nodules in 210 patients were the following: advanced age or poor surgical candidates for significant comorbidities in 37 patients, poor liver function test in 24 patients, single kidney after nephrectomy in 9 patients, poor renal function in 73 patients, association of other cancers in 13 patients, and patient preference in 54 patients. NSS was offered to patients who were relatively healthy and young enough to endure the procedure of surgery. The inclusion criteria for the cohort study were as follows: (1) clinical TNM classification of AJCC T1aN0M0; (2) single tumor; (3) absence of vascular invasion or extrarenal spread. The exclusion criteria were as follows: (1) patients with suboptimal MRI or CT images; (2) those who underwent neoadjuvant therapy; (3) lack of clinical or imaging data or follow-up information; (4) those who suffered from sepsis, severe anemia, tumor lysis syndrome before NSS or MWA; (5) had severe coagulation disorders (i.e. prothrombin time > 25 s, prothrombin activity <40%, and platelet count <50 cells × 109/L). Finally, 1477 patients (NSS: n = 1267; MWA: n = 210) were included. The NSS and MWA procedures were previously described [Citation14,Citation15].
2.2. Outcome measurements and follow-up
AKI is defined according to the AKIN criteria (≥1.5-fold increase or increase by 26.5 μmol/L in preoperative creatinine within 48 h after procedure). AKI is classified into three stages: stage 1 as creatinine increases 1.5- to 2-fold; stage 2 as creatinine increase 2- to 3-fold; stage 3 as creatinine increase >3-fold (or need for dialysis or a peak sCr > 4 mg/mL with at least a 0.5 mg/dL increase) [Citation16]. In terms of the KDIGO guidelines and relative references, the AKI recovery is defined as the sCr level fall back to within 120% of baseline creatinine level closest to 90 days after AKI episode to allow sufficient time for recovery [Citation17]. After a complete resection or ablation was achieved, routine visit was repeated at the third month and then every 6 months. Follow-ups were closed at times of death or last visits of the patients. Last follow-up date and status were recorded. Reasons for death were measured and recorded. OS and CSS were calculated using the days between NSS or MWA and death, or end of follow-up.
2.3. Preoperative variables
Perioperative variables, known to be or that could potentially be associated with AKI, were examined. These factors were chosen a priori, based on AKI literature and on our clinical experience with AKI [Citation18–20]. The collected data were as follows: (1) patient demographics (sex, age), comorbidities (CCI score), ECOG performance status, laboratory examination (baseline eGFR, blood sugar, blood uric acid, triglyceride, total cholesterol, calcium, hemoglobin, white blood cell, platelet counts, ALT, AST, ALB, TIBL and DIBL); (2) tumor features (size, laterality and pole, adjacency, blood supply and RENAL Score); (3) procedure parameter (blood loss, warm ischemia time or ablation time). Baseline eGFR was calculated by CKD-EPI equation, which was successfully used to assess renal function in elderly cancer patients. Judging standards of tumor adjacency and blood supply, were in accordance with relevant references. Tumors adjacency to the renal pelvis and bowel were defined as distance between tumor margin and bowel or renal pelvis < 5mm as measured by US) [Citation21]. Contrast-enhanced MR or CT imaging was used to assess vascularity of the tumors. Fast multiplanar spoiled gradient-recalled-echo sequences with fat saturation (125/4.2, 90° flip angle, 256 × 192 matrix, 16–25 s breath hold) or multidetector CT (Lightspeed 16; GE Medical Systems, Milwaukee, Wis, 5-mm section thickness, a pitch of 1.35:1.0, 120 kV, and 250 mA) were performed dynamically. At visual inspection, the signal intensity enhancement of the tumor greater than and equal to that of normal renal cortex from late cortical phase (40–50 s) to delayed phase (210 s) were classified as hypervascular and the tumor enhancement less than that of normal renal cortex throughout the whole phases (cortical phase, comedullary phase and delayed phase) were classified as hypovascular [Citation22].
2.4. Law of total probability for prediction of survival probability
Law of Total Probability: p(A) = p(A|B1)p(B1) + p(A|B2)p(B2) + … + p(A|Bn)p(Bn), can be understood as follows: There are many reasons for an event (all kinds of reasons are mutually exclusive); thus, the probability of the event is the sum of the probability of each reason causing the event [Citation23]. Each patient has two conditions; suffering from AKI or not, which are mutually exclusive. Based on the Law of Total Probability, survival probability = AKI risk × overall survival (OS) of AKI patients + (1 – AKI risk) × OS of non-AKI patients. AKI risk and OS can be known from separate AKI nomograms and Kaplan-Meier survival analyses for NSS and MWA.
2.5. Statistical analysis
Continuous features were summarized as means and standard deviations or medians and interquartile ranges (IQRs). Categorical data were summarized with frequency counts and percentages. The comparison for continuous variables was conducted using Student’s t-test or Wilcoxon signed rank test and Pearson χ2 test for categorical variables. Cox regression analysis and logistic model were used to test significant effects on all-cause mortality and AKI using multiple factors and the coefficient of logistic model was used to develop AKI nomogram. The prediction of AKI, obtained from the nomogram was used to compute the AUC and perform the decision curve analysis (DCA). Calibration curves were plotted to assess the calibration of the nomogram. OS and CSS were calculated using the Kaplan-Meier method and compared using the log-rank test. The ability of Law of Total Probability to predict survival probability was assessed by Group t test. All tests were two sided with a significance level set at p < 0.05 and statistical analyses were performed using SPSS 16.0.
3. Results
3.1. Comparison of patient characteristics between NSS and MWA cohorts
Before Propensity Score Matching, MWA patients were significantly older, with higher CCI score, lower baseline eGFR (p < 0.0001 for all), lower serum albumin (p < 0.05) and worse physical condition (p < 0.01) in comparison to NSS patients. RCCs were found more frequently in the right laterality in MWA patients (p < 0.0001) (). After Propensity Score Matching, all parameters were well balanced (supporting material Table 1).
Table 1. Characteristics of the study cohort before PSM.
3.2. Comparison of AKI incidence and AKI recovery between NSS and MWA cohorts
AKI incidences were 27.85% and 17.72% in NSS and MWA cohorts, respectively (p = 0.032, supporting material Table 1). Of these AKI patients, the number of patients met 1, 2 and 3 stage AKI criteria were 35 (79.55%), 6 (13.64%) and 3 (6.82%) for NSS and 24 (85.71%), 3 (10.71) and 1 (3.57%) for MWA, respectively (supporting material Table 1). Our results showed that MWA had lower incidence of moderate to severe AKI severity relative to NSS.
Nine patients in NSS cohort and five patients in MWA cohort recovered from AKI before discharge. The results showed that 43.2% (19/44) and 42.9% (12/28) AKI patients recovered to within 120% of their baseline sCr for NSS and MWA cohorts, respectively (p = 0.9728). The CKD upstaging proportions in NSS and MWA cohorts were 45.4% (20/44) and 28.5% (8/28), respectively (p = 0.3111). Patients with higher baseline eGFR and lower CCI score were more likely to recover AKI (supporting material Table 1). The median eGFR in AKI patients who recovered was 66.62 mL/min per 1.73 m2 versus 53.57 mL/min per 1.73 m2 in those who did not recover (p = 0.000), and the median CCI score was 3.89 versus 4.60 (p = 0.011, supporting material Table 2).
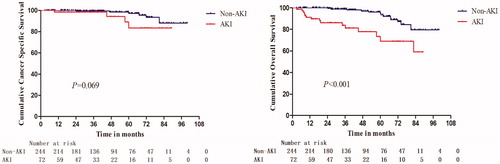
3.3. Comparison of survival outcomes between AKI and non-AKI patients
In total patients, the 1-, 3- and 5-year OS of AKI patients were 91.3%, 83.8% and 73.5%, respectively; and for non-AKI patients, they were 100.0%, 98.9% and 94.8%, respectively (p < 0.001) (). The differences of 1-, 3-, and 5-year CSS between AKI patients and non-AKI patients were not significant ( and supporting material Table 3). Multivariate analysis indicated that AKI was an independent risk factor for all-cause mortality in RCC patients (supporting material Table 4).
3.4. AKI nomogram
AKI influencing factors, such as patient, tumor and procedure, were shown in . The results showed statistically significant differences in AKI occurrence depending on age, CCI score, baseline eGFR, tumor diameter, blood loss, tumor blood supply, RENAL Score, Performance status and treatment modality. Multivariate analysis showed several factors related to AKI occurrence in 316 patients, including tumor diameter, baseline eGFR, CCI score and RENAL Score. Nomogram that incorporated the above independent predictors was developed and presented in . Separate logistic regression analysis for AKI in NSS and MWA cohorts are shown in supporting material Tables 5 and 6, respectively. Age and RENAL Score were only predictive of AKI after NSS and tumor blood supply was only associated with MWA-related AKI. Separate AKI nomograms for NSS and MWA are shown in supporting material Figures 1 and 2.
Figure 2. Nomogram for the prediction of AKI after NSS or MWA, based on multivariable logistic regression analysis. Instructions: locate the patient's baseline eGFR on the corresponding axis. Draw a line straight downward to the score axis to determine how many points toward the probability of AKI the patient receives for his/her baseline eGFR. Repeat the process for each additional variable. Add the points for each of the predictors. Locate the final sum on the total score axis. Draw a line straight up to find the patient's probability of AKI. AKI = acute kidney injury; eGFR = estimated glomerular filtration rate; CCI = Charlson comorbidity index.
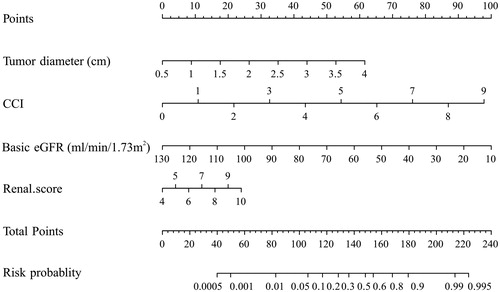
Table 2. Independent predictors of AKI.
3.5. Nomogram performance and clinical use
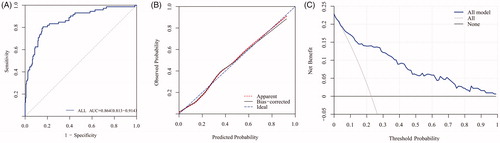
The AUC for the prediction nomogram obtained from 316 patients was 0.864 (95% CI 0.813–0.914) (). Calibration curve of the nomogram was presented in , which demonstrated that the AKI probabilities predicted by the nomogram agreed with the actual probabilities. DCA demonstrated the net clinical benefit originating from applying this model with probabilities ≥5% (). The discrimination, calibration and clinical usefulness for separate nomograms of NSS and MWA were shown in supporting material Figure 3.
Figure 3. AUC, calibration curve and decision curve analysis of AKI nomogram. A, AKI nomogram ROC curve. The x-axis indicates 1-specificity and y-axis the sensitivity. Area under the curve of ROC (AUC = 0.864) represents the great AKI discriminative ability of the nomogram. B, AKI nomogram calibration curve. The x-axis represents the nomogram-predicted probability and y-axis AKI actual probability. A perfect prediction would correspond to the 45° blue dashed line. The red dotted line represents primary cohort (n = 316) and the black solid line is bias corrected by bootstrapping (B = 1000 repetitions), indicating observed nomogram performance. C, Decision curve analysis (DCA) of AKI nomogram. DCA demonstrating the net benefit associated with the use of the nomogram-derived probability, based on multivariable logistic regression analysis, for the prediction of AKI. AKI = acute kidney injury.
3.6. Law of total probability to predict survival probability (SP)
The Law of Total Probability is introduced to estimate patient’s survival probability (described in the Materials and Methods section). Group t-test proved the accurate SP prediction ability of this formula by comparing the average SP of each cohort with actual OS (NSS: 0.943 ± 0.062 vs. 0.947 ± 0.059, t = 2.512, p = 0.087; MWA: 0.914 ± 0.079 vs. 0.915 ± 0.078, t = 2.611, p = 0.080) ().
Table 3. Accuracy assessment of predictive OSP by Law of Total Probability.
4. Discussion
Acute kidney injury is prevalent in cancer patients with incidence of 25.8–33.8%, especially in RCC patients with incidence of 49.01–54.2% [Citation24,Citation25]. The causes of AKI prevalence in cancer patients was not only related to medical interventions, such as antitumor drugs, surgery or ablation, but also related to spontaneous tumor lysis syndrome, sepsis, infection, hypercalcemia, abdominal compartment syndrome, urinary tract obstruction, vascular occlusion and contrast agents, etc. [Citation1,Citation26,Citation27]. However, the impact of AKI on survival outcomes in RCC patients is not clear and the predictors of AKI after MWA for RCC have not been studied. Therefore, the current study provides new knowledge on AKI incidence, clinical significance and prediction nomogram for T1a RCC patients. Our findings may serve as evidence for that the option of RCC treatment strategy should give priority to postprocedural AKI risk.After thermal ablation (TA) as first line of treatment for T1a RCC, some studies have investigated acute renal failure (equivalent to AKI stage 3) after TA with incidence 0–5.9% [Citation28]. However, AKI after TA has not been reported. High-power MWA used in our study had a modest AKI (17.72%) and AKI stage 3 (3.57%) incidences, lower than NSS group (27.85% and 6.82%). But subsequent multivariate logistic analysis showed that treatment modality, NSS or MWA, was not an independent risk factor for postoperative AKI occurrence.
By now, there has been no study on AKI recovery after thermal therapy. For NSS, Bravi et al. reported that 20% patients with T1N0M0 renal masses experienced AKI after NSS. The rate of patients recovering 90% of baseline function was 30% and 51% and AKI patients had CKD upstaging [Citation29]. Compared with our results about NSS, there are two speculated possible reasons accounting for the difference in the rates of AKI recovery: 1. The criteria for AKI recovery were different. Bravi et al.’s study defined AKI recovery as recovery of at least 90% of baseline eGFR after partial nephrectomy. However, sCr level falls back to within 120% of baseline creatinine level was employed in the current study. The exemplified calculation showed that AKI recovery standard adopted by Bravi et al. was more stringent. 2. The time of recovery assessment were different. Bravi selected 1 year after NSS to assess AKI recovery and we chose 3 months after MWA. Three months was recommended by AKI guidelines [Citation16]. It can reduce the chronic damage to renal function caused by comorbidities such as chronic nephritis, hyperuricemia or diabetes, etc.
Our results demonstrated that, after NSS or MWA, AKI patients had increased risk of all-cause mortality relative to non-AKI patients, but cancer-specific survival was not significantly different between these two categories. Most studies blamed that AKI can induce chronic kidney disease (CKD) and end stage renal disease, which were the direct cause of patients’ death. However, another mechanism attributed to death induced by AKI is that IL-1, TNF, and angiopoietin-2 cytokines releasing induced by AKI promote inflammation reaction, which leads to cardiac cell apoptosis, endothelial disfunction, and deteriorated microcirculatory dysfunction. They are more important pathogenic or lethal mechanism than the only eGFR decline [Citation30]. Cox regression analysis indicated that postoperative AKI was an independent risk factor for all-cause mortality. The data sufficiently demonstrates that AKI is a definite early indicator for poor survival prognosis in RCC patients.Tumor diameter, baseline eGFR, and CCI score are independent AKI predictors for both NSS and MWA. Rewa and Bagshaw indicated that the quantity and quality of preserved renal parenchyma, are the most important determinants of functional change [Citation31,Citation32]. Laguna indicated that defective quantity or quality of preserved parenchyma can result in serious deficiencies of compensatory function of residual nephrons [Citation33]. Baseline eGFR directly reflects the quality of preserved parenchyma, because a lower eGFR represents extensive glomerulosclerosis and tubular fibrosis [Citation34]. Tumor diameter indirectly determines the quantity of preserved parenchyma, as larger tumor necessitates resection or ablation of more peritumoral parenchyma for safety boundary. An intense inflammation response, after NSS or MWA, can accelerate the exposure of a latent kidney injury to hypertension, diabetes or inflammatory disease [Citation35,Citation36], which can explain why high CCI score patients are more vulnerable to post-procedural AKI.
In separate nomograms, high RENAL Score and tumor rich blood flow were independent predictors for NSS- and MWA-related AKI, respectively. Some studies reported that the ischemia time can predict the AKI occurrence after NSS [Citation37], but our results demonstrated that RENAL Score was independently predictive of AKI rather than ischemia time. Antonelli et al. and Beksac et al. studies support the above conclusion. They found that the on-clamp and off-clamp approaches for robotic partial nephrectomy showed a comparable AKI incidence and there was no significant difference in AKI incidence between those that achieved trifecta and in those where trifecta was not [Citation38,Citation39]. Bertolo et al. confirmed worse probability of maintaining ≥90% baseline renal function was found more in patients with CCI ≥ 3 (p = 0.004) and patients with PADUA score ≥8 (p = 0.023) [Citation40]. Casey and Inderbir et al. indicated that, for medially based hilar tumors which are higher RENAL nephrometry scores, robot-assisted and laparoscopic partial nephrectomies are virtually impossible if the hilum remains unclamped, because one or more distinct, higher-order arteries immediately supply the tumor or tumor-bearing segment of the kidney [Citation41]. It is quite different with laterally based tumor, as the increased intraparenchymal distance between the main renal artery and the tumor makes it less likely that a dedicated arterial branch immediately supplying the tumor. So, there are two speculations to explain why RENAL Score rather than ischemia time takes more responsibilities for AKI occurrence after NSS: 1. In present study, the time of warm ischemia was generally shorter, 22 (18, 27) minutes within the range of 25–30 min recommended by RCC management guidelines [Citation11,Citation12]. 2. AKI is a complex pathophysiological process. It is not only related to the ischemia-reperfusion injury [Citation42], but also related to the type, quantity and blood supply distribution of damaged vessels in tumor resection. Blood flow can produce heat sink effect. The peripheral renal tissue around ablation zone showed a congestive status with a temperature 40–50 °C, which can damage the non-neoplastic nephrons potentially [Citation43]. Unfortunately, so far, no research has been conducted to explore the influence of thermal sedimentation effect on blood flow temperature. Meanwhile, the present study also found that serum Cr decreased by more than 10% baseline occurred in some patients after MWA, which, from another angle, pointed out that the thermal sedimentation effect had a definitely certain action on peritumoral renal tissue. This phenomenon has never been reported before. Post et al. demonstrated that microcirculation perfusion disorder was one of the main AKI pathogenic mechanisms [Citation44]. Based on this evidence, we speculated, that the reduction of serum Cr or AKI occurrence after MWA, was the result of renal microcirculation perfusion changes caused by thermal sedimentation effect. The pathological changes of the peritumoral tissue after MWA may be helpful to reveal our clues. Damage of glomerular capillary loops and renal tubular epithelial cells or microcirculatory vasodilation without cell damage may be the corresponding renal pathological change in patients with AKI or serum Cr decline. The microcirculatory vasodilation can increase glomerular perfusion blood flow and further improve glomerular filtration rate. Heat damage or improvement in microcirculation perfusion may largely depend on the heat intensity carried away, which is necessary to study the differences of pathological changes and temperature distribution around tumor margin between AKI patients and those with significant serum Cr decrease.High AUC, excellent calibration and great clinical benefit proved that nomogram has strong AKI discriminative ability, accurate AKI prediction ability and potent clinical usefulness. Group t test definitely proved the average calculated survival probability was closely comparable to actual OS for NSS or MWA cohort. Clinicians can use AKI risk value and the Law of Total Probability to calculate patient’s survival probability for preoperative counseling. Additionally, when counseling patients, one should seriously mention that adjuvant treatment modalities that might be required after procedure must list nephrotoxicity among their side effects, especially for screening patients with high AKI risk.
This study had numerous limitations and one of them is its retrospective design with a relatively small patient series after PSM. The limited sample size might have reduced the statistical power in comparative analyses resulting in bias and coincidence. Also, stage 1 AKI accounted for the majority of AKI events in NSS and MWA cohorts. Therefore, we did not analyze the impact of AKI severity on survival outcomes according to AKI stage stratification. Finally, our survival estimates encompassed a relatively prolonged duration of 7 years, but outcomes beyond this point require further study.
5. Conclusion
AKI was an early indicator for poor overall survival in RCC patients. It can be predicted by several oncological parameters. Nomogram and Law of Total Probability can accurately predict AKI risk and SP. They may serve as tools for screening patients at high risk of AKI and poor survival prognosis after NSS or MWA.
Supplement Table 1. Characteristics of the study cohort after PSM
Download PDF (378.5 KB)Supplement table 2. Comparison of AKI predictors between non-recovery and recovery AKI patients
Download PDF (269.5 KB)Supplement table 3. Comparison of survival prognosis between AKI and non-AKI patients
Download PDF (333.1 KB)Supplement table 4. Independent risk factors for overall survival
Download PDF (348 KB)Supplement table 5. Independent risk factors for AKI after NSS
Download PDF (343.9 KB)Supplement table 6. Independent risk factors for AKI after MWA
Download PDF (299.6 KB)Disclosure statement
None of the contributing authors have any conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
Additional information
Funding
References
- Rosner MH, Perazella MA. Acute kidney injury in patients with cancer. N Engl J Med. 2017;376(18):1770–1781.
- Lim C, Audureau E, Salloum C, et al. Acute kidney injury following hepatectomy for hepatocellular carcinoma: incidence, risk factors and prognostic value. HPB. 2016;18(6):540–548.
- Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858.
- Odutayo A, Wong CX, Farkouh M, et al. AKI and long-term risk for cardiovascular events and mortality. JASN. 2017;28(1):377–387.
- Schmid M, Krishna N, Ravi P, et al. Trends of acute kidney injury after radical or partial nephrectomy for renal cell carcinoma. Urol Oncol. 2016;34(7):293.e1–293.e10.
- Xing M, Kokabi N, Zhang D, et al. Comparative effectiveness of thermal ablation, surgical resection, and active surveillance for T1a renal cell carcinoma: a surveillance, epidemiology, and end results (SEER)-medicare-linked population study. Radiology 2018;288:81–90.
- Moreland AJ, Ziemlewicz TJ, Best SL, et al. High-powered microwave ablation of t1a renal cell carcinoma: safety and initial clinical evaluation. J Endourol. 2014;28(9):1046–1052.
- Klapperich ME, Abel EJ, Ziemlewicz TJ, et al. Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology 2017; 284(1):272–280.
- Zhou W, Herwald SE, McCarthy C, et al. Radiofrequency ablation, cryoablation, and microwave ablation for T1a renal cell carcinoma: a comparative evaluation of therapeutic and renal function outcomes. J Vasc Interv Radiol. 2019;30(7):1035–1042.
- Chang JS, Park YH, Ku JH, et al. Predicting factors for death from other causes in patients with localized renal cell carcinoma. Korean J Urol. 2012;53(1):18–22.
- Ward RD, Tanaka H, Campbell SC, et al. AUA renal mass and localized renal cancer guidelines: Imaging implications. Radiographics 2018;38(7):2021–2033.
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924.
- Cho A, Lee JE, Kwon GY, et al. Post-operative acute kidney injury in patients with renal cell carcinoma is a potent risk factor for new-onset chronic kidney disease after radical nephrectomy. Nephrol Dial Transplant. 2011;26(11):3496–3501.
- Guo G, Cai W, Zhang X. Improved laparoscopic nephron-sparing surgery for renal cell carcinoma based on the precise anatomy of the nephron. Oncol Lett. 2016;12(5):3799–3803.
- Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: intermediate-term results. Radiology 2014;270(3):880–887.
- Fujii T, Uchino S, Takinami M, et al. Validation of the kidney disease improving global outcomes criteria for AKI and comparison of three criteria in hospitalized patients. CJASN. 2014;9(5):848–854.
- Sawhney S, Mitchell M, Marks A, et al. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open 2015;5(1):e006497–e006497.
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: Focus on modifiable risk factors. Circulation 2009;119(4):495–502.
- Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: Risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13(11):697–711.
- Han SS, Kim DK, Kim S, et al. C-reactive protein predicts acute kidney injury and death after coronary artery bypass grafting. Ann Thorac Surg. 2017;104(3):804–810.
- Hao G, Hao Y, Cheng Z, et al. Local tumor progression after ultrasound-guided percutaneous microwave ablation of stage T1a renal cell carcinoma: risk factors analysis of 171 tumors. Int J Hyperthermia 2018;35(1):62–70.
- Scialpi M, Di Maggio A, Midiri M, et al. Small renal masses: assessment of lesion characterization and vascularity on dynamic contrast-enhanced MR imaging with fat suppression. AJR Am J Roentgenol. 2000;175(3):751–757.
- Durrett R. Probability and examples. 4th ed. London: Cambridge University Press; 2010.
- Kang E, Park M, Park PG, et al. Acute kidney injury predicts all-cause mortality in patients with cancer. Cancer Med. 2019;8(6):2740–2750.
- Christiansen CF, Johansen MB, Langeberg WJ, et al. Incidence of acute kidney injury in cancer patients: A Danish population-based cohort study. Eur J Intern Med. 2011;22(4):399–406.
- Campbell GA, Hu D, Okusa MD. Acute kidney injury in the cancer patient. Adv Chronic Kidney Dis. 2014;21(1):64–71.
- Darmon M, Ciroldi M, Thiery G, et al. Clinical review: specific aspects of acute renal failure in cancer patients. Crit Care 2006;10(2):211–211.
- Patel HD, Pierorazio PM, Johnson MH, et al. Renal functional outcomes after surgery, ablation, and active surveillance of localized renal tumors: a systematic review and meta-analysis. CJASN. 2017;12(7):1057–1069.
- Bravi CA, Vertosick E, Benfante N, et al. Impact of acute kidney injury and its duration on long-term renal function after partial nephrectomy. Eur Urol. 2019;76(3):398–403.
- Wu VC, Wu CH, Huang TM, et al. Long-term risk of coronary events after AKI. JASN. 2014;25(3):595–605.
- Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207.
- Zabell JR, Wu J, Suk-Ouichai C, et al. Renal ischemia and functional outcomes following partial nephrectomy. Urol Clin N Am. 2017;44(2):243–255.
- Laguna MP. Re: parenchymal volume preservation and ischemia during partial nephrectomy: Functional and volumetric analysis. J Urol. 2014;191(2):347–347.
- Denic A, Ricaurte L, Lopez CL, et al. Glomerular volume and glomerulosclerosis at different depths within the human kidney. JASN. 2019;30(8):1471–1480.
- Polichnowski AJ. Microvascular rarefaction and hypertension in the impaired recovery and progression of kidney disease following AKI in preexisting CKD states. Am J Physiol Renal Physiol. 2018;315(6):F1513–F1518.
- Gao G, Zhang B, Ramesh G, et al. TNF-alpha mediates increased susceptibility to ischemic AKI in diabetes. Am J Physiol Renal Physiol. 2013;304(5):F515–F521.
- Minervini A, Campi R, Lane BR, et al. Impact of resection technique on perioperative outcomes and surgical margins after partial nephrectomy for localized renal masses: A prospective multicenter study. J Urol. 2019;203(3):496–504.
- Antonelli A, Cindolo L, Sandri M, et al. Safety of on- vs. off-clamp robotic partial nephrectomy: per-protocol analysis from the data of the CLOCK randomized trial. World J Urol. 2019. DOI:10.1007/s00345-019-02879-4
- Beksac AT, Okhawere KE, Elbakry AA, et al. Management of high complexity renal masses in partial nephrectomy: A multicenter analysis. Urol Oncol. 2019;37(7):437–444.
- Bertolo R, Fiori C, Piramide F, et al. The preoperative stratification of patients based on renal scan data is unable to predict the functional outcome after partial nephrectomy. Int Braz J Urol. 2018; 44(4):740–749.
- Hou W, Ji Z. Achieving zero ischemia in minimally invasive partial nephrectomy surgery. Int J Surg 2015;18:48–54.
- Lee C, Jang MJ, Kim BH, et al. Recovery of renal function after administration of adipose-tissue-derived stromal vascular fraction in rat model of acute kidney injury induced by ischemia/reperfusion injury. Cell Tissue Res. 2017;368(3):603–613.
- Chu KF, Dupuy DE. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat Rev Cancer 2014;14(3):199–208.
- Post EH, Kellum JA, Bellomo R, et al. Renal perfusion in sepsis: from macro- to microcirculation. Kidney Int. 2017;91(1):45–60.