Abstract
Purpose
To evaluate the efficacy and safety of ultrasonography (US)-guided radiofrequency ablation (RFA) for treating low-risk T1bN0M0 papillary thyroid cancer (PTC).
Methods
This retrospective study was approved by the ethics committee of the Chinese People’s Liberation Army General Hospital (S2019-211-01). Sixty-six patients with T1bN0M0 PTC (14 men and 52 women with a mean age of 41.0 ± 9.2 years [range, 21–61 years]), who were not eligible for or refused surgery, were included in our study. RFA was performed with the moving-shot technique, and the ablation area exceeded the tumor edge by at least 3 mm. US (including contrast-enhanced) was performed before RFA; 1, 3, and 6 months after RFA, and every 6 months thereafter. US-guided core-needle biopsy was performed 3 or 6 months after ablation to rule out recurrence.
Results
The technical success rate was 100%, and there were no major complications. The tumor volume decreased significantly; the volume reduction rate (VRR) was 99.11 ± 2.44% (range, 92.62–100%) at the final follow-up with 38 tumors (57.6%) disappearing. Significant decreases in the VRR were found at every other follow-up visit before 18 months (p < .01). Technique efficacy was obtained in 64/66 (97.0%) patients over 20.5 ± 7.4 months follow-up. Malignant cells were confirmed in 2 ablation zones (3.0%), and cervical lymph node metastasis was detected in 1 patient (1.5%). These patients underwent additional RFA and achieved good results.
Conclusion
RFA may be considered a safe and effective modality for the management of T1bN0M0 PTC in select patients.
Introduction
Thyroid cancer is the most common endocrinological malignancy; its incidence rate worldwide has increased over the last 5 decades [Citation1,Citation2]. Papillary thyroid carcinomas (PTCs) account for the majority of thyroid malignancies and have favorable prognoses with a low mortality rate [Citation3,Citation4].
The first-line treatment of primary PTC recommended by several guidelines [Citation3,Citation5,Citation6] is surgery. The American Joint Committee on Cancer (AJCC) TNM system for differentiated thyroid cancer classified T1 category (intrathyroidal tumor ≤2 cm in its greatest dimension) into T1a (intrathyroidal tumor ≤1 cm) and T1b (intrathyroidal tumor >1 cm but ≤2 cm) [Citation7]. Several studies found that patients with T1N0M0 tumors show excellent prognoses after surgery [Citation8,Citation9]. However, complications related to surgery, such as permanent recurrent laryngeal nerve injury, inadvertent hypoparathyroidism, visible scarring, and hypothyroidism that requires lifelong administration of levothyroxine [Citation10,Citation11], have considerable effects on the patients’ quality of life. In light of these complications, an increasing number of patients are unwilling to undergo surgery; therefore, image-guided thermal ablation may be a potentially attractive alternative. One of the advantages of application of thermal ablation to small thyroid cancer is to try to limit invasiveness and compensate for overdiagnosis [Citation12].
Ablation guidelines and consensus statements for patients with thyroid disease recommended RFA for benign thyroid nodules and recurrent thyroid cancers [Citation13–15]. Data on RFA for the treatment of primary PTC mainly focus on low risk papillary thyroid microcarcinoma (PTMC, i.e., T1aN0M0 PTC) [Citation16–18] and gain promising results without major complications. However, little research has been performed on the validity of RFA for T1bN0M0 tumors. Therefore, the purpose of this study was to investigate the efficacy and safety of ultrasonography (US)-guided RFA for the treatment of T1bN0M0 PTC.
Materials and methods
Patients
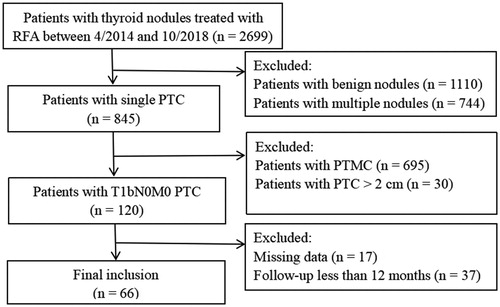
This retrospective study was approved by the ethics committee of the Chinese People’s Liberation Army General Hospital (S2019-211-01), which waived the requirement for informed consent. Sixty-six patients treated between April 2014 and October 2018 (14 men and 52 women with a mean age of 41.0 ± 9.2 years, ranging from 21 to 61 years) were included in our study (). The inclusion criteria were as follows: (1) PTC confirmed via US-guided core needle biopsy (CNB) without aggressive variants [Citation19,Citation20]; (2) solitary nodule with a greatest dimension >1 cm and ≤2 cm on US; (3) no radiologic or clinical evidence of extrathyroidal invasion, lymph node metastasis, or distant metastasis; (4) more than 12 months of follow-up; and (5) ineligibility for surgery (n = 9) or refusal to undergo it (n = 57).
Pre-ablation evaluation
US (including contrast-enhanced ultrasonography [CEUS]) was performed for each patient before ablation using the Siemens Acouson Sequoia 512 (Siemens, Mountain View, CA) scanner with a 7.0 MHz linear array transducer, the Siemens S2000 (Siemens, Mountain View, CA) scanner with a 8.0 MHz linear array transducer, or the Mindray Resona 7 (Mindray, Shenzhen, China) scanner with a 5.6–10 MHz linear array transducer. The 3 diameters of each tumor (length, width and depth), as well as volume, location, and US features were recorded. Tumor volume was expressed in mL using the ellipsoid volume formula: length × width × depth × 0.524, with measurements expressed in cm [Citation21]. US features, which included composition, echogenicity, shape, margin, and echogenic foci (), were evaluated and recorded according to the American College of Radiology Thyroid Imaging, Reporting, and Data System [Citation22].
Table 1. Ultrasound features of papillary thyroid carcinomas before radiofrequency ablation.
The tumor’s blood supply was evaluated using CEUS. A bolus of 2.4 ml SonoVue suspension (Bracco International, Milan, Italy) was injected and flushed with 5 ml of normal saline. Each contrast image was timed to be acquired for 3 min after bolus injection, and the video clip was digitally recorded to further analyze the CEUS enhancement mode. The echo intensity of the microbubbles within the tumor was compared to that in the normal thyroid parenchyma at peak enhancement, and the enhancement mode was divided into 3 patterns: low, equal, and high enhancement [Citation23] ().
Ablation procedure
One US physician (Y.K.L.) with more than 20 years of experience in thyroid US and interventional US performed the RFA procedures. The Siemens Acouson Sequoia 512 (Siemens, Mountain View, CA) scanner with a 6.0 MHz linear array transducer was used for guidance. The bipolar RFA generator (CelonLabPOWER; Olympus Surgical Technologies Europe, Hamburg, Germany) and an 18-gauge straight-type internally cooled electrode with a 1.5 cm active tip (CelonProSurge micro 100-T15; Olympus Surgical Technologies Europe) were used. Written informed consent was obtained from all patients before US-guided CNB and RFA. The informed consent for RFA emphasized that surgery is the standard treatment for PTC, and that RFA would not avoid the potential risks of recurrence, occult cervical lymph node metastasis, and distant metastasis.
RFA was performed in an outpatient US interventional room. Patients were in the supine position with the neck extended during the procedure. Skin sterilization was performed, and topical anesthesia with 1% lidocaine was administrated at the subcutaneous puncture site and the thyroid anterior capsule. Before ablation, care was taken to evaluate the approach route as well as the relationship between the tumor and vital cervical structures (i.e., the trachea, esophagus, recurrent laryngeal nerve and blood vessels). If the distance between the tumor and critical structures was less than 5 mm, a mixture of 1% lidocaine and normal saline was continuously injected using another 23 gauge needle to form at least 1 cm distance between the tumor and critical structures to prevent thermal injury [Citation16,Citation24]. After insertion of the electrode into the deepest and most remote part of the nodule under US guidance, we commenced ablation at 3 W. If a transient hyperechoic zone did not form at the electrode tip within 5–10 s, the output power was increased. The trans-isthmus approach and moving-shot technique were used during the procedure [Citation24,Citation25]. The whole nodule plus a safe margin (≥3 mm) [Citation26] were ablated to prevent marginal recurrence. The procedure was not terminated until the whole tumor transformed into a transient hyperechoic zone, and the needle track was coagulated to prevent tumor cell seeding. At the end of each procedure, the ablation zone was evaluated by CEUS; if residual enhancement was present, additional ablation was performed during the same treatment session.
Post-ablation evaluation and follow-up
After the procedure, patients were closely observed for 1–2 h with compression of the neck for 20–30 min to avoid bleeding before discharge. All patients were advised to take levothyroxine to maintain thyroid-stimulating hormone levels below 0.1–0.5 mU/L. When the ablation zone disappeared, levothyroxine was withdrawn in a tapered manner.
The size, volume, and vascularity of the ablation zones as well as the development of recurrent tumors in the thyroid were carefully evaluated by US and CEUS 1, 3, and 6 months after ablation, and every 6 months thereafter. The volume reduction ratio (VRR) was calculated as follows: VRR = ([initial volume − final volume] × 100)/initial volume. US-guided CNB was routinely performed at the center and edge of the ablation zone as well as in the surrounding thyroid parenchyma 3 or 6 months after ablation. If the volume of the ablation zone in subsequent months was larger than that at last time, a re-biopsy was performed under the assumption that active tumor cells may exist.
We also evaluated lymph nodes in the neck via US; the detection of any suspicious characteristics (i.e., microcalcifications, cystic aspects, peripheral vascularity, hyperechogenicity, and/or round shape) [Citation27] led to performing US-guided CNB to determine whether the nodes were metastatic or reactive. A CT examination was performed annually to rule out distant metastases.
Data analysis
We analyzed the technical feasibility, technical success, technique efficacy, and rate of complications at follow-up [Citation28,Citation29]. Technical feasibility was defined as the ability to target the tumor and perform the ablation as planned. Technical success was defined as the complete absence of enhancement in the treated tumor on immediate post-ablation CEUS. Major and minor complications as well as side effects were reported according to the reporting standards of the Society of Interventional Radiology. We considered it to be complete ablation if the ablation zone disappeared, or no viable malignant cells were detected in the ablation zone. Technique efficacy was related to a specific follow-up time point at which complete ablation was achieved.
All data were analyzed using SPSS for Windows version 25.0 (IBM Corp., Armonk). Continuous data are expressed as means ± standard deviations while categorical data are presented as frequencies and percentages. Wilcoxon’s signed rank test was used to compare changes in the largest diameter and volume of each index tumor before RFA and at each follow-up point. P-values less than 0.05 were considered statistically significant.
Results
RFA treatment parameters
The RFA treatment parameters are listed in . Each tumor underwent 1 or 2 sessions of RFA. Sixty-two patients (93.9%) were treated with a single RFA session, while 4 patients (6.1%) required 2 sessions. The second RFA was performed 12 months after the first ablation in 2 patients owing to the detection of malignant cells by CNB. The ablation zone showed minimal enhancement on CEUS in 2 patients, but there were no malignant cells detected on CNB. To prevent recurrence, additional RFA was performed 6 months after the first ablation in these 2 patients.
Table 2. Radiofrequency ablation treatment parameters.
Treatment response after RFA
Correct tumor targeting was achieved for all 66 patients (100%), and all the ablations were performed as planned. Immediate CEUS after ablation showed no enhancement in the ablation zones, which completely encompassed the target tumors (i.e., the technical success rate was 100%).
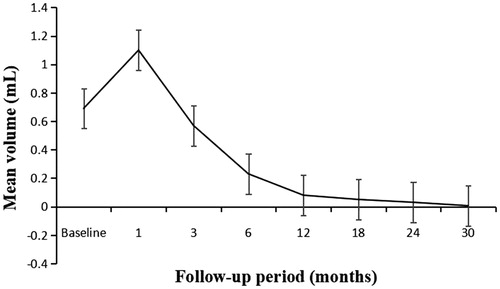
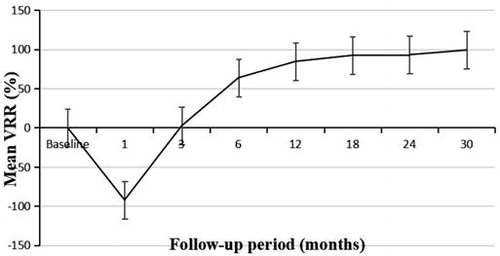
The mean follow-up duration was 20.5 ± 7.4 months (range, 12–48 months). At the 1-month follow-up, the diameters and volumes of the ablation zones were larger than those of the PTCs, then decreased gradually (). There were significant differences in the maximum diameters between baseline and each follow-up point (p < .01), except for that between baseline and 3 months after ablation (p = 0.705). The mean tumor volume decreased from 0.69 ± 0.34 ml (range, 0.25–1.76 ml) before ablation to 0.006 ± 0.016 ml (range, 0.00–0.05 ml) at the final follow-up (, ), and significant differences were found between the volume at baseline and that at each follow-up point (p < .01). The VRR changed from negative to positive 3 months after ablation (). Significant differences in the VRR were found between every 2 follow-up periods before 18 months (p < .01), and there were no significant differences in the VRR were observed between 18 months and 24 months (p = 0.5).
Table 3. Changes in mean diameter, volume and volume reduction rate at each follow-up point.
Thirty-eight ablation zones (57.6%) disappeared during follow-up (). Four ablation zones (6.1%) disappeared in 6 months, 15 (22.7%) in 12 months, 11 (16.7%) in 18 months, 4 (6.1%) in 24 months, and an additional 4 ablation zones (6.1%) had disappeared at the last follow-up. Among the 28 ablation zones that did not disappear, CNB revealed no viable neoplastic cells in 26 cases. As a result, at the last follow-up, technique efficacy rate was 97.0% (64/66 patients).
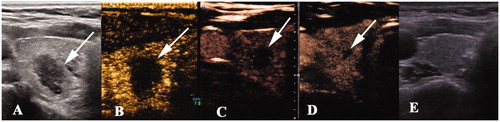
Figure 4. Radiofrequency ablation (RFA) treatment and follow-up of a 46 year-old man with papillary thyroid caner. (A) A hypoechoic nodule with an irregular margin and punctuate echo foci, size 1.1 × 1.0 × 0.7 cm, was detected in the left thyroid lobe (white arrow). (B) The nodule was covered by a hyperechoic area (white arrow) on ultrasonography during the RFA procedure. (C–F) The ablation area decreased gradually to 1.3 × 1.0 × 0.9 cm, 0.8 × 0.8 × 0.5 cm, 0.5 × 0.4 × 0.4 cm, and 0.3 × 0.2 × 0.2 cm 1, 3, 6, and 12 months, respectively, after ablation. There was no enhancement on contrast-enhanced ultrasonography. (G) Ultrasonography 18 months after ablation showed a needle track. (H) The ablation area disappeared on ultrasonography 30 months after ablation.
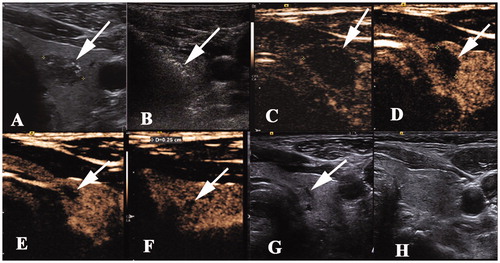
Figure 5. A 40 year-old woman with a papillary thyroid cancer in the right thyroid lobe. The images of the nodule pre- and post-ablation are shown. (A) A hypoechoic nodule (white arrow) with an irregular margin measuring 1.4 × 0.8 × 0.7 cm was detected in the right lobe. (B) One month after ablation, the ablation zone was 1.3 × 1.0 × 1.0 cm in size, which was larger in volume than that before ablation. (C–D) The ablation zone decreased gradually to 0.6 × 0.4 × 0.4 cm and 0.4 × 0.3 × 0.2 cm 3 and 6 months after ablation, respectively, and there was no enhancement on contrast-enhanced ultrasonography. (E) The ablation area disappeared on ultrasonography 12 months after ablation.
Two patients (3.0%) were found to have malignant cells on CNB at the edge of their ablation zones 6 months after undergoing RFA ablation; these patients successfully underwent a second RFA session. A metastatic lymph node was detected at ipsilateral level IV in 1 patient 3 months after ablation; this lymph node was successfully treated with RFA. No distant metastases were detected during follow-up.
Complications
All patients tolerated the RFA procedure well, and no major complications were observed. Two patients experienced moderate pain that was managed with painkillers. No hematomas, voice changes, or skin burns occurred.
Discussion
The incidence of PTC has increased in the last few decades mainly owing to the widespread use of US and US-guided fine-needle aspiration biopsy; however, the mortality rate of this disease remains stable. Determining the optimal clinical treatment for low-risk small PTC has produced considerable controversy. Surgery has been the traditional treatment option of PTC; however, it is associated with several complications that would lower the quality of life [Citation10,Citation11]. Owing to the indolent nature of PTC, active surveillance was previously proposed as a management option [Citation30] and is currently adopted by Japanese and American guidelines as an alternative to immediate surgery [Citation4,Citation35]. To date, the indication of active surveillance was limited to T1aN0M0 PTC, not including T1bN0M0 PTC. However, once diagnosed with cancer, patients may experience great anxiety and be compelled to eliminate the tumor; therefore, only a small minority of patients may accept active surveillance.
In light of the above, image-guided thermal ablation is considered an alternative approach and has several advantages over surgery in terms of treating patients with PTC. It is less invasive, can be performed in an outpatient department with local anesthesia, is less expensive, and is associated with a better quality of life [Citation26]. Moreover, image-guided thermal ablation might be a way to compensate for overdiagnosis [Citation12].
As a widely used thermal ablation method, image-guided RFA has been successfully reported in patients with malignant tumors of the liver, kidney, and lung [Citation31–33]. In patients with thyroid disease, RFA research has initially focused on the treatment of benign nodules that cause symptomatic or cosmetic problems, and excellent results with satisfactory volume reduction and improvement of symptoms have been obtained with this method [Citation34,Citation35]. A recent consensus document proposed thermal ablation as a first-line treatment for benign nodules for the first time [Citation15]. RFA has also achieved favorable results for the management of recurrent thyroid cancer [Citation36,Citation37]. Lim et al. found that 82% (50/61) of recurrent tumors among their patients completely disappeared after RFA, and that the mean serum thyroglobulin level decreased significantly; their data indicated that RFA can effectively control locoregional recurrent PTC and may replace surgery in select patients [Citation37]. Recent studies on the application of RFA for the treatment of T1aN0M0 PTC [Citation16–18] have produced promising results. In our previous study, patients achieved a mean volume reduction rate of 96% and complete tumor disappearance rate of 10.2% within 1 year. [Citation16]. Additionally, no local tumor recurrence or cervical lymph node metastasis was observed during follow-up. However, there remain very few studies that focus on T1bN0M0 PTC.
A large database study demonstrated that compare to T1a tumors, T1b tumors have more lymphovascular invasion, positive surgical margins, metastatic lymph nodes, and distant metastases [Citation8]; however, T1a and T1b tumors have similar prognoses despite undergoing different extents of surgery [Citation8,Citation38]. The 2015 American Thyroid Association guidelines reconsidered lobectomy as an acceptable option for patients with T1b–T2 tumors without extrathyroidal extension and lymph node metastasis [Citation3]. Moreover, a recent long-term follow-up study (mean follow-up, 7.4 years) found no significant difference between the progression rates of T1a and T1b tumors [Citation39]. Based on these data, we supposed that US-guided RFA may produce favorable results in select patients with T1b tumors.
Kim et al. [Citation40] provided a valuable evidence that RFA could be a safe and effective method to control low-risk small PTCs, not just to PTMCs. However, the participants in their study were elderly patients who were ineligible for surgery, and only 2 cases had PTCs larger than 1 cm. Our study is the first one to evaluate the feasibility of US-guided RFA for the management of T1bN0M0 PTC. We included 66 patients with T1bN0M0 PTC, who were ineligible for or refused surgery; their mean age was 41.0 ± 9.2 years. The technique efficacy rate was 97.0%, while the mean VRR was 99.11 ± 2.44% (range, 92.62–100%) at the 30-month follow-up. There were no major complications during the procedure. This demonstrated that US-guided RFA may be a potentially effective and safe modality for treating T1bN0M0 PTC, especially when surgery is not possible or is refused by the patient. Moreover, 4 patients underwent a second RFA ablation session without additional technical difficulties.
There were several limitations in our study in addition to those intrinsic to a retrospective investigation. Because occult malignancies and micrometastases cannot be detected with US, we may have missed them when performing RFA. Of note, the same risk may also be present in the contralateral lobe if patients undergo lobectomy. The number of patients was small, and the follow-up period was short; this may have underpowered the statistical analysis for such an indolent disease process. Therefore, further large multicenter studies involving long-term follow-up are required to confirm our results.
In conclusion, RFA may be a safe and effective alternative to surgery for the treatment of T1bN0M0 PTCs, especially in patients who are ineligible for surgery or refusal to undergo it.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020;16(1):17–29.
- Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–653.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–322.
- Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol. 2014;81 (Suppl 1):1–122.
- Gharib H, Papini E, Garber JR, et al. American association of clinical endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidlines for clinical practice for the diagnosis and management of thyroid nodules-2016 update. Endocr Pract. 2016;22(Supplement 1):1–639.
- Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): what changed and why? Thyroid. 2017;27(6):751–756.
- Anderson KL, Youngwirth LM, Scheri RP, et al. T1a versus T1b differentiated thyroid cancers: do we need to make the distinction? Thyroid. 2016;26(8):1046–1052.
- Ito Y, Masuoka H, Fukushima M, et al. Excellent prognosis of patients with solitary T1N0M0 papillary thyroid carcinoma who underwent thyroidectomy and elective lymph node dissection without radioiodine therapy. World J Surg. 2010;34(6):1285–1290.
- Linos D, Economopoulos KP, Kiriakopoulos A, et al. Scar perceptions after thyroid and parathyroid surgery: comparison of minimal and conventional approaches. Surgery. 2013;153(3):400–407.
- Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer – recent advances and future directions. Nat Rev Endocrinol. 2018;14(11):670–683.
- Mauri G, Sconfienza LM. Image-guided thermal ablation might be a way to compensate for image deriving cancer overdiagnosis. Int J Hyperther. 2017;33(4):489–490.
- Kim J, Baek JH, Lim HK, et al. 2017 thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperther. 2019;36(1):375–381.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581–1587.
- Ding M, Tang X, Cui D, et al. Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol. 2019;74(9):712–717.
- Zhang Y, Zhang MB, Luo YK, et al. Effect of chronic lymphocytic thyroiditis on the efficacy and safety of ultrasound‐guided radiofrequency ablation for papillary thyroid microcarcinoma. Cancer Med. 2019;8(12):5450–5458.
- Lloyd RV, Osamura RY, Klöppel G, et al. WHO classification of tumors: pathology and genetics of tumors of endocrine organs. Lyon: IARC Press; 2017.
- Guo Z, Ge M, Chu Y, et al. Recent advances in the classification of low-grade papillary-like thyroid neoplasms and aggressive papillary thyroid carcinomas: evolution of diagnostic criteria. Adv Anat Pathol. 2018;25(4):263–272.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Tessler FN, Middleton WD, Grant EG, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14(5):587–595.
- Zhang Y, Zhou P, Tian S, et al. Usefulness of combined use of contrast-enhanced ultrasound and TI-RADS classification for the differentiation of benign from malignant lesions of thyroid nodules. Eur Radiol. 2017;27(4):1527–1536.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18(4):615–623.
- Shin JH, Baek JH, Ha EJ, et al. Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol. 2012;2012:1–7.
- Zhang M, Tufano RP, Russell J, et al. Ultrasound-guided radiofrequency ablation versus surgery for low risk papillary thyroid micro-carcinoma: results of over 5 years follow-up. Thyroid. 2020;30(3):408–417.
- Leenhardt L, Erdogan MF, Hegedus L, et al. 2013 European Thyroid Association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. 2013;2(3):147–159.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39(7):1023–1030.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–260.
- Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34(1):28–35.
- Yuan Z, Wang Y, Zhang J, et al. A meta-analysis of clinical outcomes after radiofrequency ablation and microwave ablation for lung cancer and pulmonary metastases. J Am Coll Radiol. 2019;16(3):302–314.
- Lee DH, Lee JM. Recent advances in the image-guided tumor ablation of liver malignancies: radiofrequency ablation with multiple electrodes, real-time multimodality fusion imaging, and new energy sources. Korean J Radiol. 2018;19(4):545–559.
- Sanchez A, Feldman AS, Hakimi AA. Current management of small renal masses, including patient selection, renal tumor biopsy, active surveillance, and thermal ablation. J Clin Oncol. 2018;36(36):3591–3600.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperther. 2019;36(2):13–20.
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276(3):909–918.
- Lim HK, Baek JH, Lee JH, Kim WB, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25(1):163–170.
- Wang LY, Nixon IJ, Palmer FL, et al. Comparable outcomes for patients with pT1a and pT1b differentiated thyroid cancer: is there a need for change in the AJCC classification system? Surgery. 2014;156(6):1484–1490.
- Sakai T, Sugitani I, Ebina A, et al. Active surveillance for T1bN0M0 papillary thyroid carcinoma. Thyroid. 2019;29(1):59–63.
- Kim J, Baek JH, Sung JY, Min HS, et al. Radiofrequency ablation of low-risk small papillary thyroid carcinoma: preliminary results for patients ineligible for surgery. Int J Hyperther. 2017;33(2):212–219.