Abstract
Objective
To evaluate the magnetic resonance (MR) signal intensity changes in the sacrococcygeal region of patients with uterine fibroids treated with high intensity focused ultrasound (HIFU).
Materials and Methods
Two hundred and sixty-seven patients with uterine fibroids treated with HIFU between January and December 2016 were retrospectively reviewed. All patients underwent enhanced pre- and post-HIFU MRI. Multivariate analysis was used to assess the relationship between the factors and the signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum.
Results
Among the 267 patients, 122 (46%) had MR signal intensity changes in the sacrum and/or the soft tissue adjacent to the sacrum after HIFU. Multivariate analysis showed that the position of the uterus, the distance from the dorsal side of the fibroid to the sacrum, and the ablation efficiency were significantly correlated with MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum. Further analysis showed a significant relationship between the location of the MR signal intensity changes and uterine size, the enhancement degree of the uterus. Leg pain was only seen in patients with MR signal intensity changes both in the sacrum and the soft tissue adjacent to the sacrum.
Conclusions
The location of the uterus, the distance between the dorsal side of the fibroids to the sacrum, and ablation efficiency have a significant relationship with the MR signal intensity changes. The size of the uterus and the degree of enhancement are related to the locations of MR signal changes.
Introduction
Uterine fibroids/myomas are the most common benign tumors in the reproductive system of childbearing age women. The prevalence of uterine fibroids varies between races and is generally reported as 20–40% [Citation1]. Half of the patients with uterine fibroids presented with large menstruation volume, lumbosacral pain, constipation, and other symptoms [Citation2]. Currently, treatments for uterine fibroids include medication, surgery, uterine artery embolization (UAE), and high intensity focused ultrasound (HIFU) [Citation3]. Among these treatment modalities, medication and HIFU are noninvasive treatments. Medication can improve the symptoms, but the symptoms often return after termination of medication. HIFU is a local thermal ablation technique that destroys tumor by thermal cavitation or mechanical destruction through the absorption of ultrasound energy generated by an extracorporal transducer. The ultrasound beams penetrate the tissues of the body and are focused on the tumor to induce coagulative necrosis. As a noninvasive technique, HIFU has been widely used to treat patients with solid tumors. Many studies have shown that HIFU is safe and effective in the treatment of uterine fibroids [Citation4–9]. However, in clinical practice, we encountered some patients who complained of sacrococcygeal region pain or leg pain. In post-HIFU magnetic resonance imaging (MRI), the T2 weighted image (T2WI) shows signal changes and the contrast-enhanced MRI shows the changes in enhancement in the sacral vertebrae and/or the soft tissue adjacent to the sacral vertebrae. Recently, Liu et al. showed that complications in the far field, such as sacrum/buttock pain and leg pain, may occur in patients who have been treated by HIFU for benign uterine diseases, with an incidence rate of 10.5% (2833/27,053) for sacrum/buttock pain and 0.06% (17/27,053) for leg pain [Citation9]. Unfortunately, they did not show any details of MRI signal changes of these patients. Cun et al. reported that 135 out of 346 (39.0%) patients with a single fibroid treated with HIFU had sacral injury. Among them, only 14.1% (19/135) of the patients reported sacrum/buttock pain, and 2 patients presented leg pain. However, in the group without signal changes in the sacrum on MRI, 9.5% (21/211) of patients complained of sacrum/buttock pain [Citation10]. They further analyzed the factors that may be related to sacral injury and showed that the enhancement, the distance from the fibroid to the sacrum, and the type of fibroid were significantly related to sacral injury. However, they did not evaluate whether the patients had edema in the soft tissue adjacent to the sacrum, nor any relationship between the pain and signal intensity changes in the sacrum or the soft tissue adjacent to the sacrum. Therefore, this study was to retrospectively evaluate signal changes in the sacral vertebrae and the soft tissues adjacent to the sacrum on MRI, to further analyze the factors that may lead to sacral injury and edema of the soft tissues adjacent to the sacrum, and to explore the relationship between the pain and signal intensity changes in the sacrum or the soft tissue adjacent to the sacrum.
Materials and methods
This retrospective study was approved by the ethics committee at our institute. Before HIFU treatment, the details of the treatment were discussed with all patients, who then signed a consent form.
Subjects
From January to December 2016, 312 patients with uterine fibroids were treated with HIFU at Chongqing Haifu Hospital. Among them, 267 patients were retrospectively reviewed, 36 patients who did not undergo pre-HIFU MRI examination and 9 patients who did not undergo post-HIFU MRI examination in our hospital were excluded from this study.
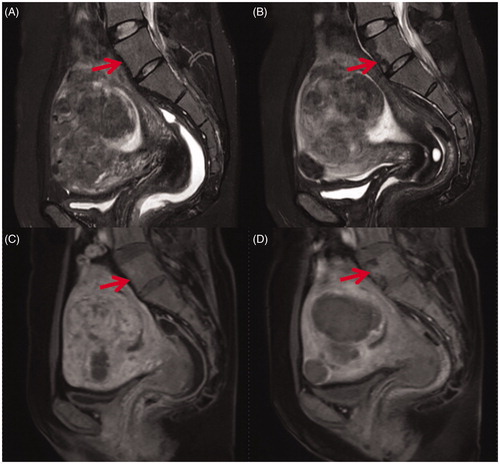
Figure 1. Sagittal view of MR images obtained from a 41-year-old patient with uterine fibroids before and 1 day after HIFU treatment. (A) Pre-HIFU T2WI image showed normal signal intensity in lumbar vertebrae (arrow); (B) Post-HIFU T2WI image showed a sheet-like hypointense area in the vertebrae of lumbar 5 (arrow); (C) Pre-HIFU contrast-enhanced image showed normal enhancement in the vertebrae of lumbar 5 (arrow); (D) Post-HIFU contrast-enhanced image showed a partial perfusion area corresponds to the hypointense area in the vertebrae of lumbar 5 on post-HIFU T2WI (arrow).
The inclusion criteria for this study were as follows: (1) a confirmed diagnosis of uterine fibroids; (2) all had clinical symptoms; (3) a demonstrated ability to communicate with the nurse or physician during HIFU procedure; and (4) agreed to undergo pre- and post-HIFU MRI examinations at our institute.
Exclusion criteria include patients treated with HIFU who did not have an MRI at our hospital.
Magnetic resonance imaging
All pre- and post-HIFU MR examinations were performed using a 1.5 T Magnetom Symphony MR system (uMR570, Shanghai United Imaging Healthcare, the model). The standardized parameters of the T2WI sequence were as follows: TR 2300 ms/TE 100 ms, voxel size 1.0 × 1.0 × 4.0 mm, thickness 5 mm. The standardized parameters for the T1-weighted image (T1WI) sequence were as follows: TR 500 ms/TE 13 ms, voxel size 1.7 × 1.3 × 5.0 mm, thickness 5 mm. The parameters for the contrast-enhanced T1WI sequence were as follows: TR 6.23 ms/TE 2.91 ms, voxel size 1.7 × 1.2 × 4.0 mm, thickness 5 mm.
Every MRI reading was performed by two experienced radiologists. The data from pre-HIFU MR images included the position of the uterus (anteverted, retroverted), the location of uterine fibroids, the type of uterine fibroids (either submucosal fibroids, subserosal fibroids, or intramural fibroids), the signal intensity of the fibroids on T2WI (hypointense, isointense, hyperintense), the signal intensity in the sacrum and the soft tissue adjacent to the sacrum, distance from the ventral side of the fibroid to the abdominal wall, distance from the dorsal of the fibroid to sacrum. According to the degree of enhancement of the uterine fibroids as compared to the myometrium within 60 s after the gadolinium injection, the degree of enhancement was classified as slight when less than that of the myometrium, moderate when similar to that of the myometrium, and significant when greater than that of the myometrium. The post-HIFU MRI examination was performed one day after HIFU. The signal intensity in the sacrum and the soft tissue adjacent to the sacrum was carefully reviewed and compared between pre-HIFU and post-HIFU MRI on T1, T2, and contrast enhanced images. The three-dimensional diameters of the non-perfused area and the fibroids were measured on the enhanced magnetic resonance image: long diameter (D1), anteroposterior diameter (D2) and transverse diameter (D3). The volume of the fibroids and the volume of the non-perfused area were calculated according to the following equation: V = 0.5233 × D1 × D2 × D3. The volume of the signal intensity changed region was measured using the software program, which was programed by the engineers from Chongqing Haifu Medical Tech Co., Ltd., to contour the signal intensity changed region in every slice of T2 weighted image of MRI, then calculated by the same program.
HIFU treatment
Before HIFU treatment, every patient was asked to undergo bowel and skin preparation. Bowel preparation included ingestion of half liquid food or liquid food for 2 days, a 12 h fast, and a mandatory enema before HIFU on the treatment day. Skin preparation included shaving the hair of the anterior abdominal wall between the umbilicus and the superior border of the symphysis pubis, then degreasing and degassing with 70% ethanol and degassed water. A urinary catheter was inserted into the bladder and normal saline was used to control the bladder volume.
The procedure of HIFU was performed using JC200, an ultrasound-guided HIFU tumor therapeutic system [Chongqing Haifu Medical Technology Co., Ltd. Chongqing, China]. This system is equipped with a therapeutic focused ultrasound transducer of 20 cm in diameter and a B mode ultrasound diagnostic probe (MyLab 70, Esaote, Genova, Italy) sits at the center of the transducer to monitor the ablation process. The patients were positioned prone on the HIFU table, with the anterior abdominal wall placed in contact with degassed water. The procedure of HIFU ablation was performed under intravenous conscious sedation. The vital signs monitored included respiration, oxygen saturation, heart rate, and blood pressure.
The procedure started with the posterior part of the fibroid, and the focus was placed at least 1 cm away from the boundary of the fibroid and at least 3 cm away from the sacrum. The sonication power was adjusted based on feedback from the patient and the grayscale changes on the ultrasonographic image. This treatment was repeated on a section-by-section basis to achieve complete ablation of the planned treatment volume; once the increased gray scale covered the treated lesion, the treatment was terminated. Treatment time, sonication power, sonication time, treatment intensity (seconds/hour, the sonication time per hour in treatment), and treatment efficiency (mm3/s, ablated fibroid volume per second) were recorded.
Statistical analysis
SPSS 22.0 (IBM, USA) statistical analysis software was used for data analysis. The normally distributed data was reported as mean ± standard deviation, and the skewed distribution data was reported as median and interquartile range. Sample t-test, rank sum test and x2 test were used for statistical comparisons of the two groups with or without MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum. In the multivariate analysis, the logistic regression was used for the two categorical variables, and the disordered multiclass categorical variables were used for the disordered multiclass logistic regression. p < .05 indicates that the difference was statistically significant.
Results
Patients and lesions
The mean age of the 267 patients was 39.7 ± 6.3 years (range: 35–44 years), and the median body mass index (BMI) was 22 (interquartile range: 20.3–23.7). Based on the post-HIFU MRI, the signal intensity changes in the sacrum and/or the soft tissue adjacent to sacrum were observed in 122 (46%) patients, 145 (54%) patients did not have any signal intensity changes in the sacrum or the soft tissue adjacent to the sacrum. The abnormal signal intensity changes in the sacrococcygeal bone occurred in the range of sacral 2 to sacral 4 in 77% of the 87 patients and occurred in the vertebral body of lumber 5, sacral 1, or sacral 5 in 3, 13, or 7% of the patients. The signal changed area was sheet-like, often seen as hyperintensity or hypointensity on T2WI, with poor or no enhancement on contrast enhanced MRI (). A significant difference was observed in the distance from the ventral side of the fibroid to the abdominal wall, the distance from the dorsal side of the fibroid to the sacrum, uterine position, fibroid location, and fibroid type between the two groups (p < .05). No significant difference was observed in the other variables between the two groups ().
Figure 2. Transverse view of T2WI and contrast enhanced MR images obtained from a 47-year-old patient with uterine fibroids before and 1 day after HIFU. (A) Pre-HIFU T2WI image showed no abnormal signal intensity in the soft tissue adjacent to the fibroid (arrow); (B) Post-HIFU T2WI image showed a hyperintense area in the right piriformis (arrow); (C) Pre-HIFU contrast-enhanced image showed normal enhancement in the in the right piriformis (arrow); (D) Post-HIFU contrast-enhanced image showed a decreased perfusion in the area corresponds to the hyperintense area in the right piriformis on post-HIFU T2WI compared with pre-HIFU contrast-enhanced image (arrow).
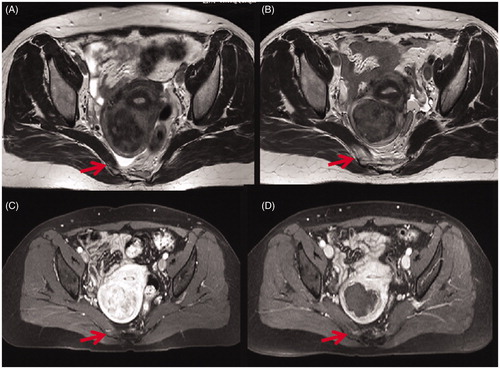
Figure 3. Transverse section through 2nd sacral vertebrae of MR images obtained from a 43-year-old patient with uterine fibroids before and 1 day after HIFU. (A) Pre-HIFU T2WI showed normal signal intensity in the sacrum and the soft tissue adjacent to the sacrum; (B) Post-HIFU T2WI showed a sheet-like hyperintense area in the sacrum and the soft tissue anterior to the sacrum (arrows); (C) Pre-HIFU contrast-enhanced image showed normal perfusion in the sacrum and the soft tissue adjacent to the sacrum; (D) Post-HIFU contrast-enhanced image showed no perfusion in the area corresponds to the signal changed area in the sacrum and the soft tissue adjacent to the sacrum on post-HIFU T2WI (arrows).
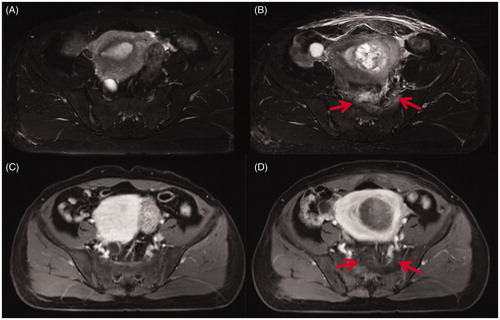
Table 1. Baseline characteristics of patients with or without MR signal changes in the sacrum and/or soft tissue adjacent to the sacrum.
Comparison of HIFU treatment parameters between the two groups
As shown in , a significant difference was observed in treatment intensity, ultrasound dosage, treatment efficiency (treated volume per second), and EEF between the patients with MR signal intensity changes and the patients without the signal changes in the sacrum and the soft tissue adjacent to the sacrum (p < .01). No significant difference was observed in NPV ratio between the two groups (p > .05).
Table 2. Comparison of HIFU treatment parameters between patients with and without MR signal changes in the sacrum and soft tissue adjacent to the sacrum.
Multivariate analysis results
The unconditional binary logistic regression analysis was used to further analyze the variables that were significantly different between the two groups. The results showed that a retroverted uterus, the distance from the dorsal side of the fibroid to the sacrum, and treatment efficiency are the independent risk factors that are related to MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum. Compared to the patients with a anteroverted uterus, HIFU treatment for uterine fibroids in patients with a retroverted uterus has a higher risk to cause injury to the sacrum and the soft tissue adjacent to the sacrum (OR = 2.678, p=.018). The distance from the dorsal side of the fibroid to the sacrum has a negative relationship with MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum. The shorter the distance from the dorsal side of the fibroid to the sacrum is, the more likely the injury to the sacrum and the soft tissue adjacent to the sacrum occur (p < .01). If the ablated volume in 1 s is higher, it is less likely to cause the MR signal intensity changes in both the sacrum and the soft tissue adjacent to the sacrum ().
Table 3. Binary logistic regression analysis to evaluate related factors to the MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum.
Comparison of the fibroids and baseline characteristics of patients with MR signal intensity changes in the sacrum and/or the soft tissue adjacent to the sacrum
According to the MR signal intensity changes in the sacrum and/or in the soft tissue adjacent to the sacrum, we further classified these patients as 3 subgroups: signal intensity changes in the sacrum (21 cases) (), signal intensity changes in the soft tissue adjacent to the sacrum (35 cases) (), and signal changes in both the sacrum and the soft tissue adjacent to the sacrum (66 cases) (). As shown in , a significant difference was observed in the uterine volume and the degree of enhancement of the fibroids among the three groups (p < .05). No significant difference was observed in uterine position, types of uterine fibroids, location of fibroids, and signal intensity on T2WI among the three groups ().
Table 4. Comparison of the fibroids and baseline characteristics of patients with MR signal intensity changes in the sacrum and/or the soft tissue adjacent to the sacrum after HIFU.
Comparison of HIFU ablation results in patients with the signal intensity changes in the sacrum and/or the soft tissue adjacent to the sacrum
As shown in , the treatment intensity (defined as sonication time per hour) was significantly higher in the group of patients with MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum than the other two groups. The treatment intensity was significantly lower in the group of patients with MR signal intensity changes only in the soft tissue adjacent to the sacrum (p < .05). No significant difference was observed in therapeutic dosage, treatment efficiency, EEF, and NPV ratio among the three groups.
Table 5. Comparison of HIFU treatment results of patients with MR signal changes in the sacrum and/or the soft tissue adjacent to the sacrum.
Analysis of the related factors to MR signal intensity changes in the sacrum or the soft tissue adjacent to the sacrum
A multivariate logistic regression analysis was used to evaluate the correlation between the factors and the MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum. In this regression model, uterine volume and treatment intensity were used as dummy variables. The results showed that when the uterine volume was greater than 719.9 cm3, compared to the group of patients with signal intensity change in the sacrum, the possibility of the signal changes in both the sacrum and the soft tissue adjacent to the sacrum was less (OR = 0.127, p=.025 < .05). The results also showed that the degree of enhancement of the fibroids is related to the signal intensity changes after HIFU. In comparison with the fibroids with moderate enhancement, the fibroids with hypoenhancement on MRI were less likely to have the MR signal changes in both the sacrum and the soft tissue adjacent to the sacrum (p < .05). No significant difference was observed between the moderate enhanced fibroids and significant enhanced fibroids ().
Table 6. Multivariate logistic regression analysis results of the factors affecting MR signal intensity changes in the sacrum and/or the soft tissue adjacent to the sacrum.
Relationship between the MR signal intensity changes in the sacrococcygeal region and the leg numbness/pain, as well as the sacral pain
In this study, the T2WI along with the post contrast images were both used to evaluate the signal changes in the sacrum, soft tissue or both. Among the 122 patients with MR signal intensity changes in the sacrum and/or the soft tissue adjacent to the sacrum, 25 cases complained of sacral pain and 3 cases reported leg pain. The incidence of sacral pain was 19% (4 cases) in the group of patients with MR signal intensity changes in the sacrum, 21.2% (14 cases) in the group of patients with MR signal intensity changes in both the sacrum and the soft tissue adjacent to the sacrum, and 20% (7 cases) in the group of patients with MR signal intensity changes in the soft tissue adjacent to the sacrum. However, leg pain was only seen in the group of patients with MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum.
As shown in , the signal intensity change volume in the soft tissue on T2WI in patients with sacral pain was significantly larger than that in patients without sacral pain. No significant difference in signal intensity change volume in the sacrum on T2WI, and no significant difference in non-perfused volume between the two groups was observed. The sample size in the group of patients with leg pain was too small to perform statistical analysis. It seems that the signal intensity change volume in patients with leg pain is similar to those without leg pain, but the location of the signal intensity change is related to leg pain ().
Table 7. Comparison of the signal intensity change volume between patients with sacral pain and those without sacral pain.
Discussion
The previous studies have demonstrated that HIFU is safe in the treatment of uterine fibroids [Citation8,Citation11,Citation12]. Although the ultrasound has good penetrability in the tissues, the phenomenon of reflection and refraction occurs when the ultrasound beams pass through the interface between different tissues [Citation13], which may cause energy to accumulate in a local tissue outside the target area, causing damage to the tissue, thus the adverse effects occurs. Recently, Fan et al. reported that MR signal intensity changes in the sacrum were observed in 37.6% of the patients with uterine fibroids after HIFU treatment [Citation14]. This study showed a percentage of 32.6% (87/267) the MR signal intensity changes in the sacrum, which was still high (). The phenomena may be explained by the high impedance interface of the bone and a large ultrasound absorption coefficient, so that the energy is deposited in a large amount in the bone region, and the rapid temperature increase then caused damage to the superficial area of the sacrum. If the increased temperature of the sacrum lasted for a longer time, then the thermal energy spread to the surrounding tissues may cause damage to the piriformis, its fascia, or the sacrocaudal ventral ligament, resulting in inflammation. Generally, the signal of hypointensity on T2WI of post-HIFU MRI is sometimes seen in the center of the sheet-like area in the sacrum, indicating necrosis (without enhancement on contrast MRI), while hyperintensity on T2WI indicates inflammation (with hypoenhancement on post-HIFU contrast MRI) ( and ). The edematous region may extend over a wide range and cause symptoms. In this study, 35 patients had only soft tissue edema adjacent to the sacrum likely due to the refraction of ultrasound at the interface resulting in a significant accumulation of ultrasound energy resulting in injury [Citation15].
Cun et al. reported that the enhancement degree of uterine fibroids, the distance between uterine fibroids and sacrum, and the type of uterine fibroids were related to MR signal intensity changes associated with the sacrococcygeal injury [Citation8]. In the present study, the significant differences in the distance from uterine fibroid to abdominal wall, the distance from uterine fibroids to the sacrum, the location of uterine fibroids, the type of fibroids and HIFU treatment results were observed between the group of patients with sacrococcygeal signal changes and those without sacrococcygeal signal changes ( and ). However, multivariate analysis showed that only the location of uterine fibroids, the distance from the dorsal side of the fibroid to the sacrum, and treatment efficiency were related to MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum (). During HIFU treatment of uterine fibroids, the distance from the dorsal side of the fibroid to the sacrum was negatively correlated with the MR signal intensity changes associated with sacral injury and edema of the soft tissue adjacent to the sacrum. Therefore, in comparing with the patients with ananteroverted uterus, the incidence of sacral injury and edema of the soft tissue adjacent to the sacrum after HIFU was significantly higher in patients with a retroverted uterus.
HIFU is a thermal ablative technique; all factors affecting the deposition of ultrasound energy in the target area will affect the ablation efficiency of HIFU treatment. In order to reduce the risk of sacrum injury and edema of the soft tissue adjacent to the sacrum, the focus of the transducer should be kept at least 30 mm away from the sacrum and the treatment efficiency should be adjusted based on the response of the patient during the procedure.
To further analyze the factors affecting the sacrococcygeal injury and the soft tissue edema, we compared the baseline characteristics and HIFU treatment results of these patients. The results showed that when the uterus volume was larger than 719.9 cm3, the patients were less likely to have MR signal intensity changes in both the sacrum and the soft tissue adjacent to the sacrum. This phenomenon may be explained by HIFU treatment for a large fibroid having a wide range for energy delivery. The ultrasonic energy deposit in the tissue per unit area in the far field was less than that of treatment for a smaller fibroid. Because of the unique bone structure of the sacrum, its absorption ability for ultrasonic energy is strong; compared to the soft tissue, the sacrum is more susceptible to thermal injury. Our study also found that HIFU treatment for the significantly enhanced fibroids have a higher risk of both sacrococcygeal injury and the soft tissue edema, compared with HIFU treatment for uterine fibroids with mild enhancement on contrast-enhanced MRI. Because the significant enhanced fibroids have rich blood flow, the heat will be taken away more easily than in the fibroids with poor blood supply, so the ultrasonic dosage of HIFU ablation for the significant fibroids is significantly higher than that for the mild enhanced fibroids. Therefore, the energy deposited in the sacrum and the soft tissue adjacent to the sacrum will also increase, and thus cause soft tissue edema and sacral injury [Citation15]. On the other hand, HIFU treatment for fibroids with poor blood supply will be easier to treat with HIFU, the energy deposition in the far field of HIFU will be limited, and the risk to cause the soft tissue edema will be reduced [Citation15]. For those who only had MR signal intensity changes in the soft tissue adjacent to the sacrum, we speculate that this phenomenon is related to the interfaces between the soft tissues adjacent to the sacrum.
In this study, we found a relatively high incidence of MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum, but only a small number of patients complained of sacral pain or leg pain after HIFU. To evaluate any relationship between the MR signal intensity changes in the pelvis and the sacral or leg pain, we further reviewed the post-HIFU MR images of the 25 cases of sacrococcygeal pain and the 3 cases of leg pain after HIFU treatment. There was no significant difference in the rate of sacral pain among the patients with signal intensity changes in the sacrum, in the soft tissue adjacent to the sacrum, or in both. However, our results showed that the signal intensity change volume in the soft tissue on T2WI in patients with sacral pain was significantly larger than that in patients without sacral pain (). Sacral pain is only related to local tissue injury or inflammation without involvement of sacral nerve. In this study, leg pain was only seen in patients with MR signal intensity changes in both the sacrum and the soft tissue adjacent to the sacrum, but we did not find any significant difference in signal intensity change volume between the patients with leg pain and those without. We carefully reviewed the MR images of the patients with leg pain and found the signal intensity change area was located in sacral 1 and sacral 2, and the soft tissue adjacent to the lumbosacral trunk (). Therefore, sacral pain is only related to local tissue injury or inflammation without the involvement of the sacral nerve, leg pain was generally caused by sacral nerve irritation after HIFU. It seems that sacral pain or leg pain is more likely related to the location of the signal changed area, not the volume. Sacral pain was relieved within 2 weeks after HIFU treatment when the edema subsided. Leg pain persisted for a longer period of time and treatment for symptoms with Celebrex (200 mg oral once a day) and physical therapy was required. The duration of leg pain was related to the area of MR signal intensity changes and pain was generally relieved 2-6 months after HIFU treatment.
Conclusions
In summary, HIFU treatment for uterine fibroids may cause MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum of the patients. The occurrence of MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum is related to uterus location, the distance from the dorsal side of the fibroids to the sacrum, and treatment intensity. In those patients who had MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum, the change in MR signal intensity area has a significant relationship with uterine volume and the degree of enhancement of fibroids. Sacral pain is seen in patients with either MR signal intensity changes in the sacrum alone, in the soft tissue adjacent to the sacrum or in both. However, leg pain only occurred in patients with MR signal intensity changes in the sacrum and the soft tissue adjacent to the sacrum. Therefore, in order to further reduce the incidence of the sacral injury and the edema of the soft tissue adjacent to the sacrum, the influencing factors must be fully considered to optimize the HIFU treatment protocol for patients with uterine fibroids. If the uterine fibroid is located on the posterior wall of the uterus with rich blood supply, and the uterine volume is smaller than 719.9 cm3, HIFU treatment for this fibroid should be very cautious.
Acknowledgement
We are grateful to Wendy Zhang from New York Medical College for helping us edit and revise this paper. Jin Bai and Lian Zhang are senior consultants to Chongqing Haifu.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–319.e20.
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2(1):16043.
- Zhang L, Zhang W, Orsi F, North American Hyperthermia Group. et al. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J hyperth. 2015;31(3):280–284.
- Zhang L, Chen WZ, Liu YJ, et al. Feasibility of magnetic resonance imaging guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies adjacent to uterus. Eur J Radiol. 2010;73(2):396–403.
- Tempany CM, Stewart EA, McDannold N, et al. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226(3):897–905.
- Chen J, Li Y, Wang Z, Committee of the Clinical Trial of HIFU versus Surgical Treatment for Fibroids, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG. 2018;125(3):354–364.
- Zhang W, He M, Huang G, et al. A comparison of ultrasound-guided high intensity focused ultrasound for the treatment of uterine fibroids in patients with an anteverted uterus and a retroverted uterus. Int J Hyperth. 2016;32(6):623–629.
- Liu Y, Zhang W, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterine diseases. Int J Hyperth. 2018;35(1):56–61.
- Peng S, Zhang L, Hu L, et al. Factors influencing the dosimetry for high-intensity focused ultrasound ablation of uterine fibroids: a retrospective study. Medicine. 2015;94(13):e650.
- Cun JP, Fan HJ, Zhao W, et al. Factors influencing MR changes associated with sacral injury after high-intensity focused ultrasound ablation of uterine fibroids. Int J Hyperth. 2019;36(1):21–28.
- Zhou M, Chen JY, Tang LD, et al. Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril. 2011;95(3):900–905.
- Zhao WP, Chen JY, Zhang L, et al. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weightedMR imaging. Eur J Radiol. 2013;82(1):e43–e49.
- Jolesz FA, Hynynen K. Magnetic resonance image-guided focused ultrasound surgery. Cancer J. 2002;8(1):S100–S112.
- Hassanuddin A, Choi JH, Seo DW, et al. Factors affecting tumor ablation during high intensity focused ultrasound treatment. Gut Liver. 2014;8(4):433–437.
- Chan O, Del Buono A, Best TM, et al. Acute muscle strain injuries:a proposed new classification system. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2356–2362.
