Abstract
Purpose
The present study retrospectively evaluated the feasibility, safety, and short-term efficacy of computed tomography (CT)-guided percutaneous microwave ablation (MWA) to treat multiple synchronous ground-glass opacities (GGOs) of the lung.
Materials and Methods
From October 2016 to May 2019, 33 patients (9 males and 24 females, mean age: 59.6 ± 10.0 years) with multiple GGOs (103 GGOs with mean size 12.3 ± 6.3 mm) were enrolled in this study. Patients underwent 66 procedures of CT-guided percutaneous MWA. The feasibility, safety, local progression-free survival, and overall survival were evaluated.
Results
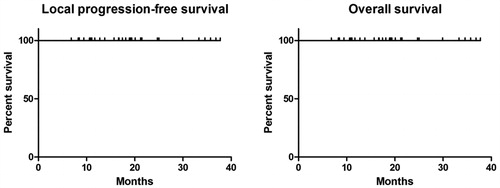
The technical success and technique efficacy rate were 100% and no MWA procedure-related deaths were reported. The median follow-up period was 18.1 (range: 6.8–37.7) months. Major complications included pneumothorax (11/66, 16.7%), pleural effusion (2/66, 3.0%), pneumonia (3/66, 4.5%), and nerve injury (1/66, 1.5%), which were well controlled by appropriate treatment. Minor complications included pneumothorax (38/66, 57.6%), pleural effusion (43/66, 65.2%), hemoptysis (13/66, 19.7%), subcutaneous emphysema (4/66, 6.1%), and hemothorax (2/66, 3.0%). Currently, all patients are alive without local progression or tumor recurrence, despite the relatively insufficient follow-up time.
Conclusion
CT-guided percutaneous MWA for the treatment of multiple synchronous lung GGOs is feasible, safe, and efficacious over short-term follow-up. It may also be employed as an alternative approach for nonsurgical candidates. A longer follow-up is warranted to evaluate the oncologic outcomes.
Introduction
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer-related mortality worldwide [Citation1]. It is typically diagnosed in the advanced stage and thus, is associated with a dismal prognosis. With the widespread use of low-dose computed tomography and high-resolution computed tomography (HRCT) for routine thoracic imaging examination, early lung cancer or precancerous lesions can be identified with increased frequency. These lesions often manifest as pulmonary ground-glass opacities (GGOs) [Citation2,Citation3]. A GGO is defined as a hazy region in the lung with increased opacity and preservation of bronchial and vascular markings on HRCT scan. It is a non-specific characteristic that may be associated with various diseases [Citation4,Citation5]. Although some cases of pneumonia, injury, edema, hemorrhage, and focal pulmonary fibrosis can also manifest as GGOs, persistent (non-transient) GGOs on serial computed tomography (CT) scans after treatment with antibiotics or steroids suggest a higher probability of malignancies, especially when increase in size or enlargement of solid components are observed inside the lesion on follow-up [Citation6].
GGOs can be radiologically classified into two categories: pure GGO (pGGOs) containing no solid component and mixed GGOs (mGGOs) containing a pGGO region as well as a consolidated region [Citation5,Citation7]. Malignant GGOs are commonly associated with lung adenocarcinoma. According to the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification, pulmonary adenocarcinomas are classified into following categories: preinvasive lesions, minimally invasive adenocarcinomas (MIAs), and invasive adenocarcinomas (IAs). Preinvasive lesions include atypical adenomatous hyperplasia (AAH) and adenocarcinoma in situ (AIS) [Citation8]. Multiple synchronous GGOs (hereinafter referred to as ‘multiple GGOs’) are defined as two or more GGOs synchronously found in the same lobe, lung, and/or bilateral lungs in a patient and are usually considered to represent multifocal origins rather than intrapulmonary metastasis [Citation9]. Currently, multiple GGOs have gained increasing interest. Despite guidelines established for the management of small pulmonary nodules including solitary GGOs, there are still major controversies regarding the diagnosis and the treatment of multiple GGOs [Citation10,Citation11].
Recently, local thermal ablation, a precise and minimally invasive technique, has been increasingly applied to treat primary or metastatic lung tumors [Citation12]. Percutaneous CT-guided microwave ablation (MWA), a local thermal ablation technique, has been increasingly performed alone or in combination with other therapies for the treatment of early and advanced primary non-small cell lung cancers [Citation13–18]. Moreover, preliminary implementation of MWA as an alternative approach for treating solitary GGOs has raised expectations regarding the management of multiple GGOs [Citation19]. However, the use of MWA for the treatment of multiple GGOs has not been reported to date. In this retrospective study, the feasibility, safety, and preliminary outcomes of MWA for treating multiple GGOs were investigated.
Material and methods
Patients and inclusion and exclusion criteria
The present retrospective study was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University. The study complied with the ethical principles of World Medical Association’s Declaration of Helsinki. Written informed consent was obtained from all patients before inclusion in the study. Definitive diagnosis and parameter determination of GGOs were made based on a thin-section HRCT scan by an experienced radiologist, following the established criteria for GGOs [Citation4]. The size of the tumor was defined as the largest axial diameter of the lesion (including the GGO area) on the lung window in HRCT. Consolidation was defined as an area of increased opacity within the GGO that completely obscured the bronchial and the vascular markings. The diameter of the consolidation area on the axial image was also measured in the lung window setting. For mGGOs, lesions with ratio of consolidation diameter to tumor diameter (CTR) of <0.5 were considered GGO-predominant tumors and lesions with CTR ≥ 0.5 were considered solid-predominant tumors [Citation20]. In this study, only pGGOs and GGO-predominant mGGOs were included. For some mGGOs, in addition to routine HRCT, positron emission tomography-CT (PET-CT) was applied to search for regional and distant metastases and to verify whether all tumors could be clinically categorized as T1 N0 M0 lung adenocarcinomas [Citation21]. Each GGO on HRCT scans performed before MWA was reviewed by two radiologists. The integral course of treatment for each patient with multiple GGOs was established by a multidisciplinary tumor board that included at least one medical oncologist, thoracic surgeon, respiratory physician, radiologist, and pathologist.
Patients who met the following criteria were included in this study: (1) Eastern Cooperative Oncology Group performance status 0–2, (2) patients aged 18 years or older and non-pregnant females, (3) persistent GGOs with highly suspicious imaging features of malignancy (no change, an increase in diameter, or presence of solid component) for at least 3 months or more on serial HRCT examinations, (4) ≥2 GGOs simultaneously found per patient, (5) GGOs present in the same lobe, ipsilateral lung, and/or bilateral lung, not abutting the large vessels or the lung hilum, (6) a CTR <0.5 for all GGOs on HRCT scans (slice thickness ≤ 1 mm) before MWA, (7) GGOs with a maximum diameter of ≤30 mm, (8) histological diagnosis of preinvasive lesions, MIA, and/or IA through percutaneous coaxial needle cutting biopsy of at least one of the most predominant (in size and solidity) lesion in each patient, and (9) unsuitability for surgery due to advanced age, poor cardiopulmonary function or other comorbidities, high anxiety, or refusal of surgery due to fear. Exclusion criteria were: (1) the presence of regional lymph node metastasis or distant metastasis verified by enhanced CT, PET-CT, and enhanced magnetic resonance imaging and (2) untreatable coagulopathies and/or platelet count <50 × 109/L. The histological diagnoses of the biopsied GGOs were either confirmed by the conventional paraffin sections in separate procedures before MWA or by frozen sections and post procedural paraffin sections in the same procedures.
Instruments and MWA procedure
CT (Lightspeed 64 V, GE Healthcare, Chicago, IL, USA) was used to guide MWA. MWA was performed with MTC-3C microwave ablation system (Vison-China Medical Devices R&D Center, Nanjing, Jiangsu, China), ECO-100A1 microwave ablation system (ECO Medical Instrument Co., Ltd., Nanjing, Jiangsu, China), or KY-2450B microwave ablation system (Canyon Medical Inc., Nanjing, Jiangsu, China), with a frequency of 2450 ± 50 MHz and adjustable continuous wave output power ranging from 0 to 100 W. For the microwave antenna, the effective length was 100–180 mm and the outside diameter was 14–20 G, with a 1.5-cm radiating tip (tapered end). Cooling of the surface temperature of the antennas was carried out with a water circulation cooling system.
Based on tumor location, size, and adjacent structures, a treatment plan was designed via immediate pre-procedural CT. The appropriate body placement, puncture site on the body surface, optimal puncture trajectory, and number of antennas were confirmed. All percutaneous MWA procedures were performed using sterile technique and under local anesthesia with the patients under moderate sedation. After achieving satisfactory anesthesia, the antenna was positioned into the initial planned site. Once proper positioning of the antenna was ascertained via CT, MWA was performed at predetermined power and duration. When the range of the ablation zone, as monitored in real time using CT, was 5–10 mm beyond the lesion boundary, the ablation procedure was terminated, followed by immediate withdrawal of the antennas from the lesion. Subsequently, the puncture wound was bandaged after disinfection. At the end of the procedure, a repeat whole-lung CT scan was performed to assess the technical success and the immediate complications. When the tumor was treated according to the protocol and the ablation zone completely overlapped or encompassed the target tumor including an ablative margin, the procedure was defined as a technical success [Citation22]. Heart rate, respiratory rate, oxygen saturation, electrocardiographic tracing, and blood pressure were continuously monitored throughout the MWA session and for an additional 6 h after the patients’ safe return to the ward.
Follow-up and outcome assessment
A non-contrast chest CT scan was performed for all patients 24–48 h after MWA to assess the potential complications and the technique effectiveness. Technique effectiveness referred to a prospectively defined time-point at which complete macroscopic ablation of the tumor, as evidenced by imaging follow-up, was achieved [Citation22]. During the periprocedural period, non-contrast or contrast-enhanced CT was performed when required, as determined by patients’ condition such as aggravation. Subsequently, contrast-enhanced chest CT was performed monthly for the first 3 months after MWA and later, every 3 months for the first year. Thereafter, the follow-up intervals were extended to 6 months.
Considering the lesion at 4–6 weeks after MWA as baseline, the local ablation effect was assessed by studying the signs and the dynamic changes in the lesion on a series of repeated contrast-enhanced CT scans. If available, PET-CT examination at 3 months after ablation was feasible for assessment of local response and distant metastasis. According to expert consensus for thermal ablation of lung tumors (edited in 2018), the local effect of the ablative response included complete ablation, incomplete ablation, and local progression [Citation12]. The tumors that were incompletely ablated or locally progressive and met the inclusion criteria were treated again with MWA. Local progression-free survival (LPFS) was defined as the interval between the initial ablation and the first radiologic evidence of local progression. Tumor recurrence other than local tumor progression was defined as distant metastasis. LPFS, tumor recurrence, and overall survival (OS) over 1 or 2 years were assessed using the results of the follow-up.
Side effects and complication assessment
Complications were assessed based on the number of ablation procedures. While considering the severity of injuries to patients according to the American Society of Interventional Radiology (SIR) criteria classification, complications were classified as ‘major’ and ‘minor’ [Citation22,Citation23]. Major complications were defined as events that led to substantial morbidity and disability such as unexpected loss of an organ, increase in the level of care, hospital admission, or substantially lengthened hospital stay (SIR classifications C–E). This category also included any cases in which blood transfusion or interventional drainage procedure was required. Any death within 30 days after image-guided tumor ablation had to be addressed (SIR classification F). All other events were considered minor complications (SIR classifications A–B).
Statistical analysis
Technical success, technique effectiveness rate, LPFS, and tumor recurrence rate were evaluated based on the tumor characteristics. Survival was calculated from the time of lung MWA. Continuous numerical variables were presented as a mean ± standard deviation and range. LPFS and OS curves were constructed with the Kaplan–Meier method. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS 17.0 Software package (SPSS, Chicago, IL, USA).
Results
Feasibility and clinical outcomes
Between October 2016 and May 2019, 33 consecutive patients (9 males and 24 females with mean age of 59.6 ± 10.0 years, range: 37–78 years) with multiple GGOs (103 GGOs including 84 pGGOs and 19 mGGOs, mean size: 12.3 ± 6.3 mm, range: 4–30 mm) underwent percutaneous CT-guided MWA in the present study. Pathological types of 51 GGOs were confirmed by coaxial cutting needle biopsy and included 5 cases of AAH, 18 cases of AIS, 21 cases of MIA, and 7 cases of IA. Detailed clinical features of patients and GGO characteristics are listed in . A PET-CT scan was performed in 12 patients before MWA. Slight accumulation of 18 F-fluorodeoxyglucose was observed only in the GGOs of 5 cases. The precise location of each GGO and the distribution pattern of multiple GGOs in each patient are also indicated specifically in . Multiple GGOs, varying in number from 2 to 8 across patients, were treated with MWA in a single or multiple (2–4) procedures (). Punctures were made, microwave antennas were placed in the appropriate positions, and ablation was completed in compliance with the planned protocol, achieving a 100% technical success rate in 66 procedures. Additionally, 9 GGOs (>2.0 cm) that were adjacent to blood vessels or those that could not be punctured along the largest diameter were ablated with double antennas to reduce heat-sinking effect, to avoid excessive ablation, and to ensure a sufficient ablative boundary. Comparison of CT images before and after therapy revealed that all 103 GGO sites were fully covered by the ablative zones 24–48 h after the initial MWA and demonstrated a 100% technique effectiveness rate. In this study, 25 patients underwent 2–4 separate MWA procedures with a median interval of 42 days (range 5–658 days) between 2 adjacent ablation procedures. The remaining 8 patients underwent a single MWA procedure ().
Table 1. Clinical features of 33 patients with multiple ground-glass opacities (GGOs) and GGO characteristics.
Table 2. Parameters of microwave ablation and the course of treatment in 66 procedures.
As of 30 November 2019, no patients were lost to follow-up and the median period of follow-up using contrast-enhanced CT after MWA was 18.1 months (range: 6.8–37.7 months) ( and ). During the follow-up, there was no local tumor progression, tumor recurrence, or death among the 33 patients. To date, the 103 ablated GGOs (66 MWA procedures) were well controlled in all patients. Due to insufficient follow-up time, the definite 1-year, 2-year, and long-term LPFS and OS rates could not be obtained from statistical analysis of the existing data (). Currently, all patients are under clinical observation and have not received further anti-tumor treatments such as stereotactic radiation therapy, chemotherapy, targeted therapy, and immunotherapy.
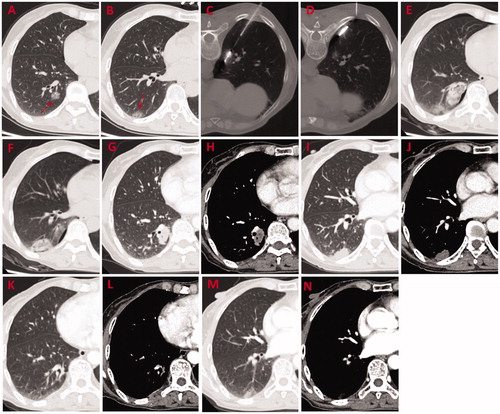
Figure 1. A 69-year-old female patient had three synchronous GGOs in three lobes of the bilateral lungs. She underwent two separate MWA procedures with an interval of 1 year. (A, B) GGO lesions (arrow) of 22 mm × 16 mm and 12 mm × 10 mm in the apicoposterior segment of the left upper lobe and in the superior segment of the left lower lobe, respectively were observed on the axial HRCT before MWA. (C) One of the GGOs in the left lung was biopsied and pathologically diagnosed as MIA before MWA. (D, E) MWA was performed on two GGOs in the left lung with an ablation power/time of 60 W/9 min and 60 W/5 min, respectively. (F, G) Reactive fibrous scars with a clear boundary formed at 4 months after MWA (H, I) Further shrinkage of the fibrous scars was visible at 12 months after MWA. (J, K) Only fibrotic streaks were visible at 30 months after MWA. (L) A GGO lesion (arrow) of 14 mm × 11 mm in the posterior basal segment of the right lower lobe was observed on HRCT and pathologically diagnosed as AIS. (M) MWA was performed on the GGO with an ablation power/time of 50 W/7.5 min. (N) Ablated zone of 38 mm × 26 mm was seen at 24 h after MWA. (O) A fibrotic streak formed in the right lower lobe at 18 months after MWA. GGOs: ground glass opacities; MWA: microwave ablation; HRCT: high-resolution computed tomography; MIA: microinvasive adenocarcinoma; AIS: adenocarcinoma in situ.
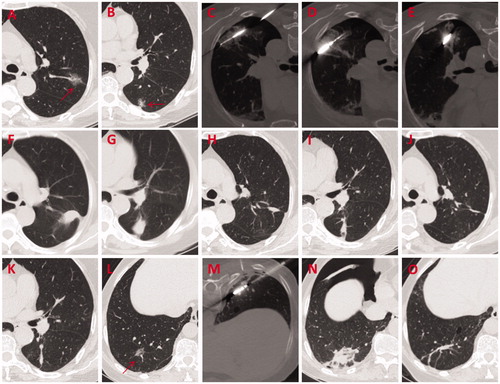
Figure 2. A 61-year-old female patient with two synchronous GGOs in the same lobe underwent MWA procedure for both lesions simultaneously. (A, B) GGO lesions (arrow) of 15 mm × 15 mm and 15 mm × 12 mm located in the posterior basal segment of the right lower lobe were observed on axial HRCT before MWA. (C, D) MWA was performed on two GGOs with an ablation power/time of 40 W/7 min and 65 W/5 min, respectively. (E, F) Ablated zones of 40 mm × 25 mm and 40 mm × 35 mm, respectively were seen at 24 h after MWA. (G–J) Shrinking ablated zones with a denser texture and a clear boundary were observed at 3 months after MWA. (K–N) Non-enhancing coarse fibrotic streaks were visible at 24 months after MWA. GGOs: ground-glass opacities; MWA: microwave ablation; HRCT: high-resolution computed tomography.
Side effects and complications
No MWA-related deaths occurred during the procedure or within 30 days after MWA. Side effects mainly included pain, postablation syndrome, cough, and pleural response (). Among the 66 MWA procedures, 21 cases (31.8%) experienced intraprocedural or postprocedural pain including 16 cases of mild pain that did not require intervention and 5 cases of severe pain that required active treatment. Postablation syndrome, which consists of low-grade fever (<38.5 °C), nausea, vomiting, and general malaise, occurred in 15 cases (22.7%). Cough was observed in 13 cases (19.7%) including 9 with mild cough and 4 with severe cough. Pleural response was mainly characterized by a slow heart rate or even sudden cardiac arrest, which occurred in 2 cases (3.0%).
Table 3. Side effects and complications during and after 66 microwave ablation procedures.
Complications are summarized in . Major complications included massive pneumothorax and a large amount of pleural effusion, requiring chest tube placement for drainage. In addition, pulmonary infection, lengthening of the hospital stay, and nerve injury leading to limb dyskinesia were also classified as major complications. Minor complications included mild pneumothorax, pleural effusion, hemothorax, pulmonary hemorrhage, and subcutaneous emphysema. These complications were asymptomatic or self-limiting and did not require invasive intervention. Among the 11 cases with pneumothorax that required chest tube placement for drainage, 4 cases underwent intraprocedural intubation, 6 cases underwent intubation 24 h after MWA, and 1 case underwent intubation 4 days after MWA. Pneumothorax resolved satisfactorily with 3 cases requiring an indwelling tube for 2 days, 4 cases for 3 days, 2 cases for 4 days, and 2 cases for 6 days. Pleural effusion was observed in 45 procedures (45/66, 68.2%), of which only 2 cases (2/66, 3.0%) received intubation. Hemoptysis was observed in 13 cases (13/66, 19.7%) and was effectively alleviated by application of conventional hemostatic drugs. Slight subcutaneous emphysema occurred in 4 cases (6.1%, 4/66) with spontaneous recovery. Three procedural cases (3/66, 4.5%) suffered from pneumonia after the treatment and recovered after administration of effective antibiotics. Slight, asymptomatic hemothorax occurred in 2 cases (2/66, 3.0%). It was absorbed spontaneously without special treatment. A case of sensory dyskinesia of the upper extremity occurred after ablation of a GGO in the apex pulmonis, denoting damage to the brachial plexus (1/66, 1.5%).
Discussion
Multiple GGOs are an increasingly frequent finding. Approximately 20–30% of the resected GGOs were found to be accompanied by multiple other smaller intrapulmonary GGOs [Citation11,Citation24]. Moreover, multiple GGOs were found to present a variety of pathologies including AAH, AIS, MIA, and even IA. They also presented different combined mutation patterns of epidermal growth factor receptor and K-ras, suggesting that each GGO seemed to arise as an independent event [Citation25–27]. Currently, surgical management of multiple GGOs detected by screening is even less well defined and no standard algorithms have been established for the selection of lesions (if any) to be treated [Citation24]. This may be due to a number of factors such as (1) difficulty in accurate localization and manual palpation when the lesions are deeper and smaller, (2) infeasibility of acquiring pathological specimens of each GGO before surgical intervention, (3) extraordinary complexity of optimal combinations of various surgical approaches (wedge resection, segmentectomy, or lobectomy) for treating lesions scattered in multiple lobes of the ipsilateral or the contralateral lung, and (4) intolerance to single-stage or multi-stage surgery due to advanced age and/or poor cardiopulmonary function in patients. In view of the aforementioned unfavorable factors for surgery, image-guided percutaneous thermoablation procedures such as radiofrequency ablation (RFA) and MWA are alternative treatments that have the advantages of fewer complications, minimal invasion, good tolerability, higher repeatability, pathological independence, and quicker recovery [Citation12].
Currently, there is no consensus regarding whether biopsies must be performed for all GGOs to be ablated. A CT-histopathological comparison study including 53 pGGOs in 49 patients who underwent surgical resection showed that about 81% (43/53) of the persistent pGGOs were attributed to AAH (n = 3), bronchoalveolar carcinoma (BAC) (n = 36), or adenocarcinoma with a predominant BAC component (n = 4) [Citation6]. With wide application of CT-guided percutaneous coaxial needle cutting biopsy (CTNB), which is a less invasive method than surgical resection, a higher positive rate of GGOs diagnosed as malignant is achieved [Citation28]. A recent study on diagnostic ability of CTNB for GGOs involving 74 patients with solitary pGGOs (n = 55) or GGO-dominant lesions (n = 19) revealed that the positive diagnosis rate of preinvasive lesions (AAH and AIS), MIA, and IA was up to 90.5% after use of a combination of pre-MWA and immediate post-MWA biopsies [Citation29]. Such a high incidence of malignancy in persistent GGOs suggests that some biopsies of GGOs are reasonably bypassed and ablations are performed directly in high-risk cases such as smaller and deeper lesions or lesions adjacent to vessels. In the present study, pGGOs (n = 84) accounted for the majority (81.6%) of the ablated lesions (n = 103), while mGGOs (n = 19) with a CTR <0.5 accounted for 18.4% of the lesions. Considering the interference posed by intrapulmonary hemorrhage obscuring the lesions after biopsy in accurate placement of microwave antennas in MWA, only 51 GGOs (49.5%) received CTNB in a separate procedure or in the same procedure before MWA in the present study.
To date, there have been only a few clinical studies on the outcome and the safety of percutaneous CT-guided thermoablation for the treatment of GGOs. Kodama et al. [Citation30] reported that 33 patients with 42 lung tumors having ≥50% GGO component received lung RFA with a mean follow-up of 42 months. All cases except one death due to brain hemorrhage were alive and OS and cancer-specific survival rates were 100% and 100%, respectively at 1 year, 96.4% and 100%, respectively at 3 years, and 96.4% and 100%, respectively at 5 years. Iguchi et al. [Citation31] reported that 16 patients with 17 lung cancer lesions with GGOs received percutaneous CT-guided RFA. All cases except one death due to recurrent cancer remained alive and OS and disease-specific survival rates were 93.3% and 100%, respectively at 1 year and 93.35% and 100%, respectively at 5 years. In another study by Yang et al. [Citation19] involving MWA treatment for GGOs, 51 patients with 51 lung adenocarcinomas showing GGOs were enrolled. The 3-year LPFS, cancer-specific survival, and OS rates were 98%, 100%, and 96%, respectively. These results suggested that thermoablation including RFA and MWA in the treatment of malignant GGOs, especially in treatment of solitary lesions, is safe and effective with noteworthy survival benefits. However, special clinical studies on the efficacy and the safety of MWA for treating multiple GGOs are scarce.
Moreover, there has been no report comparing the efficacy and the safety of RFA and MWA for the treatment of GGOs. The alveolar lumen in GGO-dominant lesions is moderately filled with cells and fluid and contains a significant amount of air [Citation32]. The higher impedance and lower electrical conductivity of the air-filled GGOs when compared with solid nodules may hinder heat dispersion in RFA and reduce local control of the GGOs. When compared with RFA, microwaves propagate effectively through air-filled lungs and offer the advantages of faster heat production, less sensitivity to tissue type, and synergistic action of multiple antennas, ensuring a higher local control rate [Citation33,Citation34]. Only 1 out of 51 lesions (2%) was observed to progress locally during follow-up in a study of MWA treatment for GGOs [Citation19]. No lesion progressed locally (0/103) in the present study, which was significantly less than the results of 2 studies involving RFA, in which 6 out of 42 (14.3%) and 3 out of 17 (17.6%) lesions, respectively progressed locally [Citation31,Citation32].
In the present study, pain (31.8%), postablation syndrome (22.7%), and cough (19.7%) were common side effects of MWA. This finding is consistent with our previous report [Citation19]. Severe pain could be alleviated by good local anesthesia of the intercostal nerve adjacent to the lesions as well as by appropriate analgesics and sedatives. Postablation syndrome could be relieved by short-term administration of nonsteroidal drugs and glucocorticoids, if necessary. Cough could be relieved by oral administration of codeine before the procedure [Citation12,Citation19]. Pneumothorax is one of the most commonly reported complications of MWA, with an incidence of 10%–67% in previous reports [Citation12,Citation19,Citation34]. The incidence of pneumothorax in the present study was 74.2%, which was slightly higher than that in previous reports. It was probably attributed to multiple punctures of pleura or combined biopsy. However, chest tube placement was required in only 16.7% of the pneumothorax cases, a finding similar to previously reported rates of 9.8%–31.9% [Citation19,Citation34,Citation35]. Pleural effusion has been reported to occur in 1%–60% of the cases [Citation12,Citation34]. Despite a slightly higher incidence of 68.2% in present study, only 3.0% of the cases required chest tube placement for drainage, a finding similar to the one reported in our previous report [Citation35]. Pneumonia (4.5%) could be effectively cured by appropriate antibiotics as previously reported [Citation19,Citation35]. There was no reported case of life-threatening invasive aspergillosis [Citation36,Citation37]. Nerve injury of brachial plexus (1.5%) has been rarely reported and the symptoms partially receded over time [Citation38]. Other minor complications including hemoptysis (19.7%), subcutaneous emphysema (6.1%), and hemothorax (3.0%), which were self-limiting or asymptomatic, could be well controlled by appropriate treatment.
To date, all patients enrolled in the present study have survived without local progression or tumor recurrence including mediastinal lymph node and distant metastasis. This result is attributed to a more indolent biological nature of multiple GGOs when compared with solitary GGOs. It has been confirmed that AAH and BAC were more frequent in multiple GGOs, while adenocarcinoma was more frequent in solitary GGOs [Citation25]. Since lymphadenectomy is inessential for 3-cm or smaller GGO-predominant lesions [Citation39], good local tumor control is explicitly crucial for favorable outcomes. Due to a similar ground-glass attenuation, the GGO margin is hardly differentiated from the ablation zone margin [Citation30]. Despite the high local control rate in this study, careful long-term follow-up of ablated tumors is necessary.
The present study has some limitations. It was a retrospective single-institution study that comprised of a small number of patients. We did not perform lung biopsy for all lesions and the follow-up duration after addressing multiple GGO lesions was relatively short. This study was not designed to compare the outcomes of MWA with those of other treatments such as surgery, stereotactic body radiation therapy, or RFA. Therefore, a prospective, multicenter, randomized, controlled study is required in future to clarify the safety and the effectiveness of MWA treatment for multiple GGOs.
In conclusion, results of the present study showed that CT-guided percutaneous MWA is a feasible, safe, and effective treatment for multiple synchronous GGOs with CTR <0.5 (GGO-predominant). It can be considered an alternative approach for nonsurgical candidates. Nevertheless, longer follow-up is warranted to evaluate the oncologic outcomes after this procedure.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
- Silva M, Pastorino U, Sverzellati N. Lung cancer screening with low-dose CT in Europe: strength and weakness of diverse independent screening trials. Clin Radiol. 2017;72:389–400.
- Lee HY, Lee KS. Ground-glass opacity nodules: histopathology, imaging evaluation, and clinical implications. J Thorac Imaging. 2011;26:106–118.
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722.
- Lococo F, Cusumano G, De Filippis AF, et al. Current practices in the management of pulmonary ground-glass opacities: a survey of SICT Members. Ann Thorac Surg. 2018;106:1504–1511.
- Kim HY, Shim YM, Lee KS, et al. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology. 2007;245:267–275.
- Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27:391–408.
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285.
- Migliore M, Fornito M, Palazzolo M, et al. Ground glass opacities management in the lung cancer screening era. Ann Transl Med. 2018;6:90–90.
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the fleischner society 2017. Radiology. 2017;284:228–243.
- Chen D, Dai C, Kadeer X, et al. New horizons in surgical treatment of ground-glass nodules of the lung: experience and controversies. TCRM. 2018;14:203–211.
- Ye X, Fan W, Wang H, et al. Expert consensus workshop report: guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition). J Can Res Ther. 2018;14:730–744.
- Yang X, Ye X, Zheng A, et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol. 2014;110:758–763.
- Tafti BA, Genshaft S, Suh R, et al. Lung ablation: indications and techniques. Semin Intervent Radiol. 2019;36:163–175.
- Liu J, Huang W, Wu Z, et al. The application of computed tomography-guided percutaneous coaxial biopsy combined with microwave ablation for pulmonary tumors. J Can Res Ther. 2019;15:760–765.
- Wei Z, Wang Q, Ye X, et al. Microwave ablation followed by immediate biopsy in the treatment of non-small cell lung cancer. Int J Hyperthermia. 2018;35:262–268.
- Solbiati LA. A valuable guideline for thermal ablation of primary and metastatic lung tumors. J Can Res Ther. 2018;14:725–726.
- Hertzanu Y, Ye X. Computed tomography-guided percutaneous microwave ablation: a new weapon to treat ground-glass opacity-lung adenocarcinoma. J Cancer Res Ther. 2019;15:265–266.
- Yang X, Ye X, Lin Z, et al. Computed tomography-guided percutaneous microwave ablation for treatment of peripheral ground-glass opacity-Lung adenocarcinoma: a pilot study. J Can Res Ther. 2018;14:764–771.
- Matsuguma H, Yokoi K, Anraku M, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: a predictor of lymph node metastasis. J Thorac Cardiovasc Surg. 2002;124:278–284.
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2016;11:1204–1223.
- Ahmed M, Solbiati L, Brace CL, et al.; For the International Working Group on Image-guided Tumor Ablation, Interventional Oncology Sans Frontières Expert Panel, Technology Assessment Committee of the Society of Interventional Radiology, and the Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273:241–260.
- Cardella JF, Kundu S, Miller DL, et al.; Society of Interventional Radiology. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189–S191.
- Sihoe ADL, Petersen RH, Cardillo G. Multiple pulmonary ground glass opacities: is it time for new guidelines? J Thorac Dis. 2018;10:5970–5973.
- Kim TJ, Goo JM, Lee KW, et al. Clinical, pathological and thin-section CT features of persistent multiple ground-glass opacity nodules: comparison with solitary ground-glass opacity nodule. Lung Cancer. 2009;64:171–178.
- Takamochi K, Oh S, Matsuoka J, et al. Clonality status of multifocal lung adenocarcinomas based on the mutation patterns of EGFR and K-ras. Lung Cancer. 2012;75:313–320.
- Liu M, He WX, Song N, et al. Discrepancy of epidermal growth factor receptor mutation in lung adenocarcinoma presenting as multiple ground-glass opacities. Eur J Cardiothorac Surg. 2016;50:909–913.
- Yu H, Zhang C, Liu S, et al. Application value of coaxial biopsy system in needle cutting biopsy for focal ground glass-like density nodule. J Can Res Ther. 2018;14:1509–1514.
- Wang J, Ni Y, Yang X, et al. Diagnostic ability of percutaneous core biopsy immediately after microwave ablation for lung ground-glass opacity. J Can Res Ther. 2019;15:755–759.
- Kodama H, Yamakado K, Hasegawa T, et al. Radiofrequency ablation for ground-glass opacity-dominant lung adenocarcinoma. J Vasc Interv Radiol. 2014;25:333–339.
- Iguchi T, Hiraki T, Gobara H, et al. Percutaneous radiofrequency ablation of lung cancer presenting as ground-glass opacity. Cardiovasc Intervent Radiol. 2015;38:409–415.
- Detterbeck FC, Homer RJ. Approach to the ground-glass nodule. Clin Chest Med. 2011;32:799–810.
- Chen CK, Chou HP, Sheu MH. Image-guided lung tumor ablation: principle, technique, and current status. J Chin Med Assoc. 2013;76:303–311.
- Welch BT, Brinjikji W, Schmit GD, et al. A national analysis of the complications, cost, and mortality of percutaneous lung ablation. J Vasc Interv Radiol. 2015;26:787–791.
- Zheng A, Wang X, Yang X, et al. Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg. 2014;98:243–248.
- Huang G, Ye X, Yang X, et al. Invasive pulmonary aspergillosis secondary to microwave ablation: a multicenter retrospective study. Int J Hyperthermia. 2018;35:71–78.
- Huang G, Liu Q, Ye X, et al. Invasive pulmonary aspergillosis: a rare complication after microwave ablation. Int J Hyperthermia. 2014;30:412–417.
- Hiraki T, Gobara H, Mimura H, et al. Brachial nerve injury caused by percutaneous radiofrequency ablation of apical lung cancer: a report of four cases. J Vasc Interv Radiol. 2010;21:1129–1133.
- Moon Y, Sung SW, Namkoong M, et al. The effectiveness of mediastinal lymph node evaluation in a patient with ground glass opacity tumor. J Thorac Dis. 2016;8:2617–2625.