Abstract
Objective
To investigate the application value of acoustic radiation force impulse imaging in preoperative prediction for efficacy of High-Intensity Focused Ultrasound uterine fibroids ablation
Methods
A prospective study was conducted on 32 women (41 fibroids) undergoing HIFU uterine fibroids ablation between January 2019 and September 2019. The virtual touch tissue quantification (VTQ) technique was used for the preoperative determination of uterine fibroids shear wave velocity (SWV). The stiffness of the preoperative uterine fibroids was graded on a virtual touch tissue image (VTI). All uterine fibroids were ablated with a single point ablation acoustic power of 400 W. All patients underwent pelvic cavity MRI examination for the measurement of the size, volume and non-perfused volume (NPV) of fibroids within the first month after HIFU ablation. The ablation rate of uterine fibroids was calculated according to the formula: ablation rate = NPV × 100/target fibroid volume. The patients were divided into two groups based on the postoperative ablation rate: ≥70% ablation rate group, and<70% ablation rate group. The preoperative SWV and VTI grade of uterine fibroids were compared between the two groups. The correlation of preoperative uterine fibroids’ SWV and VTI grade with HIFU ablation rate were analyzed using the Spearman’s correlation coefficient. The optimal cutoff points in preoperative uterine fibroids SWV of 70% ablation rate were determined by receiver operating curve (ROC) analysis.
Results
A total of 30 patients (73.17%, 30/41) showed ablation rate ≥70%, with preoperative uterine fibroids’ SWV values of (3.42 ± 0.71) m/s. Of these, 24 patients (80%, 24/30) had VTI grades II–III. On the other hand, 26.83% (11/41) showed ablation rate <70%, with preoperative uterine fibroids’ SWV values of (4.02 ± 0.69) m/s; of these, 63.6% (7/11) had VTI grade IV. The SWV values and VTI grades of preoperative uterine fibroids were significantly different in the two groups (p < 0.05). Interestingly, postoperative ablation rate was negatively correlated with preoperative uterine fibroids’ SWV values (r= −0.536, p = 0.0003) and VTI grades (r= −0.511, p = 0.001). The area under the ROC curve of preoperative uterine fibroids’ SWV values with ablation rate <70% was 0.75 at a cutoff value of 3.915 m/s (p < 0.05). Specificity was 72.7% and sensitivity was 80.1%; the positive predictive value was 72.7%, and the negative predictive value was 80%.
Conclusion
ARFI technique is an effective and feasible noninvasive ultrasound technique for the preoperative prediction of the efficacy of HIFU uterine fibroids ablation.
1. Introduction
Uterine fibroids are prevalent benign uterine tumors with an estimated incidence of 20 − 40% in women during their reproductive years [Citation1]. While most of the uterine fibroids are asymptomatic, some of them can cause a multitude of symptoms such as abnormal uterine bleeding, anemia, pelvic pressure, urinary frequency, urinary incontinence, constipation, and tenesmus, in addition to infertility and miscarriage [Citation2,Citation3]. Therefore, uterine fibroids with clinical symptoms should be actively treated. High intensity focused ultrasound (HIFU), a new noninvasive ablative technology, has been widely used in the treatment of uterine fibroids. A large number of clinical studies have demonstrated that HIFU can effectively ablate uterine fibroids and improve the clinical outcomes of patients [Citation4,Citation5]. HIFU is becoming more popular for the treatment of uterine fibroids in young women due to its minimal invasiveness, simple operation, high efficiency, high safety, and uterus physiological functions preservation.
With the development of ultrasound technology, elastography has been gradually introduced into clinical practice, which uses the mechanical characteristics (strain and elasticity modulus) of human soft tissues for clinical imaging [Citation6]. Acoustic Radiation Force pulse Imaging technology (ARFI) is a new elastography-based technology that includes the virtual touch tissue quantification (VTQ) and the virtual touch tissue imaging (VTI) .In this technique, tissue vibration is induced by acoustic radiation force, which causes deformation of tissue and shear wave propagation that are detected by ultrasonic methods, from which the viscoelastic coefficient of tissue is extracted [Citation7]. Therefore, it can be effective to identify the elastic properties of tissues by detecting the data or images to reflect the tissue hardness [Citation8,Citation9]. While ARFI technology has been widely applied for the evaluation of tumor treatment efficacy [Citation10–12], only few studies have shown its value in the preoperative prediction of HIFU ablation of uterine fibroids. In this study, we investigated the correlation between HIFU postoperative ablation rate and preoperative uterine fibroids’ SWV values and VTI grades. In addition, we evaluated the application value of acoustic radiation force impulse imaging in the preoperative prediction of the efficacy of HIFU ablation of uterine fibroids.
2. Materials and methods
2.1. Study population
The study was conducted on 32 women patients, with a total of 41 fibroids, undergoing HIFU uterine fibroids ablation between January 2019 and September 2019. The patients were 26–51 years old, with an average age of 39.1 ± 5.6 years. The diameter of uterine fibroids ranged between 32.7 and 110.2 mm, with an average diameter of 57.26 ± 19.69 mm. The inclusion criteria of patients were: (1) women of childbearing age; (2) refused the surgical operation and had a strong desire to preserve the uterus; (3) airborne ultrasound can be displayed well and uterine fibroids treatment is in a secure channel; and (4) no communication barriers. The exclusion criteria were: (1) the presence of other gynecological diseases such as vaginitis, pelvic inflammatory disease, cervical carcinoma, and other tumors; (2) the presence of connective tissue disease; (3) received ≥45 Gy of abdomen’s radiotherapy; (4) had cerebral infarction or cerebral hemorrhage 6 months before enrollment; (5) the presence of severe diseases in the heart, brain, lung, kidneys, or other organs; (6) Body Mass Index (BMI) greater than 32; (7) uterine fibroids with large numbers of necrotic areas; and (8) pregnant and lactating women. All participants signed informed consents.
2.2. Imaging techniques
Both gray-scale US and ARFI examinations were performed with SIEMENS S3000 color Doppler ultrasonic diagnostic apparatus equipped with a 6C1 convex array probe (bandwidth frequency of 2.0–5.0 MHz) and ARFI software. The morphology, size, boundary, echoes and color Doppler feature of uterine fibroids were observed and measured. Qualitative and quantitative measurement of lesion stiffness were detected using two ARFI imaging methods: virtual touch tissue imaging (VTI) and virtual touch tissue quantification (VTQ).
Before HIFU ablation, all patients underwent two-dimensional ultrasound and color-coded Doppler ultrasonography to observe uterine fibroids’ size, location, internal echotexture and blood supply. Uterine fibroids were classified based on their echogenicity into hyperechoic, isoechoic, or hypoechoic. Uterine fibroids with echogenicity similar to, higher than, or lower than that of the myometrium were classified as isoechoic, hyperechoic and hypoechoic, respectively.
VTQ: shear wave velocity (SWV) values of uterine fibroids were measured in all patients before HIFU ablation. During SWV measurement, the patients lied supine, with the probe placed directly on the lower abdomen. After observing the maximum uterine fibroids longitudinal sections, ARFI was switched to the VTQ mode. The region of interest (ROI) was then determined and placed in the center, outer upper, outer lower, inner upper and inner lower of the fibroid, adjusted based on the size of the fibroid. After that, patients were asked to hold their breath, the ‘update’ key was then pressed, which launched a high-intensity low-frequency pulse. The shear wave velocity (SWV) speed was measured in units of m/s. One SWV value can be automatically obtained after pressing “update” key one time, reflecting the elasticity of the region. The SWV related to tissue hardness in the ROI was read and recorded. The measurement was repeated 3 times in the same area, and an average value was recorded.
VTI: visualizes the relative stiffness in the selected ROI with a gray-scale picture. ARFI was switched to the VTI mode, the size of ROI was adjusted to include uterine fibroids with enough surrounding gland. After the maximum longitudinal section of uterine fibroids was displayed in the center of the screen, the patients were asked to hold their breath and the update key was pressed at the same time. VTI was performed on a dual imaging screen, with the left-sided screen showing the B-mode image and the right-sided screen showing elastography image. The stiffness of the uterine fibroids was analyzed on VTI elastography image. The VTI grade of the uterine fibroids was classified according to the VTI classification method by Wan [Citation13] as follows: Grade I, predominantly white; Grade II, predominantly white with few gray portions; Grade III, predominantly gray; Grade IV, predominantly gray with few black portions; and Grade V, completely black or predominantly black with few gray portions.
2.3. Hifu treatment
Uterine fibroids ablation was performed on a JC200 focused ultrasound tumor treatment system (Chongqing HIFU technology co, LTD), which included two major parts: a real-time ultrasound positioning monitoring equipment and a HIFU three-dimensional scanning equipment. The therapeutic parameters included: (1) therapeutic frequency of 0.97 MHz; (2) focus width of 3 mm; (3) focal length of 8 mm; (4) focal length of 162 mm; and (5) a single therapeutic power of 400 W.
All patients were given adequate bowel preparation the day before treatment and were instructed to fast for at least eight hours. The overlying skin was carefully shaved to avoid any potential interference from body hair, that may cause US wave reflection and the risk of skin damage. Before placing the patient in the prone position, a Foley catheter was inserted in all patients to expand the bladder with distilled water, which is useful for the anterior and cranial dislocation of the uterus. A balloon of purified water was placed between the HIFU transducer and the patient to compress and push the bowel loops away from the HIFU track and avoid the unpredictable presence of air bubbles, but also to reduce the thickness of the subcutaneous fat layer. Patients were carefully placed in the prone position on the HIFU table, ensuring that the skin overlying the target lesion was in contact with the water balloon and degassed water contained in the water tank, where the HIFU transducer was placed. The procedure was performed under sedation with intravenous administration of midazolam (1–10 mg) and fentanyl (25–100 mg) by an anesthetist. Intraoperative continuous intravenous infusion of oxytocin (80 U + 500ml normal saline) was performed at 40 drops/min to reduce the blood supply of uterine fibroids.
The target lesion was first detected using the airborne combined therapeutic device, which consists of a diagnostic probe for locating and monitoring lesion and a therapeutic device for launching high-intensity focused ultrasound waves. A preliminary planning scan was then performed to define the suitable safe ablation track, which was stored as a baseline in cine loop format. The entire fibroid volume was then divided into 5-mm interspersed axial US slices, which were recorded by the software of the HIFU equipment as the treatment plan. The scanning mode adopted was point to line, line to surface, and surface to body layer, where layers were scanned from deep to shallow. This process was repeated on a slice-by-slice basis to achieve complete ablation of the whole fibroid volume. The single-point ablation power was 400 w. During continuous therapy, the irradiation and pause times were 1 s each. The distance between points, the row spacing, and the ratio of irradiation to pause times were adjusted according to changes in tissue echogenicity and the response of the patient to acoustic irradiation. During ablation, the real-time scans were obtained immediately before and after individual energy exposures (i.e. HIFU sonication) and were compared to assess changes in the echogenicity of the treated region, which reflects the ablation degree. The post-sonication appearance was expected to be similar to that of an ill-defined hyperechoic area. If echogenicity of all the uterine fibroids was changed, the infusion of oxytocin was stopped for 10 min to remove the influence of oxytocin on the blood supply to uterine fibroids. SonoVue contrast ultrasound injection was used for the immediate evaluation of ablation results. The ablation was considered effective when the contrast agent could not be perfused into the tumor.
2.4. The evaluation of therapeutic effects
Enhanced MRI examination was performed with a 3.0 T Philips Achieva Magnetic Resonance Imaging scanner system within one month after HIFU ablation. All patients underwent conventional cross-sectional, coronal, and sagittal Spin Echo (SE) T1WI sequence and Turbo Spin Echo (TSE) T2WI sequence scans, in addition to lipid inhibition. Before examination, patients were instructed to drink water to fill their bladder, and the NPV of ablation was then measured. The size of postoperative uterine fibroids was measured using enhanced MRI images, including length diameter (D1), anteroposterior diameter (D2), left and right diameter (D3), and postoperative NPV. The uterine fibroids’ volume, the NPV, and the ablation rate were calculated. The volume (V) and the ablation rate were calculated using the following formulas: V = 0.5233 × D1 × D2 × D3; ablation rate = NPV × 100%/target fibroid volume.
2.5. Statistical analysis
Statistical analysis was performed using SPSS v19.0. Data were reported as mean ± standard deviation. Student’s t-test was used for statistical comparison between measurements, while Fisher’s chi-square test was used for comparison between count data. Spearman’s correlation was used for correlation analysis. Mann-Whitney rank-sum test was used for statistical analysis of nonparametric and ranked data. p < 0.05 was considered statistically significant. The Receiver Operating Characteristic (ROC) curve was plotted and used for evaluating the diagnostic ability of the classifier at a specific cutoff.
3. Results
3.1. The conventional ultrasound-based classification of preoperative uterine fibroids
Patients were divided into two groups based on the postoperative ablation rate: the ≥ 70% ablation rate group, and the <70% ablation rate group. The ≥70% ablation rate group accounted for 73.17% (30/41) of cases, while the <70% ablation rate group accounted for 26.83% (11/41) (). There was no statistically significant difference between the two groups in the conventional ultrasound classification of preoperative uterine fibroids (χ2 = 2.388, p = 0.463) ().
Figure 1. Statistical analysis of the conventional ultrasound classification of preoperative uterine fibroids.
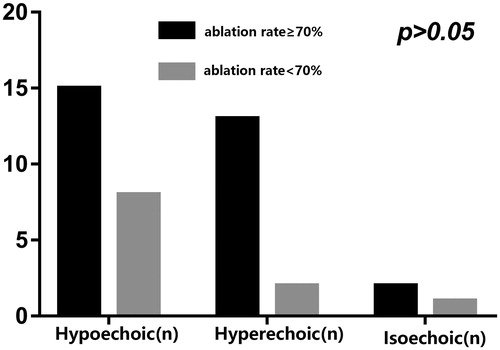
Table 1. Conventional ultrasound classification of preoperative uterine fibroids.
3.2. SWV values of preoperative uterine fibroids
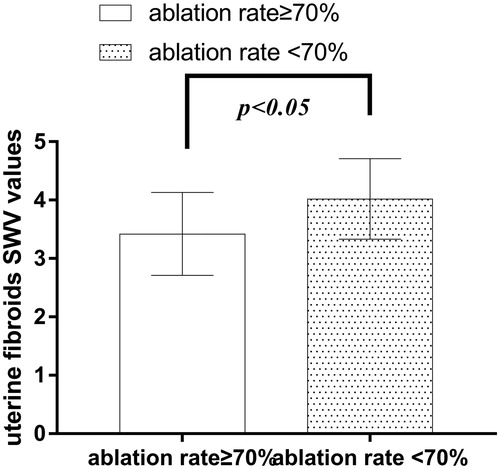
The SWV values of preoperative uterine fibroids in patients with ≥70% ablation rate ranged between 2.14 and 4.41 m/s, with an average value of 3.42 ± 0.71 m/s. On the other hand, the <70% ablation rate group showed preoperative uterine fibroids’ SWV value between 3.01 and 4.68 m/s, with an average SWV value of 4.02 ± 0.69 m/s. Interestingly, the SWV value was statistically different between the two groups (t= −2.401, p = 0.021) ().
3.3. VTI graphs of preoperative uterine fibroids
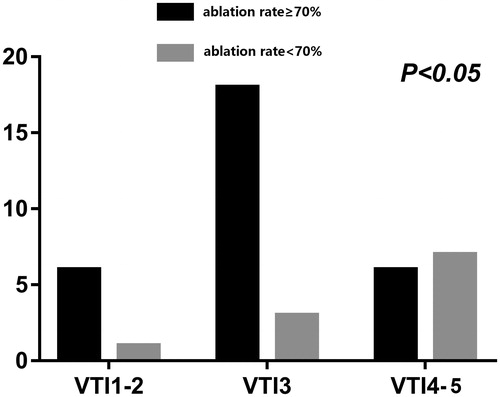
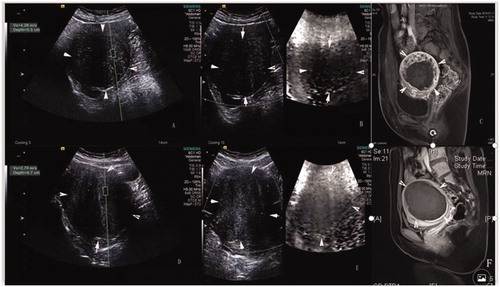
In the ≥70% ablation rate group, 80% (24/30) of patients had VTI grades II–III, whereas 63.6% (7/11) of the <70% ablation rate group had VTI grade Ⅵ. The SWV values, VTI grades, and enhanced MRIs of postoperative uterine fibroids in the two ablation rate groups are shown in .
Figure 3. The SWV values, VTI grades, and enhanced MRIs of preoperative uterine fibroids. (A) Preoperative fibroid with SWV values of 4.28 m/s and an ablation rate < 70%. (B) Preoperative fibroid with VTI grade Ⅵ and ablation rate < 70%. (C) Enhanced MRI showing residual mass with an ablation rate <70%. (D) Preoperative fibroid with SWV value of 2.70 m/s and ablation rate ≥70%. (E) Preoperative fibroid with VTI grade II and ablation rate ≥70%. (F) Enhanced MRI showing no residual mass.
Mann-Whitney rank-sum test showed statistically significant difference in VTI grades between the two groups (z = −2.587 p = 0.010) (). The VTI grades of the two groups are shown in .
Table2. Comparative of preoperative uterine fibroids VTI grade in the two groups.
3.4. The correlation between HIFU ablation rate and the SWV values and VTI grades of preoperative uterine fibroids
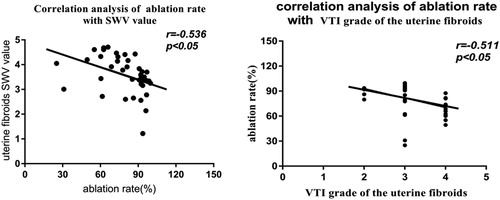
The ablation rates were negatively correlated with both the SWV values (r = −0.536, p = 0.001) and the VTI grades (r = −0.511, p = 0.0003) of preoperative uterine fibroids ().
3.5. The accuracy of preoperative uterine fibroids’ SWV values for the prediction of uterine fibroids ablation efficacy by HIFU
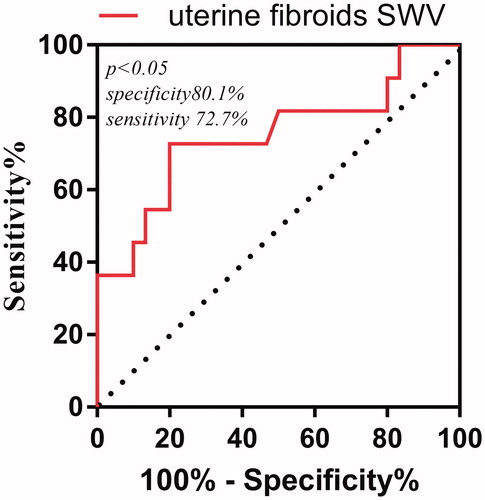
The cutoff value of SWV value for preoperative uterine fibroids with an ablation rate <70% was determined using the ROC curve. The optimal cutoff VTQ value was obtained using the Youden index (sensitivity + specificity − 1) from the ROC curve. The area under the ROC curve to predict an ablation rate < 70% was 0.75 at an SWV cutoff of 3.915 m/s (p < 0.05). The specificity was 72.7% and the sensitivity was 80.1%. The positive predictive value was 72.7%, while the negative predictive value was 80% ().
4. Discussion
HIFU is a noninvasive local tumor treatment technology that, compared with the traditional treatment method, is characterized with short treatment time, high efficacy, and mild pain. Previous studies have reported the ablation rate of HIFU as the ratio of nonperfusion area volume (NPV) to myoma volume from postoperative enhanced MRI [Citation14]. The higher the ablation rate, the better the clinical effect. Indeed, Long-term follow-up shows that the ablation rate is closely related to the postoperative uterine fibroid volume reduction rate and the improvement of clinical symptoms. Therefore, the ablation rate of fibroids has become an important indicator for the evaluation of the uterine fibroid ablation efficacy by HIFU. In this regard, it has been shown that reducing the uterine fibroids-related symptoms requires only 10% ablation of the fibroid uterine volume [Citation15]. The clinical 2-year effects of uterine fibroids volume ablation by 70% was comparable to that of hysteroscopic myomectomy [Citation16,Citation17]. Therefore, an ablation rate higher than 70% has become the criteria for the clinical evaluation of the efficacy of HIFU fibroid ablation.
The acoustic radiation force impulse (ARFI) imaging used in this study, also called acoustic palpation tissue quantitative technology, is a new technology that is based on ultrasonic elastography technology, and it includes virtual touch quantification (VTQ) and virtual touch imaging (VTI) techniques. VTQ is an ARFI technology that is based on acoustic radiation force excitation to generate a shear wave signals in biological viscoelastic tissues, which can be read using a specific electronic system. The shear wave would be limited to the internal area of the tissue due to the rapid reduction in the radiation outside the focus zone, which allows for the measurement of low-frequency shear waves’ velocity in the region of interest [Citation18]. The quantitative assessment of elastic modulus could be achieved through SWV measurement [Citation19–21]. The higher the SWV value, the greater are the elastic modulus and tissue hardness, and the smaller is the tissue strain [Citation22,Citation23]. Therefore, VTQ technology can reflect the elastic mechanical characteristics of tissues from a new perspective and can assess the hardness of tissues in a specific region. On the other hand, VTI technology is based on the application of intense acoustic radiation force to tissues with different degrees of hardness and softness, and to reflect the degree of elasticity of local tissues based on their longitudinal displacement. It is usually imaged in a grayscale form and is characterized by it noninvasive, painless, and rapid approach. The larger the elastic modulus is, the higher the tissue hardness value would be, and the greater the grayscale and VTI grades would be [Citation24]. In fact, ARFI technology has recently become a hot research topic due to its potential application in identifying tissue properties [Citation8,Citation9]. Interestingly, the application of ARFI in the evaluation of HIFU-based uterine fibroids ablation has been reported in multiple studies. Vappou [Citation25] made a preliminary study for the evaluation of VTQ after HIFU ablation and reported the feasibility of using VTQ technology to evaluate the efficacy of HIFU ablation. However, there is currently no in-depth study on the preoperative prediction of HIFU efficacy for uterine fibroids ablation. In this study, not only we analyzed the correlation of preoperative uterine fibroids’ VTI grades and SWV values with HIFU ablation rate, but also explored the value of the SWV of uterine fibroids in predicting effective ablation by mapping the ROC curve.
In this study, preoperative uterine fibroids with ≥70% ablation rate were mainly classified as hyperechoic or hypoechoic based on conventional ultrasound echo intensity, while those with <70% ablation rate were mainly hypoechoic. However, there was no significant difference in the conventional ultrasound echo intensity between the two groups, which suggests that it remains difficult to predict HIFU-based ablation efficacy ablation through conventional ultrasound echo intensity in preoperative uterine fibroids.
The preoperative SWV values and VTI grades of uterine fibroids were compared between the two groups in this study. The mean SWV value of preoperative uterine fibroids in the ≥70% ablation rate group was 3.42 ± 0.71 m/s, which was lower than that in the <70% ablation rate group, which had a mean SWV value of 4.02 ± 0.69 m/s. Similarly, the preoperative VTI grades of uterine fibroids in the ≥70% ablation rate group was less than that in the <70% ablation rate group. The VTI grade of preoperative uterine fibroids in the ≥70% ablation rate group was lower than that in the <70% ablation rate group. On the other hand, our results showed that that preoperative SWV values and VTI grades of uterine fibroids were negatively correlated with ablation rate. This could be related to differences in tissue structure composition and cell arrangement in different uterine fibroids, which can result in different viscoelastic properties between tissues, and hence, lead to differences in ablation efficacy. By studying Young’s modulus of breast malignancies, Chamming et al. [Citation26] showed that tumor hardness may increase with increasing collagen fiber content. Hence, the SWV values and VTI grades can effectively evaluate the elastic modulus of uterine fibroids. The lower the SWV value, the smaller the elastic modulus, the smaller the tissue hardness [Citation22–23], the less the interstitial components such as collagen fibers and micro-vessels, the worse the effective microcirculation of tumor tissues, the better the heat transfer effects, the higher the thermal sensitivity of tumor cells [Citation27], and the better the ablation effect would be. Finally, the ROC curve was drawn, and it was concluded that the area under the receiver operating characteristics curve of preoperative uterine fibroids SWV value for the ablation rate below 70% was 0.75 at a cutoff value of 3.915 m/s (p < 0.05). Specificity was 72.7%, sensitivity was 80.1%. Therefore, it is suggested that the ARFI technique is more advantageous than conventional ultrasound in preoperative prediction for the efficacy of HIFU uterine fibroids ablation, with its high sensitivity and specificity, which can provide positive value in preoperative prediction for the efficacy of HIFU, patient selection, and program formulation.
In this study, the ARFI was used, and the inspection method was relatively simple. After a short training period, the operation could be satisfactorily completed. Patients should be instructed to hold their breath during the elastic examination; and for patients with uterine movements, moderate pressure should be applied to fix the uterine position and shorten the distance between the probe and the lesion area as much as possible. In addition, artifact formation should be avoided as much as possible to improve the stability and quality of the image.
While this was the first attempt to predict the preoperative efficacy of HIFU uterine fibroids ablation using ARFI technology, there are many factors affecting the HIFU ablative rate that were not considered, such as target skin distance and blood flow in uterine fibroids. Therefore, further studies are required to evaluate the effects of these factors.
In summary, ARFI is a new type of ultrasonic technology that can complement conventional ultrasound examination methods by reflecting the hardness of fibroids, which can provide a potential preoperative predictor for the efficacy of uterine fibroids ablation using HIFU. In addition, it provides a useful basis for the preoperative screening and formulation of patients during HIFU treatment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48(2):312–324.
- Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104:393–406.
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95–114.
- Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermo ablative technique. Am J Obstet Gynecol. 2003;189(1):48–54.
- Zhang L, Zhang W, Orsi F, et al. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperthermia. 2015;31(3):280–284.
- Garra BS. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007;23:255–268.
- Gennisson JL, Deffieux T, Fink M, et al. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94(5):487–495.
- Zhan J ,Jin JM ,Diao XH, et al. Acoustic radiation force impulse imaging (ARFI) for differentiation of benign and malignant thyroid nodules—a meta-analysis. Eur J Radiol. 2015; 84:2181–2186.
- Kim JE, Lee JY, Bae KS, et al. Acoustic radiation force impulse elastography for focal hepatic tumors: usefulness for differentiating hemangiomas from malignant tumors. Korean J Radiol. 2013;14(5):743–753.
- Cho SH, Lee JY, Han JK, et al. Acoustic radiation force impulse elastography for the evaluation of focal solid hepatic lesions: preliminary findings. Ultrasound Med Biol. 2010;36:202–208.
- Kang J, Kwon H, Cho J, et al. Comparative study of shear wave velocities using acoustic radiation force impulse technology in hepatocellular carcinoma: the extent of radiofrequency ablation. Gut Liver. 2012;6(3):362–367.
- Xu X, Luo L, Chen J, et al. Acoustic radiation force impulse elastography for efficacy evaluation after hepatocellular carcinoma radiofrequency ablation: a comparative study with contrast-enhanced ultrasound. Biomed Res Int. 2014;2014:1–7.
- Wan J, Wu R, Yao M, et al. Acoustic radiation force impulse elastography in evaluation of triple-negative breast cancer: a preliminary experience. Clin Hemorheol Microcirc. 2018;70(3):301–310.
- Lee JY, Chung HH, Kang SY, et al. Portable ultrasound-guided high-intensity focused ultrasound with functions for safe and rapid ablation: prospective clinical trial for uterine fibroids-short-term and long-term results. Eur Radiol. 2020;30(3):1554–1563.
- Fennessy FM, Tempany CM, McDannold NJ, et al. Uterine leiomyomas: MR imaging–guided focused ultrasound surgery—results of different treatment protocols. Radiology. 2007;243(3):885–893.
- Olive DL. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2008;111(3):775; author reply 775–6.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstetr Gynecol. 2005;105:877–881.
- Bai M, Du L, Gu J, et al. Virtual touch tissue quantification using acoustic radiation force impulse technology: initial clinical experience with solid breast masses. J Ultrasound Med. 2012;31(2):289–294.
- Karlas T, Lindner F, Tröltzsch M, et al. Assessment of spleen stiffness using acoustic radiation force impulse imaging (ARFI): definition of examination standards and impact of breathing maneuvers. Ultraschall in Med. 2014;35(01):38–43.
- Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol. 2003;29:1715–1723.
- Gallotti A, D’Onofrio M, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. Radiol Med. 2010;115(6):889–897.
- Hall TJ. AAPM/RSNA physics tutorial for residents: topics in US: beyond the basics: elasticity imaging with US. Radiographics. 2003;23(6):1657–1671.
- Ophir J, Céspedes I, Ponnekanti H, et al. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13(2):111–134.
- You Q-Q, Xu M, Yao M-H, et al. Diagnostic value of acoustic radiation force impulse for BI-RADS category 4 breast lesions of different sizes. Clin Hemorheol Microcirc. 2018;70(2):143–154.
- Vappou J, Bour P, Marquet F, et al. MR-ARFI-based method for the quantitative measurement of tissue elasticity: application for monitoring HIFU therapy. Phys Med Biol. 2018;63(9):095018.
- Chamming’s F, Latorre-Ossa H, Le Frère-Belda MA, et al. Shear wave elastography of tumour growth in a human breast cancer model with pathological correlation. Eur Radiol. 2013;23(8):2079–2086.
- Dudar TE, Jain RK. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res. 1984;44:605–612.