 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
To evaluate the efficacy and safety of microwave ablation (MWA) for cervical metastatic lymph nodes (LNs) post resection of papillary thyroid cancer (PTC).
Materials and methods
From November 2015 to November 2018, 14 patients with 38 cervical metastatic LNs treated by MWA were included in this retrospective study. Wilcoxon signed rank test was used to compare the changes of LN and serum thyroglobulin levels pre- and post-ablation.
Results
The technical success rate in this study was 100% (38/38). The mean follow-up time was 23.6 ± 9.3 months. On pre-ablation contrast-enhanced ultrasound, 25 LNs showed high-enhancement, 8 LNs showed iso-enhancement, and 5 LNs showed low-enhancement. The median largest diameter of LNs at pre-ablation and 3, 6, 9, 12, 18, 24, and 36 months post-ablation was 11.5 mm and 9.5, 9.0, 8.0, 8.0, 8.0, 7.0, and 6.0 mm, respectively. The median volume of LNs at pre-ablation and 3, 6, 9, 12, 18, 24, and 36 months post-ablation were 251.2 mm3 and 206.7, 167.2, 166.2, 155.7, 153.9, 153.9, and 113.1 mm3, respectively. The largest diameter and the volume of the cervical metastatic LNs at the last post-ablation was significantly smaller than the pre-ablation level (p = .0016; p = .0018). Serum Tg level at the last post-ablation (median 1.25 ng/mL) was significantly lower than the pre-ablation level (median 8.35 ng/mL) (p = .001). There were no complications.
Conclusion
MWA is a safe and effective novel treatment option for cervical metastatic LN that emerge post resection of PTC.
Introduction
In the past few decades, the growth in the incidence of thyroid cancer has outpaced that of any other malignancy in several countries worldwide [Citation1,Citation2]. According to GLOBOCAN 2018 cancer morbidity and mortality estimates provided by the International Cancer Research Agency, there were 567,233 new cases of thyroid cancer and 41,071 deaths due to thyroid cancer in 2018 [Citation3]. Papillary thyroid cancer (PTC) is the commonest thyroid cancer subtype and, in most patients, conventional treatment (i.e., thyroidectomy with or without prophylactic central neck dissection, radioiodine ablation, and suppression of serum thyroid-stimulating hormone with levothyroxine) is effective [Citation4,Citation5]. PTC is typically an indolent disease with a high cure rate; however, metastasis to cervical lymph nodes (LN) is frequently observed after the resection of the PTC [Citation6]. The American National Thyroid Association guidelines recommend that patients with cervical metastatic LN should undergo repeated surgery and/or radioiodine ablation [Citation7]. However, reoperation poses a technical challenge due to the distortion of the normal tissue plane as a consequence of postoperative fibrosis, and this is associated with a high incidence of complications. In a recent study, the rates of transient hypoparathyroidism, permanent hypoparathyroidism, and transient nerve injury were 56.6%, 10%, and 4.6%, respectively, after reoperation [Citation8]. Therefore, a less invasive technique and an alternative treatment modality to surgical resection is required to solve this problem.
Image-guided ablation is increasingly used as a minimally invasive treatment modality for patients with benign and malignant tumors; it has been shown to achieve good clinical results with a low incidence of complications [Citation9–11]. Laser ablation and radiofrequency ablation (RFA) have been proven to be effective in the treatment of cervical metastatic LN from PTC since several years [Citation12–14]. However, few studies have investigated the outcomes of MWA treatment of cervical metastatic LN from PTC [Citation15,Citation16]. Therefore, the purpose of this study was to evaluate the efficiency and safety of MWA for cervical metastatic LNs arising after surgical resection of PTC.
Materials and methods
Patients
According to the medical records, this retrospective study included 14 patients with 38 cervical metastatic LN from PTC who were treated by MWA at China–Japan Friendship Hospital (Beijing, China) between November 2015 and November 2018. The inclusion criteria were: (1) history of total thyroidectomy or subtotal thyroidectomy for PTC; (2) history of prophylactic central neck dissection; (3) postoperative levothyroxine treatment for the suppression of serum thyroid-stimulating hormone; (4) enlarged LNs in the neck detected on ultrasound (US) and diagnosed as metastasis from PTC by using fine-needle aspiration biopsy (FNAB); (5) patients refusal to undergo repeated neck dissection; and (6) post-ablation follow-up for more than 12 months. The exclusion criteria were: (1) children or pregnant women; (2) patients with distant metastasis; (3) patients with serious bleeding tendencies; and (4) patients with contraindications for the use of the US contrast agent. The protocol of this retrospective study was approved by the institutional ethics committee of the China–Japan Friendship Hospital (Beijing, China). Because of the retrospective nature of the study, patient consent for inclusion was waived.
Equipment and operators
The B-mode US and FNAB was undertaken with the Aplio 500 (Toshiba, Tokyo, Japan) or the GE LOGIQ E9 (GE Healthcare, Pittsburgh, PA, USA) system. The contrast-enhanced US (CEUS) and MWA were carried out with the GE LOGIQ E9 system, which was equipped with a linear probe that had a bandwidth of 9.0 MHz. A microwave generator and a 17-gauge (G) internally cooled antenna with a 0.4-cm tip (Intelligent Basic Type Microwave Tumor Ablation System, Nanjing ECO Microwave System, Nanjing, China) were used for the ablation procedures. A second-generation US contrast agent SonoVue (sulfur hexafluoride microbubbles, Bracco, Milan, Italy) was used in this study. The US contrast agents are usually injected as a 2.0 ml bolus (equivalent to 0.03 ml/kg, assuming 70 kg body weight) through a 20 G cannula in a median cubital vein (usually positioned in the left arm), and flushed with 10 ml sterile saline [Citation17].
All US examinations and CEUS were carried out by two radiologists, each of whom had more than 3 years of experience in thyroid US imaging. The FNAB was undertaken by a radiologist, who had previously conducted more than 50 FNAB. All MWA procedures were carried out by a radiologist, who had performed more than 100 ablation procedures.
Ablation procedures
Patients were placed supine, and their necks were exposed fully. The ablation site was routinely sterilized and draped with sterile towels. All patients underwent a pre-MWA CEUS. Before the ablation, CEUS examinations were used to evaluate the extent of the target LN and its enhancement mode. Thereafter, we administered local anesthesia (1% lidocaine), and injected isolation fluid around the target LN. The isolation fluid comprised lidocaine mixed with normal saline (1:3, lidocaine concentration: 0.5%), which was injected into the surrounding area of the target LN. For example, if the target LN was located on the side of the trachea or in front of the esophagus, we injected the isolation fluid outside the trachea or around the esophagus such that the lymph nodes were anteriorly raised and laterally to avoid heat damage to the recurrent laryngeal nerve or the trachea and esophagus. If the target LN was located in the carotid sheath, the isolation fluid was injected into the carotid sheath in order to fill the area around the lymph node to prevent damage to the vagus nerve, carotid artery, and internal jugular vein. This technique can provide heat insulation and further induce local anesthesia through the effect of lidocaine on the recurrent laryngeal and vagal nerves. For the MWA, we inserted the needle tip of the 17 G MWA antenna into the target lesion under US guidance. The power for the ablation was kept at 30 W. The moving-shot, pull-back or fixed applicator technique is used based on the tumor characteristics [Citation18]. The ablation was terminated when a transient hyperechoic echotexture was seen throughout the target LN. We used post-MWA CEUS to evaluate the extent of ablation. The ablation was considered complete if unenhanced areas on CEUS covered the ablated LN. An additional ablation was immediately undertaken if there was an enhancement area within the target LN. After complete ablation of one LN, MWA was undertaken for the other LN. The pre-ablation B-mode US, CEUS examination, ablation process, and post-ablation CEUS examination are depicted in .
Figure 1. A 47-year-old woman who had undergone a total thyroidectomy 2 years ago for papillary thyroid cancer underwent microwave ablation (MWA) of cervical metastatic lymph nodes (LN). A, Pre-MWA, B-mode ultrasonography (US) showed hypoechoic LN (arrow); B, Fine-needle aspiration biopsy (FNAB) process of the LN (arrow); C, Pre-MWA, the contrast-enhanced US (CEUS) showed uneven and highly enhanced patterns (arrow); D, Spacer fluid (arrow) was injected to surround the LN; E, Hyperechoic (arrow) pattern in the LN during MWA; F, Post-MWA, the CEUS showed no enhancement (arrow) in LN.
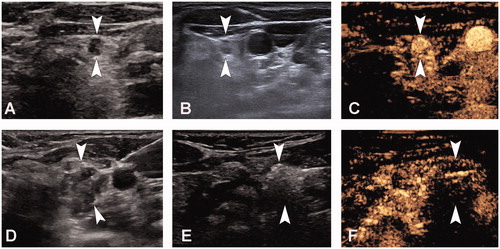
In this study, all patients were hospitalized for the MWA. After ablation, each patient was carefully evaluated for all possible complications, including nerve damage, skin burns, hematoma, tracheal damage, sound conditions, and esophageal perforation, among others.
Follow-up after MWA
Technical success was defined as complete absence of enhancement at CEUS at the end of every procedure [Citation12]. Complications were recorded using the standard for image-guided thyroid ablation [Citation18]. All patients were followed up quarterly (every 3 months) in the first year after MWA, biannually (every 6 months) in the second year, and annually thereafter. Data from the patient examination at each follow-up were recorded in detail. In accordance with the principles of follow-up examination in patients with thyroid cancer, physical examination, blood tests (serum thyroglobulin [Tg], platelet counts, coagulation tests, etc.), and imaging examination were performed to exclude recurrent and distant metastases. For the ablated area, a routine neck B-mode US examination was undertaken, and both CEUS and FNAB was carried out if target LN metastasis or recurrence was suspected. The size of the target LN (including length, width, and depth) was recorded during the US examination. The volume of the LN was calculated with the following equation:
where V is the volume; a, the largest diameter, and b and c are the other two perpendicular diameters [Citation19]. The technical success rate in this study was defined as the number of complete ablations of LN divided by the total number of LN enrolled. The complication rate was defined as the number of patients with complications, divided by the total number of patients enrolled.
Statistical analysis
Descriptive statistics are presented as mean ± standard deviation (SD) or median for continuous variables, according to normality for the continuous variable, and as frequency (percentage) for categorical variables. To compare the changes of size – volume of LN pre- and post-ablation – we used the Wilcoxon signed rank test. Pre-ablation serum Tg levels were compared with those at the last follow up post-ablation using the Wilcoxon signed rank test. All statistical analyses were conducted using the SPSS software package (SPSS Statistics, version 26.0; SPSS Inc., Chicago, IL, USA). A p-value of less than .05 was considered statistically significant.
Results
Patient characteristics
Demographic characteristics of the 14 patients (age range [mean ± SD] 28–73 [46.9 ± 11.9] years) enrolled in this study are summarized in . There were 3 (21.4%) men and 11 (78.6%) women. For the primary tumor, 5 (35.8%) patients had left lobe tumor, 1 (7.1%) patient had an isthmus tumor, and 8 (57.1%) patients had right lobe tumor. Of the 14 patients included, 2 underwent 2 surgical resections. The number of LNs in our study population ranged from 1 to 7, and the mean was 3. The follow-up time ranged from 12 to 36 months, and the mean follow-up time was 23.6 ± 9.3 months. Three patients were followed up for 12 month, 3 patients were followed up for 18 months, 4 patients were followed up for 24 months, and 4 patients were followed up for 36 months. The pre-ablation serum Tg levels ranged from 0.65 to 168.3 ng/mL (median 8.35 ng/mL).
Table 1. Clinical characteristics of the study population.
LN characteristics
The characteristics of the LNs are summarized in . There were 15 LNs (39.4%) in the left neck and 23 LNs (60.5%) in the right neck. There were 2 (5.3%), 3 (7.9%), 12 (31.5%), 15 (39.5%), and 6 (15.8%) LNs at levels I, II, III, IV, and VI, respectively. The pre-ablation largest diameter of LNs ranged from 4 to 48 mm (median 11.5 mm). The volume of the cervical metastatic LNs ranged from 33.5 to 30144.0 mm3 (median 251.2 mm3). The ablation time of LNs ranged from 10 to 685 s (median 29 s). In the pre-ablation CEUS, 25 LNs showed high-enhancement, 8 LNs showed iso-enhancement, and 5 LNs showed low-enhancement.
Table 2. Characteristics of cervical metastatic lymph nodes.
Treatment outcome of MWA and complications
All cervical metastatic LNs in the 14 patients showed complete absence of enhancement at post-ablation CEUS; this indicated that the lesions were completely ablated in one procedure. The technical success rate in this study was 100% (38/38). The changes in the largest diameter and volume of the LNs at the pre-ablation and post-ablation examinations, at each follow-up point, are shown in . The largest diameter of LNs pre-ablation and at 3, 6, 9, 12, 18, 24, and 36 months post-ablation were 11.5 mm and 9.5, 9.0, 8.0, 8.0, 8.0, 7.0, and 6.0 mm, respectively. The volume of LNs at pre-ablation and at 3, 6, 9, 12, 18, 24, and 36 months post-ablation were 251.2 and 206.7, 167.2, 166.2, 155.7, 153.9, 153.9, and 113.1 mm3, respectively. The largest diameter and volume of cervical metastatic LNs post-ablation at each follow-up time-point was significantly smaller than the corresponding pre-ablation levels (p < .05). One patient developed new cervical metastatic LNs at the 6-month follow-up after the first ablation. Re-ablation was undertaken on this recurrent LN after confirming the diagnosis. The median serum Tg level at the last follow-up (1.25 ng/mL [interquartile range 0.37–2.50 ng/mL]) was significantly lower than the pre-ablation level (8.35 ng/mL [interquartile range 1.97–17.62 ng/mL]; p = .001). Finally, 44.7% (17/38) of the lesions had completely disappeared at the last follow-up.
Table 3. Changes in cervical metastatic lymph nodes post-ablation at each follow-up.
No complications were reported in this study, nor was there any observable local infection, hematoma, skin burn, or damage to the recurrent laryngeal nerve, vagus nerve, trachea, or esophagus.
Discussion
The results of our retrospective study show that MWA is a feasible, effective and safe treatment for cervical metastatic LN that emerge post resection of PTC. It provides a novel alternative treatment option for these patients. Over the mean follow-up period of 23.6 months, none of the LNs showed local recurrence. No complications occurred in our study.
Image-guided thermal ablation has recently been recommended as a safe and effective alternative treatment option for high-risk patients or patients who refuse surgery. Previous studies have shown good outcomes of RAF in the treatment of cervical metastatic LN and local recurrence from PTC [Citation14,Citation20,Citation21]. Laser ablation has also been shown to be effective for cervical metastatic LNs of PTC since several years; it allows for precise ablation of the target, entails use of smaller needles, and is probably cheaper [Citation12,Citation13]. MWA represents one of the novel thermal ablation techniques; it can achieve very high temperature in a very short time, thus enabling larger and faster ablations. MWA has been successfully used to treat a variety of cancers, such as liver, lung, kidney, and thyroid cancers [Citation22–25]. However, few studies have assessed the use of MWA treatment for cervical metastatic LN from PTC. We conducted this retrospective study to explore the efficacy and safety of MWA for cervical metastatic LN post resection of PTC.
Our findings show that MWA is an effective therapy for cervical metastatic LNs that emerge post resection of PTC. We analyzed the main reasons for the therapeutic effectiveness of MWA. First, a prominent advantage of MWA is its high thermal efficacy, which facilitates larger area ablation in a shorter duration. Compared with other ablation modalities, MWA is less dependent on the electrical conductivities of tissues and is less limited by the electrical impedances of tissues [Citation26,Citation27]. Second, we used high-frequency US real-time guidance for the MWA. High-frequency US can clearly image LNs (e.g., LNs measuring 3–4 mm). Further, US guidance provides real-time guidance, with precision of approximately 1 mm. In addition, 44.7% (17/38) of the lesions disappeared completely at the last follow-up. This further confirms the effectiveness of MWA. In the present study, 1 patient presented with a new metastatic LN at the 6-month follow-up after the first MWA. There are similar reports in the literature. Mauri et al. [Citation12,Citation13] reported that laser ablation is a feasible, safe, and effective therapy for cervical metastatic LNs that emerge post resection of PTC. The reported local control rates in the LNs at 1 year and 3 years were 86.9% (40/46) and 100% (25/25), respectively [Citation13]. Monchik et al. [Citation28] reported that 3 patients (3/16) had new metastatic lesions after RFA and percutaneous ethanol injection treatment. Moreover, Baek et al. [Citation29] reported that 2 patients (2/10) had new lesions in the neck after RFA. Serum Tg level was shown to be a valuable indicator of the efficacy of ablation in patients with total thyroidectomy [Citation20,Citation30]. In the present study, serum Tg levels were still detected in a few patients after MWA treatment. However, the levels were significantly lower than the pre-ablation level, which indicates successful ablation of the target lymph node. However, in patients with a history of subtotal thyroidectomy, small amounts of Tg may be produced by the residual thyroid tissue post-ablation. Even patients who have undergone total thyroidectomy may still have micrometastatic lymph nodes that are undetectable on US. Therefore, post-ablation regular monitoring of serum Tg level and US examination are necessary to detect potential metastatic lymph nodes.
The present study shows that MWA therapy is a safe method for the treatment of cervical metastatic LN post resection of PTC. In this study, no complications occurred during the follow-up period. In a study of 2743 patients who underwent total thyroidectomy with concomitant unilateral lateral neck dissection, 30.5% patients developed hypoparathyroidism/hypocalcemia, 8.3% patients developed vocal cord paralysis, and 1.2% patients required tracheotomy [Citation31]. Therefore, when compared with surgical resection, MWA may be a safer therapeutic method. We analyzed that some strategies may increase the safety of MWA. First, the LN has an inherent envelope structure that hinders heat conduction. When it is ablated, the temperature inside the lymph node is high while the temperature outside the lymph node is relatively low; this may reduce complications. Second, we used continuous saline injection for lesion isolation during ablation. The specific heat capacity of water is large enough to prevent a rapid increase in its temperature. The continuous saline injection maintains flow, which can better modulate the temperature and thereby may reduce the occurrence of complications. Third, after saline injection, the target lesion can be effectively separated from the surrounding important tissue structures by a safe distance, which can further reduce the occurrence of complications. Fourth, we used high-frequency US real-time guidance, which can clearly display the important surrounding structures, such as nerves, and they can effectively be avoided during puncture, thereby reducing procedure-related complications. Finally, during the MWA, we used low-power (30 W) short-duration repeat ablation, to ensure that the local temperature was high and the surrounding temperature is low; thus, safety was further ensured.
This study has a few limitations. First, repeat FNAB was not performed for all visible lymph nodes post-ablation for cytological and Tg test. However, if target LN metastasis or recurrence was suspected, FNAB was carried out for cytological test. Second, this study had a small sample size and a limited number of LNs underwent MWA. Therefore, large-sample studies are urgently needed to verify the findings of this study. Third, the follow-up time for this study was limited, and we will continue to follow-up these patients for a better understanding of the treatment outcomes.
Conclusion
When used with multiple safety strategies, especially precision puncture and hydrodissection technique, MWA is a safe and effective treatment for cervical metastatic LN that emerge post resection of PTC. MWA provides a novel alternative treatment option for selected patients.
Acknowledgments
We would like to thank all participants for their support in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Carling T, Udelsman R. Thyroid cancer. Annu Rev Med. 2014;65(1):125–137.
- Katanoda K, Matsuoka J. Incidence rates of thyroid cancer in the world from the Cancer Incidence in Five Continents XI. Japanese J Clin Oncol. 2019;49(6):587–588.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- McLeod DS, Sawka AM, Cooper DS. Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet (London, England). 2013;381(9871):1046–1057.
- Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (London, England). 2016;388(10061):2783–2795.
- Johnson NA, Tublin ME. Postoperative surveillance of differentiated thyroid carcinoma: rationale, techniques, and controversies. Radiology. 2008;249(2):429–444.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
- Medas F, Tuveri M, Canu GL, et al. Complications after reoperative thyroid surgery: retrospective evaluation of 152 consecutive cases. Updates Surg. 2019;71(4):705–710.
- Mauri G, Nicosia L, Della Vigna P, et al. Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography. 2019;38(1):25–36.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperthermia. 2019;36(2):13–20.
- Zhuo L, Zhang L, Peng LL, et al. Microwave ablation of hyperplastic parathyroid glands is a treatment option for end-stage renal disease patients ineligible for surgical resection. Int J Hyperthermia. 2019;36(1):29–35.
- Mauri G, Cova L, Tondolo T, et al. Percutaneous laser ablation of metastatic lymph nodes in the neck from papillary thyroid carcinoma: preliminary results. J Clin Endocrinol Metab. 2013;98(7):E1203–7.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39(7):1023–1030.
- Guang Y, Luo Y, Zhang Y, et al. Efficacy and safety of percutaneous ultrasound guided radiofrequency ablation for treating cervical metastatic lymph nodes from papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2017;143(8):1555–1562.
- Teng D, Ding L, Wang Y, et al. Safety and efficiency of ultrasound-guided low power microwave ablation in the treatment of cervical metastatic lymph node from papillary thyroid carcinoma: a mean of 32 months follow-up study. Endocrine. 2018;62(3):648–654.
- Zhou W, Chen Y, Zhang L, et al. Percutaneous microwave ablation of metastatic lymph nodes from papillary thyroid carcinoma: preliminary results. World J Surg. 2019;43(4):1029–1037.
- Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version). Ultraschall in Med. 2018;39(02):154–180.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010;194(4):1137–1142.
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276(3):909–918.
- Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25(1):163–170.
- Vogl TJ, Nour-Eldin NA, Hammerstingl RM, et al. Microwave Ablation (MWA): basics, technique and results in primary and metastatic liver neoplasms – review article. Fortschr Röntgenstr. 2017;189(11):1055–1066.
- Jiang B, McClure MA, Chen T, et al. Efficacy and safety of thermal ablation of lung malignancies: A Network meta-analysis. Ann Thorac Med. 2018;13(4):243–250.
- Choi SH, Kim JW, Kim JH, et al. Efficacy and safety of microwave ablation for malignant renal tumors: an updated systematic review and meta-analysis of the literature since 2012. Korean J Radiol. 2018;19(5):938–949.
- Teng D, Sui G, Liu C, et al. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol. 2018;144(4):771–779.
- Vorlander C, David Kohlhase K, Korkusuz Y, et al. Comparison between microwave ablation and bipolar radiofrequency ablation in benign thyroid nodules: differences in energy transmission, duration of application and applied shots. Int J Hyperthermia. 2018;35(1):216–225.
- Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20(1):11–22.
- Monchik JM, Donatini G, Iannuccilli J, et al. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg. 2006;244(2):296–304.
- Baek JH, Kim YS, Sung JY, et al. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol. 2011;197(2):W331–6.
- Jeon SJ, Kim E, Park JS, et al. Diagnostic benefit of thyroglobulin measurement in fine-needle aspiration for diagnosing metastatic cervical lymph nodes from papillary thyroid cancer: correlations with US features. Korean J Radiol. 2009;10(2):106–111.
- Rocke DJ, Mulder H, Cyr D, et al. The effect of lateral neck dissection on complication rate for total thyroidectomy. Am J Otolaryngol. 2020:102421. doi:10.1016/j.amjoto.2020.102421
