Abstract
Background & aims
Very few data are available in literature about the role of radiofrequency ablation (RFA) in intrahepatic cholangiocarcinoma (ICC) and previous studies are mainly case reports and case series on a very small number of patients and nodules. In this study, we aimed to evaluate effectiveness and safety of RFA for the treatment of unresectable ICC.
Methods
This is a retrospective observational cohort study comprising all consecutive patients treated with RFA for unresectable ICC at Policlinico Sant’Orsola Malpighi Hospital, Bologna, Italy. Primary endpoint was Local Tumor Progression-Free Survival (LTPFS) while Overall Survival (OS) was also assessed as secondary endpoint.
Results
From January 2014 to June 2019, 29 patients with 117 nodules underwent RFA. Technique effectiveness 1 month after RFA was 92.3%; median LTPFS was 9.27 months. Univariate analysis and multivariate analysis showed that LTPFS was significantly related to tumor size ≥20 mm. At a median follow up of 39.9 months, median OS from the date of RFA was 27.5 months, with an OS of 89%, 45% and 11% at 1, 2 and 4 years, respectively. Number of overall lesions and the sum of their diameter at the moment of the first RFA significantly affected OS in multivariate analysis. Minor and major complication rates were 14% and 7%, respectively.
Conclusion
Tumor size ≥20 mm was associated with lower LTPFS, representing a potential useful threshold value. A careful evaluation of tumor burden appears as a crucial element in choosing the best therapeutic strategy in unresectable ICC.
Introduction
Biliary tract cancer (BTC) is the second most common primary liver malignancy, accounting for approximately 10–15% of all hepatobiliary cancers [Citation1]. BTC includes a heterogeneous group of neoplasms usually divided in ampulla of Vater cancer, gallbladder cancer, extrahepatic cholangiocarcinoma and intrahepatic cholangiocarcinoma (ICC), which represents at least 20% of all BTC [Citation2]. Although traditionally considered a rare malignancy in Western countries, the incidence and mortality rate of ICC are on the rise worldwide and are predicted to increase in the near future [Citation3,Citation4]. While radical surgery with clear margins is the only potentially curative therapy and is associated with 5-year overall survival rates between 15% and 40%, most patients with ICC are not candidates for curative resection because of advanced cancer at the time of initial presentation or underlying comorbidities [Citation5]. The results of systemic chemotherapy in patients with advanced or metastatic disease are often disappointing with regard to toxicity, time to recurrence, and survival [Citation6]. Moreover, although a high number of patients affected by ICC have a liver-only disease, clinicians inevitably encounter difficulties in choosing the best therapeutic strategy for patients with high-volume lesions and/or vascular or biliary extensions of the tumor [Citation7]. The application of different locoregional therapies in ICC has been increasingly used over the last 15 years, and the best evidence and most promising results are currently available for transarterial radioembolization (TARE), hepatic artery infusion (HAI), transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) [Citation8]. RFA is a locoregional therapy that has been reported to be effective in the local control of hepatic malignancies in patients considered unsuitable for surgical resection [Citation9,Citation10]; however, since ICC is a relatively rare tumor, data on the efficacy and safety of RFA in ICC are very limited and mainly obtained through case series [Citation11]. In this study, we aimed to evaluate the efficacy and safety of percutaneous ultrasound-guided RFA for the complete removal of unresectable, non-metastatic ICC.
Materials and methods
Patients
The local Institutional Review Board provided this study approval. An observational cohort study was conducted, in which all consecutive percutaneous ablations performed to treat ICCs at Policlinico Sant’Orsola Malpighi, Bologna, Italy, between January 2014 and June 2019 were noted. All patients signed an informed consent form prior to RFA; moreover, all patients signed a further consent form concerning the enrollment in the current study. The decision to perform RFA was determined by institutional tumor board which included medical oncologists, gastroenterologists, radiologists, radiotherapists and internationally renowned liver and transplant surgeons. All the patients included in our study were considered unresectable due to technical limitations or underlying comorbidities. Exclusion criteria included abnormal coagulation status or liver function (defined as prothrombin activity <40% and/or a platelet count of <40,000/mm3), poor performance status (Eastern Cooperative Oncology Group Performance Status rating of grade 2–4), presence of vascular invasion, metastatic disease, ICC equal or more than 5 cm in maximum diameter or more than three ICC lesions. Cholangiocarcinoma was staged according to the AJCC staging classification (8th edition, 2017) [Citation12] and only stage IA, IB and II ICCs were included. The diagnosis of ICC was based on histologic results of image-guided percutaneous needle biopsy performed during the first RFA procedure.
Radiofrequency ablation technique and follow-up
Radiofrequency ablation was performed with local or general anesthesia and under ultrasonographic guidance. A single or a cluster needle was used, depending on tumor size, location and depth. The overall duration of radiofrequency has been variable according to size and number of lesions (range 10–90 min) and radiofrequency current was emitted by a 200 W generator set to deliver maximum power with the automatic impedance control method. The ablation electrodes used included RITA XL/XLi electrodes (Angiodynamics, Latham, NY), LeVeen electrodes (Boston Scientific, Natick, Mass), and Valley Laboratory electrodes (Covidien, Mansfield, Mass). All ablations were performed according to the manufacturer’s protocol. All patients were given a prophylactic antibiotic (cefazolin 1 g or ampicillin 1 g) intravenously in order to decrease the risk of hepatic abscess or other infections. The procedure required a median hospitalization period of 3 days. A contrast-enhanced ultrasound (CEUS) examination was performed the day after the RFA and a computed-tomography (CT) scan 1 month later. Subsequently, the patients were examined with CEUS, enhanced CT or magnetic resonance imaging (MRI) every 3 months in the first 2 years after RFA and about every 6 months at 2 years after RFA. Patients who experienced local relapse and who were deemed treatable with RFA were treated with the same procedure; instead, patients who experienced extrahepatic recurrence (n = 7) or local recurrence in which RFA was not feasible (n = 3) received first-line chemotherapy. None of the patients underwent surgery after ICC relapse.
Definitions
We used the reporting standards of the Society of Interventional Radiology to define terminology and reporting criteria [Citation13]. Technical efficacy was defined as treatment of the tumor and complete replacement by ablation zones after assessment during the CT control 4 weeks after RFA. Local tumor progression-free survival (LTPFS) was defined as the time interval between initial RFA and the first radiographic evidence of local tumor progression. Overall survival (OS) was defined as the time between initial RFA and the patient’s death of any cause or the last follow-up.
Complications were graded as major or minor according to the standard terminology and reporting criteria in tumor ablation [Citation14]; major complications were defined as any event that resulted in increased level of care, including substantial morbidity or disability, or result in an extended hospital stay. All other complications were classified as minor. Any case of transient self-limiting low-grade fever, pain and general malaise was defined as post-ablation syndrome.
Statistical analysis
LTPFS and OS were estimated using the Kaplan-Meier method. Median follow up was calculated with reverse Kaplan-Meier method. ROC curve was used to find the best cutoff, using the status at 6 months (disease control vs progression) as state variable. Cox proportional hazard model was used to evaluate factors independently associated with LTPFS and OS. The normality assumption was verified with the Shapiro-Wilk test and log10-transformed when appropriate. Variables included in the final multivariate model were selected according to their clinical relevance and statistical significance in a univariate analysis (p ≤ .10). The multivariate model was designed using the backward stepwise method. Internal validation of the final multivariate model for LTPFS was performed on the ICI pooled cohort with a bootstrap sample procedure (n = 1000 samples). Performance of the final model was further quantified by the Harrell C index and validated with bootstrap resampling procedure to calculate bias corrected C index.
Results
We identified 29 patients treated with RFA (9 men and 20 women; mean age 63, range 35–84 years), which were eligible for inclusion and analysis in this study. In total, 117 lesions (35 lesions at first presentation, 62 local relapses and 20 intrahepatic recurrences) were treated with RFA.
Baseline patient and tumor characteristics are summarized in .
Table 1. Baseline characteristics 29 patients who underwent RFA from January 2014 to June 2019.
Technique effectiveness
Technique effectiveness was 92.3% (108/117) after the first CT scan performed 1 month after-RFA.
LTPFS analysis
Among all nodules, median LTPFS was 9.27 months (range: 7.34–11.15). LTPFS was correlated to lesion size (log10 transformed) both in univariate and multivariate analysis (HR 64.84, 95% CI 18.07–232.61, Supplementary Material). Harrel C index for the model was 0.698 (bias corrected C index 0.697).
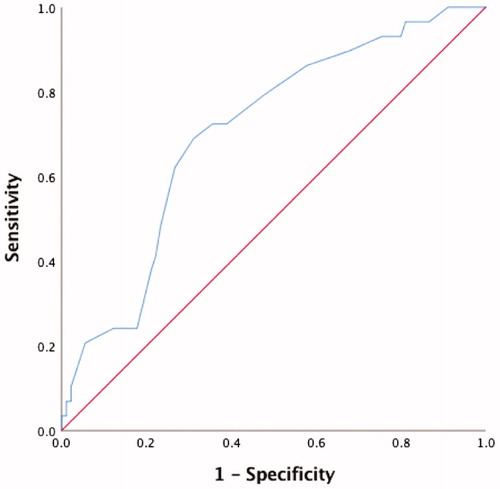
The ROC curve built on the 6 months LTPFS probability showed an AUC of 0.712 (95% CI 0.61–0.82) for the largest diameter of the nodule (p.001, ). Based on ROC curve we choose 20 mm as the best cutoff (sensitivity 69%, specificity 70%).
Figure 1. Receiver operating characteristic (ROC) curve on the 6 months Local Tumor Progression-Free Survival (LTPFS). Area under the curve (AUC) was 0.712 (95% CI 0.61–0.82) for the largest diameter of the nodule (p.001). Based on ROC curve we choose 20 mm as the best cutoff (sensitivity 69%, specificity 70%).
LTPFS was significantly longer in patients with tumor size less than 20 mm than in those equal or more than 20 mm (). Median LTPFS was 6.6 months (range 5.1–8.2) for lesions ≥20 mm and 12.9 months (range 11.5–14.2) in tumors <20 mm. Univariate and multivariate analysis showed that tumor size less than 20 mm (p <.0001) was an independent prognostic factor of LTPFS (HR 3.68; 95% CI 2.37–5.73; p < .001; ). The model was validated with a bootstrap resampling procedure that confirmed the association. C index for the correlation between tumor size ≥20 mm and PFS was 0.657 (bias corrected C index 0.652).
Figure 2. Local Tumor Progression-Free Survival (LTPFS) of tumors < 20 mm and ≥ 20 mm in diameter. Median LTPFS was 6.6 months (range 5.1–8.2) for lesions ≥ 20 mm and 12.9 months (range 11.5–14.2) in tumors < 20 mm.
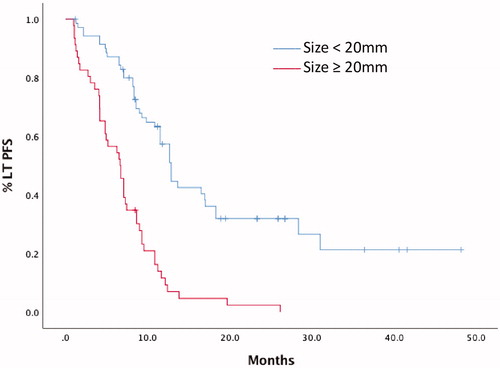
Table 2. Univariate and multivariate analysis for factors affecting LTPFS.
OS analysis
At a median follow up of 39.9 months, median OS from the date of RFA was 27.5 months (95% CI 24.3–30.6), with an OS of 89%, 45% and 11% at 1, 2 and 4 years (), respectively. ECOG – PS (p = .57), overall number of sessions of RFA (p = .19), Meld score (Model for End-Stage Liver Disease) more than 9 points (p = .54) and diameter of the biggest lesion at the first RFA (p = .21) did not significantly affect OS at univariate analysis (). On the contrary, number of lesions (p = .028) and the sum of their diameters at the first RFA (p = .04) significantly affected OS in multivariate analysis. Given the small sample size, we did not find a cutoff neither for the number of lesions or the sum of lesion diameters.
Figure 3. Overall Survival (OS) from the date of Radiofrequency Ablation (RFA) for all patients included.
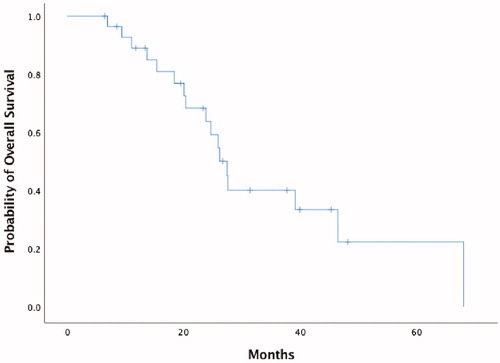
Table 3. Results of univariate and multivariate analysis for factors affecting OS.
Complications
Minor and major complication rates were 14% (16 of 117) and 7% (8 of 117), respectively. Major complications included liver abscess (n = 3), pleural effusion (n = 2), biloma (n = 2), intrahepatic hematoma (n = 1). The complications were successfully managed with percutaneous drainage and/or intravenous antibiotics. Four of the 29 patients experienced post-ablation syndrome that resolved without any treatment. Additional symptoms were alleviated after conservative treatment. No deaths occurred within 30 days after the procedure.
Discussion
Choosing the best therapeutic strategy in ICC is an increasing challenge worldwide as the diagnosis often carries extremely poor prognosis [Citation15]. While surgery still represents the cornerstone of ICC management, only one-third of tumors are amenable to surgical resection at the time of diagnosis; post-surgery recurrence remains high [Citation16,Citation17]. Unlike the majority of cancers, most ICC-related deaths are due to local disease progression rather than distant metastases, supporting the use of locoregional therapies and the efficacy of several non-surgical management strategies that have been widely investigated in the last 20 years [Citation18]. Since Slakey first described the use of RFA in ICC in 2002 [Citation18], few studies have investigated the use of RFA in ICC, where the outcomes have been less optimal than those observed in hepatocellular carcinoma [Citation19,Citation20]. However, RFA has recently emerged as a minimally invasive treatment option for hepatic malignancies and as an alternative to resection, especially for patients who have small lesions and/or are unfit for surgical resection [Citation21].
With regard to size, some authors consider RFA a useful technique to provide local control of small, localized, unresectable lesions; in patients with hepatocellular carcinomas (HCCs) ≤2 cm, for instance, RFA is considered an alternative to hepatic resection because of their comparable long-term efficacy [Citation22,Citation23]. However, the complete ablation rate achieved with RFA declines sharply in the case of larger lesions. In agreement with the previous findings after RFA in HCC [Citation24], tumor size seems to represent a basic parameter that impacts technique effectiveness in ICC. In two studies, Carrafiello [Citation25] and Kim [Citation26] suggested that RFA may provide local control in patients with tumors smaller than about 3.5 cm; in the first study Carrafiello et al., in particular, reported a technique effectiveness of 66%. This value is probably related to the small size of the case series (n = 6) which included two large tumors (> 5 cm). Fu et al. [Citation27] analyzed data from 17 patients with 26 nodules of ICC with a median nodule size of 3.8 cm; the early success rate after the first RF ablation at 1 month was 96.2%. In another retrospective analysis by Butros et al. [Citation28], technique effectiveness, defined as complete ablation with no evidence of residual tumor on the 1-month follow-up imaging study, was achieved in 89% of tumors (8/9 nodules). In our study, technique effectiveness was achieved for 108 of the 117 nodules (92.3%), with no procedure-related mortality, and our data were altogether comparable to those in literature. To our knowledge, our retrospective report is one of the largest studies on RFA in patients affected by ICC.
In our study the median LTPFS was 9.27 months, in line with previous reports of RFA in ICC. In agreement with other retrospective reports on a lower number of patients and nodules, our study showed that tumor size less than 20 mm was significantly associated with higher LTPFS. Therefore, our results suggested that 20 mm could be considered a threshold value to consider. In fact, univariate and multivariate analysis identified tumor size equal or more than 20 mm as prognostic of shorter LTPFS. Kim et al., on the other hand, examined 20 patients affected by 29 nodules of ICC and reported that tumors <15 mm size showed significantly longer LTPFS than tumors > 15 mm [Citation29]. In the same series of 20 patients, Kim et al. stated that the median time between RFA treatment and death was 27.4 months. In our series, a median overall survival of 27.5 months and a 1-, 2-, and 4-year survival rate of 89%, 45% and 11% from the time of RFA was achieved.
Given the minimal invasiveness with low morbidity and mortality rates and short hospital stays, RFA is considered a safe technique, with some remarkable exceptions. With regard to ICC, scarce data are available about the safety profile of RFA in this neoplasm. A recent meta-analysis and systematic review by Han [Citation30] evaluated seven studies on RFA in 84 nodules of ICC and major complications occurred in four studies. Kim et al. [Citation29] reported one liver abscess 1 month after the RFA session and the patient died of sepsis 3 months after RFA despite percutaneous drainage and antibiotics administration. In Fu’s study [Citation27], one patient developed symptomatic massive pleural effusion which was successfully managed with thoracentesis. In our study, we reported eight major complications (8/117, 7%); three patients developed liver abscess after RFA and the complication was successfully managed with percutaneous drainage and antibiotics. No deaths occurred within 30 days after the procedure.
Our study had notable limitations. First, it was a retrospective study and its nature precluded us from making strong statements regarding the prognostic and therapeutic implications of RFA in ICC. Second, the study included a widely varied patient population from a single institute and the total number of patients analyzed was relatively small. Another limitation was the lack of histopathologic evaluation to verify complete nodule destruction after RFA. Finally, the analysis of OS was burdened by several limitations, including the small number of overall patients and the inclusion of lesions at different stages (first presentation, local relapse and intrahepatic relapse).
Nevertheless, despite the presence of the limitations stated above, we believe our study can provide useful information on the role of RFA in ICC in terms of LTPFS and OS. Unfortunately, no level 1 data currently support the use of this loco-regional treatment in ICC and very few data are available in this context. We recognize the need for prospective randomized studies with larger samples in order to provide further information concerning RFA in ICC and to ensure different therapeutic options in this highly lethal and increasingly frequent disease.
Conclusions
In this retrospective study we evaluated the efficacy and safety of RFA in the treatment of patients affected by unresectable ICC. Although some limitations affected this study, the application of RFA led to a remarkable local tumor control with low post-procedure morbidity and no peri-interventional mortality. RFA for unresectable ICC resulted in a median LTPFS of 9.27 months and a median OS of 27.5 months after the procedure. Given the current lack of randomized studies, a key element in choosing the best therapeutic strategy in unresectable ICC would be to opt for a careful and individual decision-making, taking into account the benefits and risks of this effective and minimally invasive yet poorly studied technique.
Authors’ contributions
GB: Made substantial contributions to conception of the study, drafted the article was involved in revising the article critically for important intellectual content; AR: made substantial contributions to conception of the study, performed data analyses and drafted the article; FGD: performed data analyses, was involved in revising the article critically for important intellectual content and gave final approval of the version to be published; CF, GE and MC: critically revised the article; GF: collected data, made substantial contributions to conception of the study and revised the article; ST: performed data analyses and made substantial contributions to conception of the study; AP: collected data, helped to draft and critically revised the article; SDL, FA, VM and ADR: revised the article and performed revision of the literature; CS: was involved in revising the article critically for important intellectual content and gave final approval of the version to be published. All Authors critically revised the article, approved the final version to be published, and agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tariq NU, McNamara MG, Valle JW. Biliary tract cancers: current knowledge, clinical candidates and future challenges. CMAR. 2019;11:2623–2642.
- Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39(S1):19–31.
- Smittenaar CR, Petersen KA, Stewart K, et al. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;115(9):1147–1155.
- Njei B. Changing pattern of epidemiology in intrahepatic cholangiocarcinoma. Hepatology. 2014;60(3):1107–1108.
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–1289.
- Rizzo A, Frega G, Ricci AD, et al. Anti-EGFR monoclonal antibodies in advanced biliary tract cancer: a systemic review and meta-analysis. In Vivo. 2020;34(2):479–488.
- Adeva J, Sangro B, Salati M, et al. Medical treatment for cholangiocarcinoma. Liver Int. 2019;39(S1):123–142.
- Sommer CM, Kauczor HU, Pereira PL. Locoregional therapies of cholangiocarcinoma. Visc Med. 2016;32(6):414–420.
- Labib PL, Davidson BR, Sharma RA, et al. Locoregional therapies in cholangiocarcinoma. Hepat Oncol. 2017;4(4):99–109.
- De Baere T, Deschamps F, Briggs P, et al. Hepatic malignancies: percutaneous radiofrequency ablation during percutaneous portal or hepatic vein occlusion. Radiology. 2008;248(3):1056–1066.
- Haidu M, Dobrozemsky G, Schullian P, et al. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Intervent Radiol. 2012;35(5):1074–1082.
- Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol. 2018;7(5):52–52.
- Goldberg SN, Grassi CJ, Cardella JF, et al. Society of Interventional Radiology Technology Assessment Committee and the International Working Group on Image-Guided Tumor Ablation. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7):S377–S390.
- Ahmed M, Solbiati L, Brace CL, et al. Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. J Vasc Interv Radiol. 2014;25(11):1691–1705.e4.
- Dhanasekaran R, Hemming AW, Zendejas I, et al. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep. 2013; 29(4):1259–1267.
- Ercolani G, Vetrone G, Grazi GL, et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 2010;252(1):107–114.
- Chun YS, Javle M. Systemic and adjuvant therapies for intrahepatic cholangiocarcinoma. Cancer Control. 2017;24(3):1073274817729241.
- Slakey DP. Radiofrequency ablation of recurrent cholangiocarcinoma. Am Surg. 2002;68(4):395–397.
- Lee MW, Raman SS, Asvadi NH, et al. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: a 10-year intention-to-treat analysis. Hepatology. 2017;65(6):1979–1990.
- Kulik L, Heimbach JK, Zaiem F, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: a systematic review and meta-analysis. Hepatology. 2018;67(1):381–400.
- Sweeney J, Parikh N, El-Haddad G, et al. Ablation of intrahepatic cholangiocarcinoma. Semin Intervent Radiol. 2019;36(04):298–302.
- Cho YK, Kim JK, Kim MY, et al. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49(2):453–459.
- Serra C, Cucchetti A, Felicani C, et al. Assessment of radiofrequency ablation efficacy for hepatocellular carcinoma by histology and pre-transplant radiology. Liver Transpl. 2019;25(1):88–97.
- Magistri P, Tarantino G, Ballarin R, et al. The evolving role of local treatments for HCC in the third millennium. AR. 2017;37(2):389–401.
- Carrafiello G, Lagana D, Cotta E, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33(4):835–839.
- Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;196(2):W205–W209.
- Fu Y, Yang W, Wu W, et al. Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol. 2012;23(5):642–649.
- Butros SR, Shenoy-Bhangle A, Mueller PR, Arellano RS. Radiofrequency ablation of intrahepatic cholangiocarcinoma: feasability, local tumor control and long-term outcome. Clin Imaging. 2014;38(4):490–494.
- Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol. 2011;80(3):e221–e225.
- Han K, Ko HK, Kim KW, et al. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol. 2015;26(7):943–948.
