Abstract
Purpose
To evaluate the safety and effect of microwave ablation (MWA) compared with transcatheter arterial embolization (TAE) for the treatment of large hepatic hemangiomas.
Materials and methods
A total of 135 patients with symptomatic or/and enlarging hepatic hemangiomas (5–10 cm) from two centers underwent either MWA (n = 82) or TAE (n = 53) as first-line treatment. We compared the two groups in terms of radiologic response, clinical response, operative time, postoperative analgesic requirements, hospital stay and complications.
Results
MWA had a significantly higher rate of complete radiologic response (89.0% vs. 37.7%, p<.001) and complete clinical response (88.6% vs. 69.2%, p=.046), fewer minor complications (43.9% vs. 66.0%, p=.019), shorter time of using analgesics (p<.001) and shorter hospital stays (p=.003) than did TAE. The operative time and major complications were comparable between the two groups.
Conclusion
Both MWA and TAE are safe and effective in treating patients with large hepatic hemangiomas. MWA had a higher rate of complete response than did TAE, and it was associated with fewer minor complications, faster recovery and shorter hospital stay.
Introduction
Hepatic hemangioma is the most common benign tumor in the liver, with an incidence rate of up to 7.0%. It is often found incidentally on abdominal imaging performed for other indications [Citation1]. Although medical intervention is often not needed for the majority of hepatic hemangiomas, therapeutic interventions are indicated for large and rapidly growing hemangiomas, which may cause obvious symptoms [Citation2–4].
Traditionally, surgical resection (SR) is the standard treatment for symptomatic large hepatic hemangiomas [Citation3,Citation5]. Although effective, SR is associated with large trauma, substantial recovery time and morbidity [Citation4,Citation6]. With the development of minimally invasive procedures, transcatheter arterial embolization (TAE) [Citation7–16], radiofrequency ablation (RFA) [Citation17–23] and microwave ablation (MWA) [Citation24–26] have been used to treat hepatic hemangiomas. They have been proven effective and less invasive than surgery. However, to date, there is still a lack of research to evaluate the benefits or disadvantages of TAE compared with MWA for the management of hepatic hemangiomas. Therefore, we conducted this study to compare the clinical outcomes of TAE versus MWA for the treatment of large hemangiomas.
Methods
Patients
This retrospective study was performed at two institutions. Written informed consent was obtained from all patients before each treatment, and approval by the institutional review boards was waived.
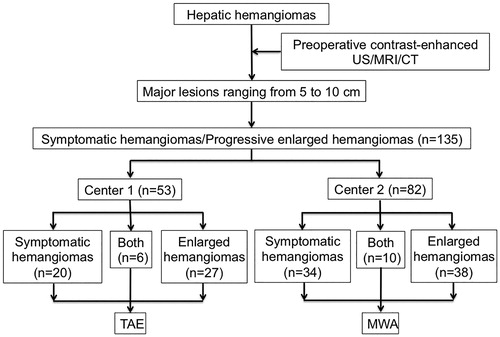
A total of 135 patients with large hepatic hemangioma (ranging from 5 to 10 cm) who received TAE (n = 53) or MWA (n = 82) as first-line therapy at the two centers between August 2010 and November 2018 were enrolled in this study. The diagnosis of hepatic hemangioma was based on two coincidental radiologic findings on contrast-enhanced ultrasound, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI), showing typical features: peripheral nodular enhancement followed by centripetal filling. The number, size and location of hemangiomas were recorded before treatment. The criteria for patient selection in the present study were the following: (a) single or multiple lesions, with a major lesion ranging from 5 to 10 cm; (b) patients had obvious symptoms related to a hemangioma (such as abdominal distension, abdominal pain, anorexia, nausea and belching) or had progressive enlargement of a hepatic hemangioma (enlarged more than 0.5 cm within 1 year); (c) liver function status of Child-Pugh class A; (d) prothrombin activity >40% and a platelet count of more than 40 × 109/L; (e) received MWA or TAE as first-line treatment; and (f) patients refused for SR. The flowchart of patient inclusion is shown in .
Ultrasound-guided percutaneous MWA procedure
MWA was performed using a 2,450-MHz MTC-3C microwave generator and 18-cm-long, 14-gauge internally cooled electrode (Vision Medical, Nanjing, China). Power output was set at 80–100 W. All patients had a percutaneous approach to ablation under general anesthesia, using ultrasound (MyLab 60; Esaote, Genoa, Italy) to identify lesions and monitor treatment effect. According to our protocol, one or two electrodes were inserted into the lesion parallel to each other with an inter-electrode distance of 2.5–3.0 cm in the same plane. The distal margin of the lesion was ablated first, and then the electrodes were withdrawn 1.5–2 cm for multipoint ablations. Multiple overlapping ablations were created until the targeted lesions were completely covered by the gasification zone on ultrasound. During the ablation, preventive measures (preoperative catheterization, a 20-mg furosemide intravenous injection, and a 125-mL sodium bicarbonate solution intravenous infusion when 50% of the hemangiomas had been ablated) were performed to prevent the incidence of acute kidney injury after October 2013.
TAE procedure
The femoral artery was punctured under local anesthesia using the Seldinger technique. After a celiac and superior mesenteric arteriography through a 5-French catheter (Cook, Bloomington, IN), the hepatic artery was catheterized. Hepatic arteriography was performed to confirm the arteries feeding the hemangiomas. Superselective cannulation of the feeding arteries was performed using a 3-French microcatheter (Microferret, Cook, Letchworth, UK). Then, the emulsion consisting of pingyangmycin (PYM, Zhejiang Hisun Pharmaceutical Co., Ltd., Taizhou City, China) and iodized oil (Lipiodol, Andre Gurbet, Aulnaysous-Bois, France Guerbet, France) was slowly injected for embolization under fluoroscopic guidance, followed by injection of gelatin sponge particles (1–2 mm in diameter) (Gelfoam1, Upjohn, Kalamazoo, MI), until the tumor-feeding arteries were saturated. The PLY-in-lipiodol emulsion was prepared by emulsifying 2 mL of 5% glucose solution with 8 mg of PYL in 20 mL of iodized oil. After embolization, angiography was performed to assess the extent of vascular embolization and identify the presence of any residual lesion staining.
Perioperative evaluation and postoperative follow-up
The perioperative evaluation included the operative time, liver function, time of analgesic use, hospital cost, hospital stay and procedure-related complication. Postoperative follow-up evaluation included radiologic response and clinical response. Radiologic response was assessed by contrast-enhanced CT or MRI and clinical response was assessed by patient symptoms. All patients underwent contrast-enhanced CT or MRI within 2 months after treatment to evaluate the treatment effect. Complete radiologic response was defined as no obvious enhancement lesions on the enhanced CT or MRI images. Incomplete radiologic response was identified as irregular, peripherally enhancing foci in the treated zone. For patients with complete response, subsequent CT or MRI examinations were repeated at 6-month intervals for 2 years. For patients with incomplete response, repeat treatment was considered if the residual lesion significantly progressed during the 6-months imaging follow-up interval. Complete clinical response was defined as disappearance of hemangioma-related symptoms, such as abdominal distension, abdominal pain, anorexia, nausea and belching. Incomplete clinical response was defined as partial relief of symptoms based on follow-up records. No clinical response was defined as persistent symptoms without remission.
Complications
Complications of treatment were described using the reporting standards of the Society of Interventional Radiology [Citation27]. A major complication was defined as an event that leads to substantial morbidity and disability that increases the level of care, results in hospital admission, or substantially lengthens the hospital stay. This includes any case in which a blood transfusion or interventional drainage procedure is required. All other complications were considered minor.
Statistical analysis
Continuous parameters were expressed as means ± standard deviation, while categorical variables were expressed as a number and percentage. Differences in the categorical data were analyzed using the χ2 test and linear by linear association. Continuous variables between groups were compared using Student’s t-test and analysis of variance. All statistical analyses were performed using the SPSS 22.0 statistical software (SPSS, Chicago, IL). Statistical significance was defined as a p value <.05.
Results
The baseline characteristics of patients are shown in , which shows that 82 patients received MWA, and 53 patients were treated with TAE. There was no significant difference in the baseline demographics and other clinical characteristics between the groups ().
Table 1. Baseline characteristics of patients between the two study groups.
Perioperative results
Both MWA and TAE were successfully performed in all patients. The perioperative data of the two groups are shown in . There was no significant difference in operation time between the two groups (p=.09). Comparison of one-day postoperative liver function shows that the average TB levels were comparable between the two groups (p=.25), but the levels of ALT (p<.001) and AST (p<.001) were significantly higher in MWA group than in the TAE group. The increased ALT and AST levels in both groups declined consistently to normal levels within 1 week. Patients undergoing MWA had a significantly shorter hospital stay (p=.003), shorter time of using analgesics (p<.001) and higher hospital cost (p<.001) when compared with the TAE group, as shown in .
Table 2. Perioperative data between two groups.
Radiologic and clinical response
Patients were followed for a period of 22.9 ± 3.4 months (ranging from 6 to 24 months) in the TAE group and 22.7 ± 3.5 months in the MWA group (ranging from 6 to 24 months). The first post-treatment follow-up CT or MRI showed a significant higher rate of complete radiologic response in the MWA group (73/82, 89.0%) than in the TAE group (20/53, 37.7%) (p<.001) (). Incomplete response was found in nine patients in the MWA group, including six patients with hemangiomas abutting the diaphragmatic dome and three patients with hemangiomas abutting the hepatic hilum. However, none of them needed to receive a second session of MWA. Of the 33 patients with incomplete response in the TAE group, eight patients received a second session of TAE, two patients received additional MWA, and the remaining 23 patients underwent follow-up without additional treatment. The last follow-up imaging showed that the mean maximal diameters of the treated tumors were 4.32 ± 1.45 cm in the MWA group and 4.75 ± 2.78 cm in the TAE group, both of which were significantly smaller than those seen in pretreatment images (p<.001) ( and ).
Figure 2. Pre-MWA (a) and one-year post-MWA and (b) CT examinations show a significant reduction in size of hemangioma after MWA treatment.
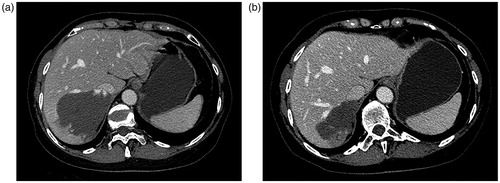
Figure 3. Pre-TAE (a) and one-year post-TAE and (b) CT examinations show a significant reduction in size of hemangioma after TAE treatment.
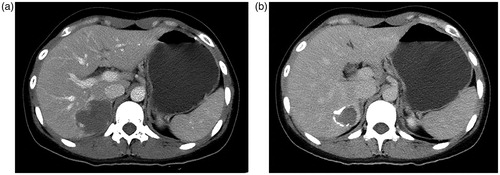
Table 3. Postoperative follow-up outcomes.
No significant difference was found in clinical response (including disappearance and improvement of symptoms) between the two groups (p=.14) (). However, the complete clinical response rate was significantly higher in the MWA group (88.6%) than in the TAE group (69.2%) (p=.046). In the TAE group, of the 26 patients who had hemangioma-related symptoms, complete resolution of symptoms was observed in 18 patients. Improvement of symptoms was observed in six patients and treatment was not required. Two patients complained of persistent abdominal pain requiring oral analgesics for pain control. In contrast, all 44 patients with symptomatic hepatic hemangiomas had either a complete resolution (n = 39) or improvement (n = 5) of their symptoms after MWA.
Complications
There were no procedure-related deaths in either of the two groups. The complications are shown in . Eight (9.8%) patients in the MWA group experienced major complications requiring specific treatment, including two patients with symptomatic pleural effusion treated by percutaneous drainage; one patient with diaphragmatic hernia treated by diaphragmatic repair; three patients with acute renal dysfunction, and two patients with jaundice treated by conservative therapy. In the TAE group, major complications were observed in six (11.3%) patients. A hepatic abscess was observed in one patient and it required percutaneous drainage and anti-infection therapy. Two patients experienced ischemic cholecystitis and fully recovered after anti-infection therapy. Three patients had jaundice and fully recovered after conservative therapy. There was no significant difference in major complication rates between the groups (p=.49). The incidence of minor complications and adverse events was significantly higher in the TAE group than in the MWA group (p=.019), as shown in .
Table 4. Complications after treatment.
Discussion
Our data showed that both MWA and TAE were safe and effective for treating large hepatic hemangiomas. Patients treated with MWA had a higher rate of complete response than those treated with TAE. In addition, MWA was associated with fewer minor complications, faster recovery and shorter hospital stays in comparison with TAE treatment.
Although SR is the most effective therapy for large hepatic hemangiomas, it is a highly invasive procedure and associated with morbidity and mortality rates of up to 31.3% and 3%, respectively [Citation4,Citation6,Citation28]. Thus, more minimally invasive treatments are needed as an alternative to surgical treatment. Several previous studies have emphasized the role of TAE as an alternative treatment for hemangiomas [Citation7–16,Citation29], which is less invasive than surgery. The PYM-lipiodol emulsion is considered to be one of the most widely used agents in TAE for hemangiomas. The mechanism of PYM-lipiodol emulsion in treating hepatic hemangiomas is the image-guided delivery of embolic and sclerosing agents into the blood sinuses, resulting in formation of microthrombi and finally causing atrophy and fibrosis of the hemangiomas. However, the treatment is not considered as curative because recurrence is common due to vascular recanalization [Citation14,Citation15]. Recently, energy-based techniques, such as RFA and MWA, which were historically used to treat hepatocellular carcinoma, have been used in treating hepatic hemangiomas [Citation17–26]. Our previous studies also have demonstrated the efficacy and safety of MWA as a new therapeutic modality for large hepatic hemangiomas [Citation26,Citation30,Citation31]. However, to the best of our knowledge, this is the first study to compare the technical and clinical outcomes of MWA and TAE in treating large hepatic hemangiomas.
In the present study, we found a significantly higher rate of both complete radiologic and complete clinical response in the MWA group than in the TAE group, with comparable major complications between the two groups. More importantly, of the incomplete-response patients, 10 in the TAE group needed an additional TAE or MWA procedure; in contrast, none of patients in the MWA group needed an additional medical intervention. In addition, MWA therapy was associated with fewer minor complications, faster recovery and shorter hospital stays than those for TAE treatment. In our experience, MWA treatment for large hepatic hemangiomas has several advantages compared with TAE. First, ultrasound-guided MWA is a more precise and controllable therapy for hepatic hemangiomas than is TAE. Second, a well-performed MWA for hepatic hemangioma leads to complete tumor necrosis, making it more efficient and effective than TAE. Third, unlike TAE, MWA is effective for both hypervascular and hypovascular hemangiomas. Thus, although the clinical response (complete and incomplete response) between the two groups was comparable, current results supported that MWA might become an alternative to TAE for therapy of large hepatic hemangiomas.
The main disadvantage of MWA for the treatment of large hepatic hemangiomas is hemolysis attributable to the generous blood supply of hepatic hemangiomas. Hemolysis can lead to various degrees of hemoglobinuria, hemolytic jaundice, anemia or even renal damage accordingly, depending on its severity. It is worth noting that intraoperative quick infusion followed by intravenous use of 125 mL of sodium bicarbonate solution and 20 mg of furosemide was effective for preventing acute renal dysfunction. In our study, three early patients who did not receive preventive measures experienced acute renal dysfunction. However, none of the later patients suffered acute renal dysfunction. Furthermore, to prevent injury to the surrounding organisms, such as the diaphragm, gastrointestinal tract, gall bladder and biliary tract, it is not necessary to pursue complete ablation of a hemangioma. In this study, all nine patients with incomplete ablation had tumors located abutting either the diaphragmatic dome or hepatic hilum. However, none of them need further treatment because of the small size and slow growth of the residual tissue.
The present study has some limitations. First, it is a retrospective study involving the experience of two centers, and the results may not be generalizable to patients in other centers. Second, the number of patients in this study is relatively small, which may limit the quality of this research. Further, prospective studies are required to confirm the findings of this study.
In conclusion, our results showed that both MWA and TAE appear to be safe and effective treatments in patients with large hepatic hemangiomas. However, MWA had a higher rate of complete response than did TAE, and MWA was associated with fewer minor complications, faster recovery and shorter hospital stays.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hasan HY, Hinshaw JL, Borman EJ, et al. Assessing normal growth of hepatic hemangiomas during long-term follow-up. JAMA Surg. 2014;149(12):1266–1271.
- Kumashiro Y, Kasahara M, Nomoto K, et al. Living donor liver transplantation for giant hepatic hemangioma with Kasabach–Merritt syndrome with a posterior segment graft. Liver Transpl. 2002;8(8):721–724.
- Lerner SM, Hiatt JR, Salamandra J, et al. Giant cavernous liver hemangiomas: effect of operative approach on outcome. Arch Surg. 2004;139(8):818–821.
- Miura JT, Amini A, Schmocker R, et al. Surgical management of hepatic hemangiomas: a multi-institutional experience. HPB (Oxford). 2014;16(10):924–928.
- Cheng WL, Qi YQ, Wang B, et al. Enucleation versus hepatectomy for giant hepatic haemangiomas: a meta-analysis. Ann R Coll Surg Engl. 2017;99(3):237–241.
- Zhang W, Huang ZY, Ke CS, et al. Surgical treatment of giant liver hemangioma larger than 10 cm: a single center's experience with 86 patients. Medicine (Baltimore). 2015;94(34):e1420.
- Deutsch GS, Yeh KA, Bates WB, et al. Embolization for management of hepatic hemangiomas. Am Surg. 2001;67(2):159–164.
- Giavroglou C, Economou H, Ioannidis I. Arterial embolization of giant hepatic hemangiomas. Cardiovasc Intervent Radiol. 2003;26(1):92–96.
- Szejnfeld D, Nunes TF, Fornazari VA, et al. Transcatheter arterial embolization for unresectable symptomatic giant hepatic hemangiomas: single-center experience using a lipiodol–ethanol mixture. Radiol Bras. 2015;48(3):154–157.
- Tarazov PG, Polysalov VN, Ryzhkov VK. Embolization of the hepatic artery in hemangiomas of the liver. Vopr Onkol. 1990;36(5):583–587.
- Zeng Q, Li Y, Chen Y, et al. Gigantic cavernous hemangioma of the liver treated by intra-arterial embolization with pingyangmycin-lipiodol emulsion: a multi-center study. Cardiovasc Intervent Radiol. 2004;27(5):481–485.
- Li Y, Jia Y, Li S, et al. Transarterial chemoembolization of giant liver haemangioma: a multi-center study with 836 cases. Cell Biochem Biophys. 2015;73(2):469–472.
- Cao X, He N, Sun J, et al. Interventional treatment of huge hepatic cavernous hemangioma. Chin Med J (Engl). 2000;113(10):927–929.
- Firouznia K, Ghanaati H, Alavian SM, et al. Management of liver hemangioma using trans-catheter arterial embolization. Hepat Mon. 2014;14(12):e25788.
- Sun JH, Nie CH, Zhang YL, et al. Transcatheter arterial embolization alone for giant hepatic hemangioma. PLoS One. 2015;10(8):e0135158.
- Ozden I, Poyanli A, Onal Y, et al. Superselective transarterial chemoembolization as an alternative to surgery in symptomatic/enlarging liver hemangiomas. World J Surg. 2017;41(11):2796–2803.
- Wen SQ, Wan M, Len KM, et al. Safety and efficacy of laparoscopic radiofrequency ablation for hepatic hemangiomas: a multicenter retrospective study. Ann Hepatol. 2018;17(2):268–273.
- Gao J, Ding X, Ke S, et al. Radiofrequency ablation in the treatment of large hepatic hemangiomas: a comparison of multitined and internally cooled electrodes. J Clin Gastroenterol. 2014;48(6):540–547.
- Park SY, Tak WY, Jung MK, et al. Symptomatic-enlarging hepatic hemangiomas are effectively treated by percutaneous ultrasonography-guided radiofrequency ablation. J Hepatol. 2011;54(3):559–565.
- Gao J, Ji JS, Ding XM, et al. Laparoscopic radiofrequency ablation for large subcapsular hepatic hemangiomas: technical and clinical outcomes. PLoS One. 2016;11(2):e0149755.
- Zhang X, Yan L, Li B, et al. Comparison of laparoscopic radiofrequency ablation versus open resection in the treatment of symptomatic-enlarging hepatic hemangiomas: a prospective study. Surg Endosc. 2016;30(2):756–763.
- Gao J, Ke S, Ding XM, et al. Radiofrequency ablation for large hepatic hemangiomas: initial experience and lessons. Surgery. 2013;153(1):78–85.
- Gao J, Fan RF, Yang JY, et al. Radiofrequency ablation for hepatic hemangiomas: a consensus from a Chinese panel of experts. World J Gastroenterol. 2017;23(39):7077–7086.
- Ziemlewicz TJ, Wells SA, Lubner MA, et al. Microwave ablation of giant hepatic cavernous hemangiomas. Cardiovasc Intervent Radiol. 2014;37(5):1299–1305.
- Liu F, Yu X, Cheng Z, et al. Risk factors for hemoglobinuria after ultrasonography-guided percutaneous microwave ablation for large hepatic cavernous hemangiomas. Oncotarget. 2018;9(39):25708–25713.
- Tang XY, Wang Z, Wang T, et al. Efficacy, safety and feasibility of ultrasound-guided percutaneous microwave ablation for large hepatic hemangioma. J Dig Dis. 2015;16(9):525–530.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202.
- Brouwers MA, Peeters PM, de Jong KP, et al. Surgical treatment of giant haemangioma of the liver. Br J Surg. 1997;84(3):314–316.
- Furumaya A, van Rosmalen BV, Takkenberg RB, et al. Transarterial (chemo-)embolization and lipiodolization for hepatic haemangioma. Cardiovasc Intervent Radiol. 2019;42(6):800–811.
- Wang Z, Tang X, Qi X, et al. Feasibility, safety, and efficacy of ultrasound-guided percutaneous microwave ablation for giant hepatic hemangioma. Int J Hyperthermia. 2018;35(1):246–252.
- Tang X, Ding M, Lu B, et al. Outcomes of ultrasound-guided percutaneous microwave ablation versus surgical resection for symptomatic large hepatic hemangiomas. Int J Hyperthermia. 2019;36(1):632–639.