?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
In this study, we developed a novel nitinol-actuated surgical instrument to conduct laparoscopic renal denervation for the treatment of resistant hypertension. We investigated whether shape and temperature settings of nitinol specimens fit well into the design goals. Furthermore, we conducted a pilot study to validate the mechanical and physiological performance of nerve ablation without damaging the renal artery.
Method
Tensile tests were performed to observe temperature-dependent thermomechanical properties and the original shape of nitinol specimens was set considering our design goal. We performed strain gage experiments to measure bending strain. We developed surgical instrument and operated laparoscopic renal denervation in a swine model. Subsequent impedance spectroscopy experiments were conducted to measure changes in impedance magnitudes during the operation and histological analyses were performed to visualize thermogenic damage to arteries and nerves.
Results
Tensile testing showed that the shape memory effect begins above 37 °C. Measured strains on nitinol surfaces were 2.10% ± 0.769%, below the strain limit of 8%. Impedance spectroscopy experiments showed decreases in magnitude in all six trials. After operation of laparoscopic renal denervation following the protocol, renal arteries and nerves were harvested and thermogenic damage was observed in nerves but not arteries.
Conclusion
We developed a novel nitinol-actuated surgical instrument with which to perform laparoscopic renal denervation. The feasibility of our device was verified using thermomechanical analyses of nitinol, and assessments of mechanical and physiological performance. Our device could be used in other laparoscopic procedures that require large degrees of freedom while restricting to trocar size.
Introduction
Hypertension (HTN) is the primary preventable cause of premature death, and was present in 1.39 billion people in 2010, accounting for 31.1% of all adults globally. HTN has become increasingly prevalent since 2000 [Citation1,Citation2]. Furthermore, approximately 10% of hypertensive patients suffer from resistant HTN, which is diagnosed as systolic/diastolic blood pressure (BP) ≥ 140/90 mmHg despite concurrent use of three or more antihypertensive medications, or four medications regardless of BP [Citation3,Citation4]. Patients with resistant HTN have a higher incidence of cardiovascular diseases, such as heart failure and atherothrombosis, and these are causes of cardiovascular morbidity and mortality [Citation5,Citation6] and chronic kidney disease [Citation7]. Moreover, resistant HTN is associated with target-organ damage in the heart, the kidneys, and the blood vessels [Citation8,Citation9]. Effective treatments for resistant HTN decrease the incidence of cardiovascular disease and improve physical and mental function [Citation10].
Among risk factors, sympathetic nervous system (SNS) overactivity has been identified as a main contributor to the initiation, maintenance, and progression of HTN, as indicated by increased adrenergic activity [Citation11–13]. Increased efferent renal sympathetic nerve activity inhibits renal excretion, increases renal renin secretion, and stimulates the renin-angiotensin-aldosterone system (RAAS) [Citation14]. Excitation of RAAS causes vasoconstriction and increases extracellular fluid volumes. These changes play critical roles in the pathophysiology of and resistant HTN [Citation11].
Since the mid-20th century, many attempts to treat resistant HTN have been performed by disrupting the SNS. Among radical interventions, thoracolumbar sympathectomy was reportedly effective in hypertensive patients, but was accompanied by high morbidity and mortality [Citation15,Citation16]. Thus, renal denervation (RDN) was proposed as an alternative intervention for attenuating renal sympathetic nerve overactivity, entailing selective ablation of nerves around renal arteries [Citation17]. Moreover, catheter-based RDN achieved targeted ablation of renal nerves. In these procedures, a catheter is inserted via a small incision and generates radiofrequency (RF) energy to cause thermal damage to renal nerves, leading to BP reductions in hypertensive patients [Citation18,Citation19]. Yet in blinded and randomized trials (SYMPLICITY HTN-3 trials) RDN failed to meet efficacy endpoints of BP reductions compared with a sham-procedure [Citation18,Citation20].
Failure in sham-controlled trials likely reflects incomplete and non-uniform denervation [Citation21], due to (1) lack of expertise in catheter-based renal denervation procedure [Citation21], (2) the presence of multiple or accessory renal arteries [Citation22,Citation23], (3) distortion of energy delivery [Citation24], and (4) the presence of nerves beyond the thermal lesion depth. RF energy from few ablation points can reach up to 2 mm from luminal surfaces [Citation25]. Considering one- to two-thirds of renal nerves are located beyond 2 mm from luminal surfaces [Citation26], these nerves most likely remained intact in these sham-controlled clinical trials [Citation27].
In our previous study, we presented a novel laparoscopic renal denervation system for the treatment of resistant HTN [Citation28]. In this feasibility study, we focused on (1) preventing tearing and physical damage to arteries, (2) targeting of RF energy inside the electrode to prevent thermogenic damage to other organs, (3) achieving sufficient thinness for insertion through a trocar to operate in laparoscopic procedures, and (4) accommodating various vessel sizes. Here we report further developments of this surgical instrument design and introduce a novel actuated nickel-titanium alloy (nitinol) wrap for blood vessels. This instrument meets the design goals outlined above because of the following features: (1) the ability to shape-set for safe actuation, (2) formation of a loop owing to high elastic limit, (3) increased degrees of freedom, (4) and the flexible printed circuit board (FPCB)-pulling method, which will be discussed later.
Nitinol is the most popular shape memory alloy and has distinctive nonlinear thermomechanical properties that are derived from reversible transformations between martensite and austenite phases. Heating of nitinol to above the austenite finish temperature Af can recover the residual strain from martensitic transformation below Af. This phenomenon is called the shape memory effect [Citation29]. After loading nitinol at temperatures above the Af, complete recovery of strain during the unloading process is called superelasticity [Citation29]. These properties of nitinol are suitable for our goals.
In addition to desirable mechanical properties, nitinol shows kink resistance, stress hysteresis, temperature dependence of stress, and fatigue resistance [Citation30–32]. Furthermore, in comparison with conventional materials (such as stainless steel), nitinol is biocompatible, is of relatively low cost, and has a relatively high recoverable strain limit of 8%. In comparison, stainless steel has a recoverable strain limit of only 1% [Citation33]. Crucially, nitinol facilitates laparoscopic approaches because it can be inserted into the body with a minimum profile and then changes its shape with body heat [Citation34]. To wrap renal arteries during ablation of surrounding renal nerves, nitinol can be deformed into a straight configuration at room temperature and recovers its preferred shape in the body. Furthermore, during laparoscopic surgery, restricted manipulation reduces the freedom for translation, axial rotation, and relative rotation. Deformation into the original shape at the distal tip can increase degrees of freedom [Citation35].
To address the four design requirements of laparoscopic surgical instruments for renal denervation, we verified nitinol’s temperature and shape setting by testing shape changes and tensile at three different temperatures. We then conducted strain gage (SG) experiments to show the formation of a loop at the nitinol tip that can be used to focusing RF energy inside. After assembling the surgical instrument with manufactured nitinol specimens, we performed impedance spectroscopy experiments to determine whether the device can pull FPCB by squeezing an artery and thereby accommodate various vessel sizes. Subsequently, we operated the laparoscopic renal denervation instrument in a swine model and verified safe separation after the laparoscopic procedure. Finally, we analyzed staining results showing that our device deactivates renal nerves even after the survival period.
Principle of operation
Principle of operation
Our concept of laparoscopic renal denervation features two sequential stages of denervation: (1) surgical denervation during dissection of a renal artery when the surgeon inserts the device beneath an artery and (2) thermal denervation of nerves distributed close to the artery and beyond the surgeon’s view. Thermal ablation plays a significant role in deactivating nerves that remain intact during the surgical procedure while preventing physical damage to the artery. To denervate renal sympathetic nerves around the artery using a laparoscopic approach, complete coverage of renal arteries is necessary to induce thermal lesions onto nerves. Therefore, our electrosurgical device was designed:
To safely wrap a renal artery.
To ablate renal sympathetic nerves.
To accommodate a variety of artery diameters.
To be safely removed after denervation.
To achieve these goals, electrode-pulling operations with nitinol were operated in four sequential processes. First, nitinol was inserted in-vivo between a renal artery and a vein. As the body heats the nitinol to around 37 °C, partial austenitic transformation allows nitinol to bend slightly (). When RF-generated heat is then conveyed into nitinol to a temperature of 50 °C, nitinol self-wraps around the artery. Hence, nitinol serves as an actuator while parallel electrodes are attached within the FPCB to generate heat, which is the main energy source. The diameter of the recovered nitinol was larger than that of the renal artery (). The FPCB was then pulled to narrow the gap between the FPCB and the artery, which was filled with saline solution or air. This mechanism for gently wrapping the artery with FPCB can be adapted to a range of artery sizes, covering all possible dimensions (). Finally, RF-generated-heat was used to ablate renal nerves, thus achieving renal denervation. After surgery, arteries were separated from the device through a slight gap in the nitinol. Nitinol shape setting and manufacturing processes are discussed below.
Figure 1. Principle of operation. (a) Nitinol slightly bends to be inserted between a renal artery and vein due to in-vivo temperature. (b) RF generated heat on parallel copper-coated-gold electrodes is conducted to nitinol and reach the superelastic phase to fully recover one loop. (c) By pulling FPCB, the electrode adjusts the diameter of a renal artery.
We avoid direct contact of nitinol with the artery primarily to prevent injury to the artery. Nitinol is rigid and could penetrate the artery, whereas FPCB is too flexible to damage arteries. Furthermore, the diameter of the FPCB can increase or decrease easily with pushing and pulling, whereas the loop diameter of nitinol can only change during shape setting at high temperatures.
Materials and methods
Nitinol manufacturing process
A 70-mm nitinol plate with a thickness of 0.3 mm and a shape-set 5-mm loop at the tip was manufactured (Fort Wayne Metals, Fort Wayne, IN, USA). With a nitinol width of 4 mm, the laparoscopic container could be inserted through a standard 5-mm port. Af was set around 50 °C so that the original shape could be partially recovered by biological heat and fully recovered by additional RF-generated-heat. The chemical components required for the present temperature settings are listed in .
Table 1. The chemical composition of used nitinol specimens.
The original shape in the high temperature parent phase was set by heating constrained nitinol, with respect to our design requirements. One loop was considered an ideal shape, but because separation after renal denervation must be performed without tearing the artery, the nitinol tip did not form a full loop. Because diameters of main and branching swine renal arteries were 5.4 ± 0.6 and 3.8 ± 0.5 mm, respectively [Citation36], two semicircles with diameters of 10.8 and 5.4 mm were chosen for the original shape.
Heating of constrained nitinol at 500 °C for 5 min can change its original shape [Citation37,Citation38]. Therefore, nitinol was fixed and heated at 500 °C using a heat gun (D26411, DeWalt, Towson, MD, USA). The heat treatment time was 5 min and the airflow speed was 450 L/min. After water quenching, nitinol was removed from the fixture.
Mechanical analysis
Tensile tests
To investigate thermomechanical behaviors of nitinol, universal tensile tests were conducted using a universal testing machine with a heat chamber (WL2100; Withlab, Gunpo, Gyeonggi-do, Korea). Tensile tests were performed at 27 °C (room temperature), 37 °C (in vivo temperature), and 50 °C (shape-recovery temperature). For each of the three temperature settings, three nitinol specimens were tested. Therefore, total of nine nitinol specimens were used. Nitinol specimens were held with a film-type grip, and were extended from the initial length of 30 mm to a maximum strain of 6%, and were then compressed back to the strain-free state. Strain rates in each process were kept constant at 10−3/s [Citation39].
Stainless steel is often used in medical device development, but has different mechanical behaviors from those of nitinol. Nitinol has much higher elasticity than stainless steel and is characterized by hysteresis [Citation30,Citation32]. To compare thermomechanical properties of nitinol and stainless steel in our former surgical instrument design, nine stainless steel specimens with the same geometric specifications as nitinol were manufactured using wire-cutting electrical discharge machining (EDM; ACE-W535, Daewoo Heavy Industries Co., Korea), and universal tensile testing was performed following the same protocol as for nitinol.
Strain gauge measurements
Shape memory alloys are mostly used in cardiovascular and orthopedic applications. For cardiovascular applications, shape memory alloys contribute to the prevention of pulmonary embolism using manufactured self-expanding stents and atrial septal occlusion devices. Shape memory alloys are used as spinal vertebra spacers and orthopedic staples in orthopedic applications [Citation40]. Given that nitinol requires higher deformation properties for our purposes than for other purposes, we performed the strain analysis. In the martensitic phase (relatively low temperature), nitinol can be deformed to approximately 8% of strain without inducing irreversible or permanent residual strain. To ensure that nitinol remained under maximum strain during deformation, we performed SG experiments.
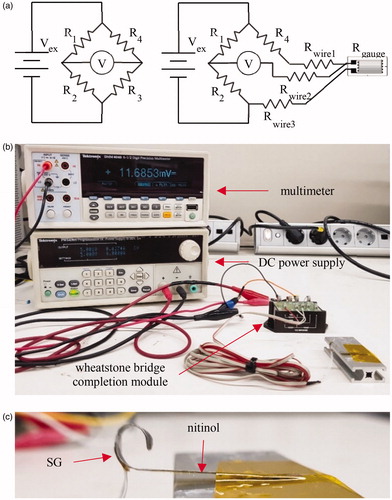
In strain tests, three-wire, pre-wired, and quarter-bridge SGs (KFH-06-120-D16-11L1M2S, Omega Engineering, Stamford, CT, USA) were attached over the bending surface of nitinol using cyanoacrylate adhesive (Z70, HBM, Darmstadt, Germany). Following excitation from a DC power supply (PWS4305, Tektronix, Beaverton, OR, USA) at 5 V, which is below the module’s maximum excitation voltage of 10 V, output voltage was measured using a 6-1/2 digit precision multimeter (DMM-4040, Tektronix, Beaverton, OR, USA). To detect small strain in the order of a few milli-strain, many SG circuit configurations include a Wheatstone bridge. Therefore, a SG, a power supply, and a multimeter were connected to a Wheatstone bridge completion module (BCM-1, Omega Engineering, Stamford, CT, USA) using screw terminals ().
Development of the surgical instrument
Our electrosurgical device comprises an electrosurgical tip and an actuator, as shown in .
Figure 3. (a) Design of the entire electrosurgical device, which is composed of the electrosurgical tip and actuator. (b) The ISO view and (c) side view of the electrosurgical tip model and (d) thermocouple probe located between nitinol and polyimide film, indicated by a red arrow. The shape transformation of nitinol at (e) 27 °C (room temperature), (f) 37 °C (in-vivo temperature), and (g) 50 °C (RF-generated-heat applied), and (h) after pulling FPCB, in which tube with a diameter of 4 mm is inserted to imitate a renal artery. For rearward manipulation of the electrosurgical tip, (f) tip extrusion lever, of which operation to the left extrudes the electrosurgical tip, and vice versa, and FPCB pulling lever, of which operation to the left pulls FPCB, and vice versa, were used. In actual operation, tip extrusion lever is pushed to the left, which is then fixed by lock, and the FPCB pulling lever is pulled to the right, i.e., shown in .
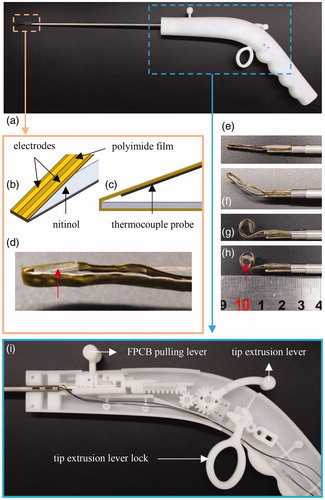
The electrosurgical tip bends renal arteries to ablate surrounding renal nerves. Parallel copper-coated-gold electrodes of 50-µm thickness and 0.8-mm width with a gap of 2 mm were attached over a 25-µm thick polyimide film. This structure is referred to as a FPCB. Furthermore, a 50-mm long polyimide film was extended forward from the front end and was attached to the opposite nitinol surface, thus securing the connected polyimide film with electrodes during pulling of the FPCB (). A T-type thermocouple probe (TT-T-40-SLE-100, Omega Engineering, Stamford, CT, USA) was then fixed behind the FPCB to measure the temperature of the arterial wall over the FPCB (). Shape transformation of the electrosurgical tip in a gradual temperature rise is illustrated ().
The actuator at the rear manipulates the extrusion of the electrosurgical tip and pulling of the FPCB. Simple back-and-forth manipulation of the tip extrusion lever, which was connected to the tip via a rack-and-pinion gear, retracted and extruded the electrosurgical tip at the front end. After forming a complete nitinol loop, the FPCB pulling lever was manipulated to the right to allow contact with the cylinder (). The gap between the FPCB and the silicone tube was covered by pulling the FPCB (). All components were printed using a Project MJP 2500 (3 D Systems, Rock Hill, SC, USA) 3 D printer.
A pilot study in a swine survival model
In-vivo evaluation in a swine survival model
A female pig of 41 kg was injected preoperatively with 0.06–mL/kg Tardomyocel comp. III antibiotic, and were sedated using intramuscular injections of zoletil (5 mg/kg) and xylazine (2 mg/kg). Preoxygenation and tracheal intubation were performed using a facial mask, and the depth of anesthesia was maintained under 2.0%–2.5% isoflurane with normoxia (SpO2, 98%) and normocapnia (EtCO2, 38–40 mmHg). Intravenous infusions of normal saline were used to maintain central venous pressure between 6 and 8 mmHg.
Three-port laparoscopy was performed to alleviate future complications. After incising the retroperitoneum, renal denervation was performed on the left renal artery in the right lateral recumbent position, and the pig’s posture was then changed to conduct renal denervation on the opposite side. All used surgical instruments were sterilized with ethylene oxide gas before surgical operation.
Single day intravenous Cefazolin was used as a postoperative antibiotic, at a dosage of 15 mg/kg three times a day. Meloxicam as an analgesic was administered as single intramuscular injection at a dosage of 0.4 mg/kg body weight only during general anesthesia, but not after anesthesia.
On the 1st/3rd/7th day, and 1st month after the procedure, blood tests were performed on the jugular vein to check for inflammation, infection, renal function, electrolyte abnormalities and other general conditions. The pig was raised in a metallic cage at 22 °C ± 2 and provided with drinking water at anytime. To reduce the stress of pig during rearing, we also provided toys for pig to promote its welfare and ensure animal rights.
After seven weeks of survival, open surgery was performed to conduct impedance spectroscopy measurements and harvest tissues for assessments of the safety of laparoscopic renal denervation.
Impedance spectroscopy
In general, impedance spectroscopy measures the impedance spectrum over a defined frequency range, which is used to calculate the electrical properties of the material [Citation41]. Impedance spectroscopy was utilized to unveil the electrical characteristics of blood vessels by attaching electrodes [Citation42]. Here, we used impedance spectroscopy to evaluate contact of the FPCB with the artery through changes in nitinol shapes and pulling of the FPCB. During an open survival surgery before euthanasia using one swine model, our surgical instrument was actuated on the left and right femoral arteries. The femoral artery whose diameter is comparable to the renal artery [Citation43] was used for the impedance experiment, which might cause physical damage to the artery and surrounding nerves during the experiment. We chose the femoral artery for the impedance experiment to preserve the renal artery which will be used for the histology later. Impedance spectroscopy experiments were performed by (1) inserting the instrument beneath an artery and measuring impedance (), (2) inducing shape recovery and measuring impedance (), and (3) pulling the FPCB until it contacts the artery and measuring impedance (). All measurements were conducted ten times in the impedance range of 10 kHz–3 MHz. A total of 201 sampling points were assessed using an impedance analyzer (E4990A, Keysight Technologies, USA), a function generator (AFG 3101, Tektronix, OR, USA), a RF power amplifier (LZY- 22+, Mini-Circuits, NY, USA), two bi-directional couplers (ZABDC50-150HP+, Mini-circuits, NY, USA), two power sensors (U2004A, Keysight Technologies, USA), a vector network analyzer (E5061B, Keysight Technologies, USA), and a RF switch (USB-4SPDT-A18, Mini-circuits, NY, USA). All instruments and temperature controls were manipulated independently using a computer with a customized control software program (LabVIEW, National Instruments, TX, USA). The RF frequency used to generate heat at the second step of the protocol, that is, inducing shape recovery using RF-generated heat, was 1 MHz.
Figure 4. EIS was conducted on (a) left swine femoral artery of which diameter was 3 mm. Experiment followed the protocol of (b) to insert the instrument beneath an artery and measure impedance, (c) to induce shape recovery and measure impedance, and (d) to pull FPCB until it contacts an artery and measure impedance (thermocouple probe was tightened during pulling the FPCB).
An anesthesia protocol identical to that in the preclinical study discussed in (1) In-vivo Evaluation in a Swine Survival Model was used. After six weeks of the survival experiment, renal arteries and their connective tissues were harvested and then euthanasia was conducted. All animal experiments were approved by the Institutional Animal Care and Use Committee at Seoul National University Hospital (SNUH-IACUC) and animals were maintained in the facility accredited by AAALAC International (#001169) in accordance with Guide for the Care and Use of Laboratory Animals 8th edition, NRC (2010).
Histological analysis
All renal arteries, including ablated segments, were fixed in 4% formalin, were paraffin-embedded, and were sectioned at a thickness of 1 mm. For each section, four serial slides with a thickness of 4-μm were cut at 200-μm intervals. Sections were scanned using a Leica SCN400F (Leica Microsystems Inc., Buffalo Grove, IL, USA) microscope.
Hematoxylin and Eosin (H&E) and Masson’s trichrome (MT) staining were used to visualize arterial and nerve damage. Nerve functions were further analyzed using immunohistochemical staining with a mouse monoclonal anti-tyrosine hydroxylase antibody (TH; Accurate Chemical, Westbury, NY). The TH antibody stains renal sympathetic nerves and is frequently used in analyses of nerve regeneration [Citation44]. Scores of one to four were assigned from TH staining results [Citation45].
Statistical analysis
All statistical analyses were performed using the SPSS v25 software (IBM, NY, USA).
Results
Nitinol shape transformation
Bending of nitinol during heating was recorded at 27 °C (room temperature), 37 °C (in-vivo temperature), and 50 °C (the shape-recovery temperature), respectively (). The temperature was precisely controlled using a refrigerated and heated bath circulator (RW3-P, Jeio Tech, Korea).
Figure 5. Shape of nitinol at (a) 27 °C (room temperature), (b) 37 °C (in vivo temperature), and (c) 50 °C (shape-recovery temperature).

Nitinol of desired chemical composition achieved a gradual shape change with increased temperature. Because shape recovery did not occur at room temperature, the nitinol specimen remained in the desired flat shape. At in-vivo and shape-recovery temperatures, we observed partial and complete shape-recovery, respectively.
Mechanical analysis
Tensile tests
Considering reductions in cross sectional areas during tension and compression, true stress σT and strain εT were calculated as follows:
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4)
where
[Pa] is engineering stress,
[N] is load,
[m2] is initial cross sectional area,
[mm/mm] is engineering strain,
[mm] is initial length, and
[mm] is length of change during tensile tests. At the three different temperatures, true stress-strain curves were plotted for nitinol and stainless steel specimens with the same geometry (). Slipping of the grip occurred during testing of one nitinol specimen at 50 °C, and the plot was excluded due to distortion.
Figure 6. True stress-strain curve of (a) stainless steel and (b) nitinol at three different temperatures; 27 °C (room temperature), 37 °C (in-vivo temperature), and 50 °C (shape-recovery temperature).
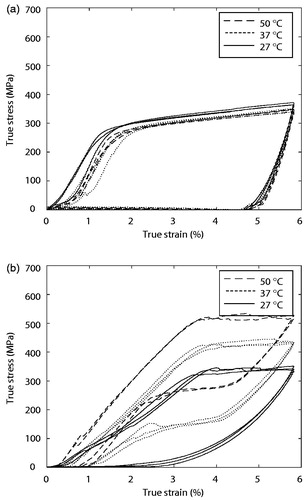
Austenitic moduli EA [MPa], martensitic moduli EM [Mpa], upper plateau strength (UPS) [Mpa], lower plateau strength (LPS) [MPa], and residual elongation Elr [mm/mm] were derived from the plot. Features were calculated using ASTM standard F2516-07ϵ. EM is elastic modulus during loading, EA is elastic modulus during unloading, UPS is the stress at 3% strain during loading, LPS is the stress at 2.5% strain during unloading, and Elr is the difference between strain levels at a stress of 7 MPa [Citation46]. lists the measurements of five thermomechanical properties at the three characteristic temperatures of stainless steel specimens, while for nitinol specimens.
Table 2. Mean and s.d. of mechanical properties of stainless steel in three different temperatures.
Table 3. Mean and s.d. of mechanical properties of nitinol in three different temperatures.
Unpaired and two-tailed t-tests were performed to compare thermomechanical properties within and between and . For nitinol specimens, all parameters showed significant change according to temperature change (p < .05), except for EM (p > 0.05 when temperature increased from 25 °C to 50 °C) and Elr (p > .05 when temperature increased from 37 °C to 50 °C). For stainless steel specimens, whose LPS analysis was excluded due to the absence of a lower plateau for their temperature independence, no parameters changed significantly (p > .05) except for UPS (p < .05 when temperature increased from 37 °C to 50 °C). Furthermore, for comparison of five parameters between stainless steel specimens and nitinol specimens, all indicators exhibited significant change, except for EM at 27 °C and 37 °C.
Regardless of temperature, UPS of stainless steel specimens was 263 ± 32.8 Mpa and Elr was 4.78 ± 0.08%. The thermomechanical properties of nitinol specimens were much more dynamic. Both UPS and LPS values increased upon heating, indicating increased stiffness of nitinol with increasing temperature. To achieve increasing UPS, EA increased also. Furthermore, Elr drastically decreased from 2.027% to 0.5522% and 0.5629%, indicating that the deformation was almost recovered at temperatures above 37 °C.
Strain gauge measurements
Strain is recorded as the change in length relative to the original length, and bending strain is expressed as follows:
(5)
(5)
where
is displacement from neutral axis,
is bending radius, and
is strain at
SGs are commonly used to measure material strains. In these measurements, the SG is closely attached to the test material, and the relationship between changes in resistance and strain are recorded. The ensuing gage factor is defined as follows:
(6)
(6)
where
[Ω] is the original resistance of SG,
[Ω] is the change in resistance of SG,
[mm] is original length of SG,
[mm] is the change in length of SG, and
[mm/mm] is the strain of SG or test specimen. Voltage outputs of the Wheatstone bridge are calculated as follows:
(7)
(7)
The terms
and
[Ω] are denoted in and
[V] is excitation voltage.
Because is equal to half of the thickness (0.15 mm) and
is equal to the bending radius (2.7 mm), the theoretical bending strain calculated from (5) was 5.56%. Furthermore, the measured voltage across the Wheatstone bridge was converted to change in resistance of SG using (7), and strain was then calculated using (6) with a gauge factor of 1.51. Three nitinol specimens were tested, which were utilized to assemble surgical instruments later, and the average and standard deviation of ten repetitive measurements were 2.10% ± 0.769%.
A pilot study in a swine survival model
Laparoscopic renal denervation
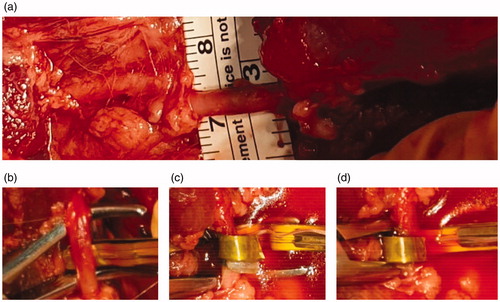
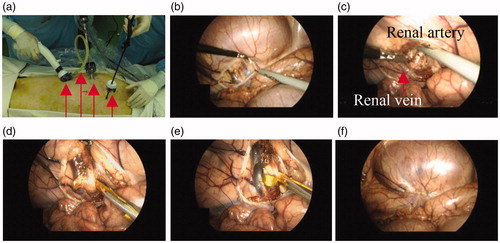
The laparoscopic renal denervation procedure was conducted in pigs using the developed surgical instrument, and the feasibility of nitinol-actuated laparoscopic renal denervation was assessed. Anesthetized pigs were initially placed in the right lateral position for denervation of nerves around the left artery. Four trocars were inserted into the abdomen to aid access of laparoscopic instruments (). Images were captured using a laparoscopic camera during surgery (). A total of four denervation procedures were performed on the left, involving proximal and distal points of the main and branch artery. A target temperature of 65 °C and duration of 70 s were used. After changing the posture of the animal and switching the electrosurgical instrument to another one, two additional denervation procedures were conducted on the right and a total of six points in the entire procedure were ablated. The retroperitoneum was then incised to approach the renal hilum, and the renal artery, around which nerves were to be ablated, was dissected from the renal vein. The electrosurgical device was inserted between the renal artery and the renal vein and RF energy was applied along the parallel electrodes to induce shape changes in the nitinol. After renal denervation, the retroperitoneum was sutured to alleviate future complications. Owing to an incomplete loop that nitinol recovers, a flexible artery could be removed from the device through a small gap. If the artery was too big to be removed through the gap, laparoscopic instruments such as a grasper were additionally utilized to widen the gap. This is possible because nitinol is cooled down after denervation and the strength of nitinol specimens decreases.
Figure 7. Images captured via laparoscopic camera during surgery. (a) Four trocars were used, of which red arrows indicate electrosurgical instrument, CO2 injection tube, laparoscopic camera, and grasper, from left, respectively. (b) Incision of a retroperitoneum. (c) A renal artery, of which surrounding nerves would be ablated, and a renal vein were dissected. (d) The electrosurgical device was inserted between a renal artery and a renal vein and (e) RF energy was applied along the parallel electrodes, which induced Nitinol shape transformation. After RDN, (f) the retroperitoneum was sutured to alleviate complications.
Impedance spectroscopy
Impedance spectroscopy was performed in three steps during wrapping of a swine femoral artery, which has a similar diameter to swine renal arteries. Diameters were measured using a medical paper ruler and were 3 mm for the left femoral artery () and 4 mm for the right femoral artery. Excluding the unusual impedance at 10 kHz, we plotted magnitudes and phases at 200 different frequencies ().
Figure 8. Magnitude of impedance measured on (a) left femoral artery, (b) left femoral artery again, and (c) right femoral artery in threefold steps. (d) The magnitude was averaged over all frequency spectrum (n = 200). The whiskers represent the standard deviation. A paired t-test was conducted and symbol *** indicates the significant difference between two subsequent steps (p < .001). (LT1: first trial on left femoral artery, LT2: second trial on left femoral artery, and RT: trial on right femoral artery).
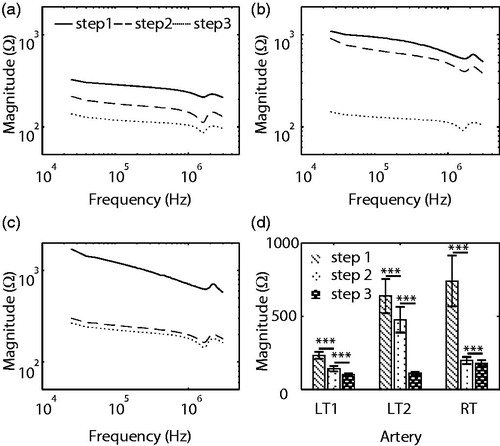
Using two-tailed and paired t-tests at each frequency (n = 200), we determined changes in magnitudes in three trials (). As manipulations proceeded from steps 1 to 2 and 2 to 3, magnitudes decreased in all six cases (p < .001).
Histological analysis
After 42 days of survival, renal arteries, including connective tissues and renal nerves, were harvested just before euthanasia. Although arterial damage was mild in MT staining, significant nerve damage was observed in treated areas (). Furthermore, digestion chambers, indicators of thermal damage, were observed ().
Figure 9. Masson’s trichrome (MT), anti-tyrosine hydroxylase antibody (TH), and Hematoxylin and Eosin (H&E) stain results from renal arteries harvested in 42 days from laparoscopic renal denervation. MT staining slide of (a) treated and (b) non-treated renal artery section (bars = 1 mm), and magnified slide of (c) treated and (d) non-treated area, of which damaged nerves are indicated by arrows (bars = 200 μm). TH stain of (e) treated renal artery section, where arrows indicate the score 1 stains, and (f) non-treated renal artery section, where arrows indicate the score 3 stains (bars = 500 μm). (g) Comparison of the degree of TH staining between two groups (n = 36). The whiskers represent the standard deviation. An unpaired t-test was conducted. (p < .001). H&E stain of renal artery slide from (f) treated zone, where n and digestion chambers are observed (bar = 200 μm).
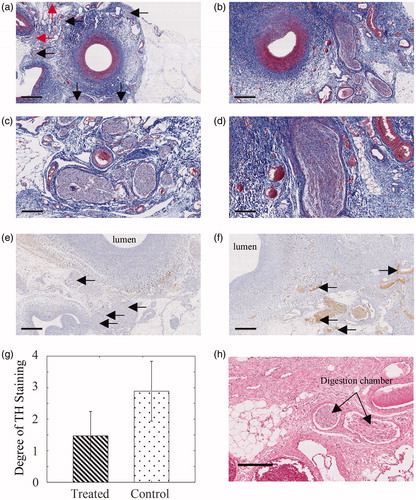
TH staining of two neighboring slides was assessed using scores of 1–4. The arrows in indicate staining scores of 1, and the arrows in indicate staining scores of 3. A total of 36 nerves in order of size on a treatment slide were selected, with the control group consisting of 36 nerves at neighboring slides. Owing to the wide gap between two neighboring slides, nerves in each group could not be matched with the naked eye and two-tailed and unpaired t-tests were performed. Degrees of TH staining were 1.47 ± 2.89 for the treated group and 0.774 ± 0.950 for the control group, with a significant difference between them (p < .001, ).
Discussion
We developed a surgical instrument for laparoscopic renal denervation. To perform actuation at the surgical tip, we manufactured nitinol specimens to meet our design goals. Because these nitinol specimens do not undergo shape recovery and remain in the desired configuration at room temperature, surgical instruments with nitinol tips can move freely inside a trocar. When inserted into body, partial shape recovery governs the direction at which nitinol bends, thus preventing the hazards of bending in the opposite direction or physical damage. Finally, when the temperature reaches 50 °C, complete shape recovery is achieved and the device wraps around the artery. This discrete shape change under the user’s temperature control could become an important technique in laparoscopic procedures. Laparoscopic procedures involving sophisticated actuation require large degrees of freedom. In addition, surgical instruments that increase degrees of freedom through the use of numerous components can become cumbersome, thus hampering trocar insertion. In contrast, nitinol can be inserted into the trocar before shape recovery and can then perform complicated actuation in situ without other components. Depending on the required procedure, the shape recovered by the nitinol specimen can be modified by shape setting. For example, if denervation on a large blood vessel is required, a large loop can be shape-set.
Our bipolar configuration of electrodes creates an electric circuit through an artery so nerves along electrodes are subject to thermal injuries. We intentionally shape-set the original configuration of an incomplete loop. It was an inevitable choice to ensure that the device is removed safely after denervation, which was considered more critical than that heat is distributed less at exposed parts. But considering the actuation of bipolar electrodes maximizes the temperature between the electrodes [Citation47], it can be predicted that heat is concentrated at the arterial wall between parallel electrodes Our tensile tests showed that the temperature setting of the nitinol specimen matched our requirements well. Specifically, the appearance and completion of the shape memory effect occurred at 37 °C and 50 °C. Statistical analyses of thermomechanical properties showed the temperature dependence of nitinol specimens as well as the temperature independence of stainless steel specimens. Exceptional cases of EM and Elr of the nitinol and UPS of the stainless steel could be mainly attributed to initial elongation independent of temperature, equal residual elongation above Af the temperature shape recovery begins, and slipperiness at initial elongation, respectively.
At 50 °C, dramatic increases in UPS and LPS values indicate hardening at higher temperatures. Elr values also returned to nearly 0% at temperatures above 37 °C, thus relieving the residual strain through the so-called shape memory effect. In contrast, the mechanical properties of stainless steel specimens were merely influenced by temperature. Moreover, the timing of the shape memory effect can be controlled with nitinol, offering benefits over materials with temperature-independent properties, such as stainless steel.
SG experiments revealed congruence of theoretical and experimental bending strains, and both were below 8%, which is the elastic limit of nitinol. Hence, nitinol specimens are stable and do not suffer permanent deformation, and unpredicted or partial shape recovery will rarely occur during surgery. Considering that human arteries are larger than swine arteries, the required bending strain will be less, allowing increased stability in clinical trials. In addition, according to (5), nitinol specimens with thicknesses of 0.3 mm can stably wrap blood vessels with a minimum radius of 1.875 mm.
Contact between electrode and arteries is a significant issue in renal denervation procedures, because RF energy is delivered to the contact zone under these conditions. Moreover, partial contact might cause uneven energy distributions. Impedance spectroscopy experiments showed a trend toward contact between electrodes and arteries during our manipulations, as indicated by decreased impedance in all six cases. Although the relationships between contact ratios and impedance have not been shown, a declining trend could be used to monitor contact during surgery.
The present histological analyses show structural and functional damage to nerves following our procedures, as visualized using TH and H&E staining, respectively. Because damaged nerves were uniformly distributed, we conclude that our device can damage renal nerves in most directions from the artery. Moreover, some of these nerves were located beyond 2 mm [Citation25] from the lumen, and this distance is considered the thermal lesion depth of catheter-based renal denervation. Nonetheless, our device ensures ablation beyond 2 mm, where red arrows in indicate damaged nerves.
The primary purpose of our study was to show the feasibility of the nitinol-actuated surgical instrument to conduct laparoscopic renal denervation. As the pilot, this study included one swine while nitinol specimens were repeatedly tested for six denervation procedures to assess the suitability of using the nitinol to accomplish our design goals. The results of the in vitro experiment and in vivo experiment demonstrated that nitinol-actuated laparoscopic renal denervation is possible in swine, which can be applied to humans with modification of the shape setting. In addition, our novel concept to wrap various sizes of blood vessels can be applied to surgical treatments other than renal denervation, such as thoracic sympathectomy.
However, the sample size of our study was insufficient to assure physiological effects of this surgical instrument. Further analyses of clinical effectiveness are planned with BP monitoring in a large-scale survival model. We suggest that laparoscopic renal denervation could be conducted by focusing energy inward inside the loop, thereby deactivating renal sympathetic nerves.
Conclusion
We propose a novel laparoscopic renal denervation procedure for the treatment of resistant HTN. We tested shape and temperature settings of our device to ensure that it meets the requirements. Moreover, mechanical experiments showed that nitinol specimens work stably under activation and have thermomechanical properties at the desired temperatures. Hence, our nitinol based surgical instrument was utilized in the preclinical study. Moreover, impedance spectroscopy experiments showed a trend toward contact between electrodes and arteries during manipulations. Finally, we performed laparoscopic renal denervation and confirmed thermogenic damage to nerves but not arteries in histological analyses. Additional research validating physiological effectiveness with BP and hormone monitoring is further required using our developed device, of which concept can be applied to surgical treatment of autonomic nervous disorders.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bloch MJ. Worldwide prevalence of hypertension exceeds 1.3 billion. J Am Soc Hypertens. 2016;10(10):753–754.
- Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–450.
- Rossignol P, Massy ZA, Azizi M, et al. The double challenge of resistant hypertension and chronic kidney disease. The Lancet. 2015;386(10003):1588–1598.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510–e526.
- Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635–1642.
- Kumbhani DJ, Steg PG, Cannon CP, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013;34(16):1204–1214.
- Wolley MJ, Stowasser M. Resistant hypertension and chronic kidney disease: a dangerous liaison. Curr Hypertens Rep. 2016;18(5):36.
- Gupta AK, Nasothimiou EG, Chang CL, et al. Baseline predictors of resistant hypertension in the Anglo-Scandinavian Cardiac Outcome Trial (ASCOT): a risk score to identify those at high-risk. J Hypertens. 2011;29(10):2004–2013.
- Liu JE, Roman MJ, Pini R, et al. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med. 1999;131(8):564–572.
- Bunker JP. The role of medical care in contributing to health improvements within societies. Int J Epidemiol. 2001;30(6):1260–1263.
- Tsioufis C, Kordalis A, Flessas D, et al. Pathophysiology of resistant hypertension: the role of sympathetic nervous system. Int J Hypertens. 2011;2011:642416.
- Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54(4):690–697.
- Papademetriou V, Doumas M, Tsioufis K. Renal sympathetic denervation for the treatment of difficult-to-control or resistant hypertension. Int J Hypertens. 2011;2011:1–8.
- Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 Suppl B):9–20.
- Morrissey D, Brookes V, Cooke W. Sympathectomy in the treatment of hypertension review of 122 cases. The Lancet. 1953;261(6757):403–408.
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152(16):1501–1504.
- Dibona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298(2):R245–R253.
- Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–1401.
- Lüscher TF, Mahfoud F. Renal nerve ablation after SYMPLICITY HTN-3: confused at the higher level? Oxford: Oxford University Press; 2014.
- Flack JM, Bhatt DL, Kandzari DE, et al. An analysis of the blood pressure and safety outcomes to renal denervation in African Americans and Non-African Americans in the SYMPLICITY HTN-3 trial. J Am Soc Hypertens. 2015;9(10):769–779.
- Esler M. Illusions of truths in the Symplicity HTN-3 trial: generic design strengths but neuroscience failings. J Am Soc Hypertens. 2014;8(8):593–598.
- Verloop WL, Vink EE, Spiering W, et al. Renal denervation in multiple renal arteries. Eur J Clin Invest. 2014;44(8):728–735.
- Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC: Cardiovasc Interv. 2013;6(10):1085–1091.
- Esler M. Renal denervation: not as easy as it looks. Sci Transl Med. 2015;7(285):285fs18
- Vink EE, Goldschmeding R, Vink A, et al. Limited destruction of renal nerves after catheter-based renal denervation: results of a human case study. Nephrol Dial Transplant. 2014;29(8):1608–1610.
- Choe W-S, Song WH, Jeong CW, et al. Anatomic conformation of renal sympathetic nerve fibers in living human tissues. Sci Rep. 2019;9(1):4831.
- Henegar JR, Zhang Y, Hata C, et al. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens. 2015;28(7):909–914.
- Ye E, Baik J, Lee S, et al. Design and simulation of novel laparoscopic renal denervation system: a feasibility study. Int J Hyperthermia. 2018;35(1):9–18.
- Sun QP, Hwang KC. Micromechanics modelling for the constitutive behavior of polycrystalline shape memory alloys—I. Derivation of general relations. J Mech Phys Solids. 1993;41(1):1–17.
- Stoeckel D. Nitinol medical devices and implants. Minim Invasiv Ther Allied Techno. 2000;9(2):81–88.
- Duerig T, Pelton A, Stöckel D. The utility of superelasticity in medicine. Biomed Mater Eng. 1996;6(4):255–266.
- Morgan N. Medical shape memory alloy applications—the market and its products. Mat Sci Eng-A. 2004;378(1–2):16–23.
- Liu J, Hall B, Frecker M, et al. editors. Compliant articulation structure using superelastic nitinol. ASME 2012 Conference on Smart Materials, Adaptive Structures and Intelligent Systems; 2013. American Society of Mechanical Engineers Digital Collection.
- Stoeckel D, Melzer A. The use of Ni-Ti alloys for surgical instruments. In: Vincenzini P, editor. Materials in clinical applications. Mirandola: Techna Srl; 1995. p. 791.
- Cuschieri A, Buess G, Périssat J. Operative manual of Endoscopic surgery 2. Springer Science & Business Media; 2013.
- Sakaoka A, Koshimizu M, Nakamura S, et al. Quantitative angiographic anatomy of the renal arteries and adjacent aorta in the swine for preclinical studies of intravascular catheterization devices. Exp Anim. 2018;67(2):291–0125.
- Marchand C, Heim F, Durand B, et al. Nitinol stent for percutaneous heart valve implantation: Material shape setting. Mater Manuf Processes. 2011;26(2):181–187.
- Gilbert HB, Webster RJ. IIIRapid, reliable shape setting of superelastic nitinol for prototyping robots. IEEE Robot Autom Lett. 2016;1(1):98–105.
- Nemat-Nasser S, Guo W-G. Superelastic and cyclic response of NiTi SMA at various strain rates and temperatures. Mech Mater. 2006;38(5–6):463–474.
- Machado L, Savi M. Medical applications of shape memory alloys. Braz J Med Biol Res. 2003;36(6):683–691.
- Macdonald JR. Impedance spectroscopy. Vol. 41. New York: Wiley New York; 1987.
- Packard RRS, Luo Y, Abiri P, et al. 3-D electrochemical impedance spectroscopy mapping of arteries to detect metabolically active but angiographically invisible atherosclerotic lesions. Theranostics. 2017;7(9):2431–2442.
- Sindram D, Martin K, Meadows JP, et al. Collagen–elastin ratio predicts burst pressure of arterial seals created using a bipolar vessel sealing device in a porcine model. Surg Endosc. 2011;25(8):2604–2612.
- Carriel V, Garzón I, Alaminos M, et al. Histological assessment in peripheral nerve tissue engineering. Neural Regen Res. 2014;9(18):1657–1660.
- Sakakura K, Ladich E, Edelman ER, et al. Methodological standardization for the pre-clinical evaluation of renal sympathetic denervation. JACC Cardiovasc Interv. 2014;7(10):1184–1193.
- ASTM, editor. Standard test method for tension testing of nickel-titanium superelastic materials. West Conshohocken: ASTM; 2008.
- Ma H, Su Y, Nathan A. Cell constant studies of bipolar and tetrapolar electrode systems for impedance measurement. Sens Actuators, B. 2015;221:1264–1270.