Abstract
Laser interstitial thermal therapy (LITT) is a minimally invasive therapy that have been used for brain tumors, epilepsy, chronic pain, and other spine pathologies. This therapy is performed under imaging and stereotactic guidance to precisely direct the probe and ablate the area of interest using real-time magnetic resonance (MR) thermography. LITT has gained popularity as a treatment for glioma because of its minimally invasive nature, small skin incision, repeatability, shorter hospital stay, and the possibility of receiving adjuvant therapy shortly after surgery instead of several weeks as required after open surgical resection. Several reports have demonstrated the usefulness of LITT in the treatment of newly-diagnosed and recurrent gliomas. In this review, we will summarize the recent evidence of this therapy in the field of glioma surgery and the future perspectives of the use of LITT combined with other treatment strategies for this devastating disease.
KEYWORDS:
Introduction
Laser interstitial thermal therapy (LITT) is a minimally invasive therapy that have been used for brain tumors, epilepsy, chronic pain, and other spine pathologies [Citation1–4]. This therapy is performed under imaging and stereotactic guidance to precisely direct the probe and ablate the area of interest under real-time MR thermography [Citation5]. This MR sequence detects the differential temperature-specific proton resonance frequency in water molecules and therefore provides the ability to predict the lesion extent by measuring the change in temperature over time [Citation5]. LITT is particularly useful for deep-seated tumors having difficult accessibility for open surgical approaches. Nonetheless, some reports suggest that hyperthermia induced by LITT may have synergistic effects with ionizing radiation or may disrupt the blood-brain barrier (BBB) facilitating the delivery of chemotherapy [Citation6,Citation7].
The mechanism of tumor tissue damage by LITT is related to the temperature and the time the tissue is exposed to that temperature. Glioma cells are particularly sensitive to heat damage due to the local hypoxia and the acidic environment compared with the surrounding normal brain tissue [Citation8]. The first area immediately surrounding the LITT probe can reach temperatures as high as 60 °C that would cause coagulative necrosis in the core of the ablated area. For instance, with temperatures in the range between 50 °C and 80 °C for less than 10-min there is tumor necrosis by means of protein denaturation. In the second more external area, immediately surrounding the first area, there is still permanent damage of the cancerous tissue associated with interstitial fluid. Finally, there is also a third marginal area that represents viable brain tissue with edema [Citation9]. After the ablation, this marginal area would increase in size during the first 2 weeks because of the presence of central liquefactive necrosis associated with peripheral edema [Citation9]. The appearance on the MRI of the central zone consist on T1 and T2 hyper and hypointensity respectively and this is correlated with damage of cellular membranes of the tumor tissue, hemoglobin degradation, and leakage from the red blood cells that gives the T1 hyperintense appearance. On the other hand, the peripheral zone demonstrates the typical appearance of vasogenic edema with T1 hypointense and T2 hyperintense areas. This area correlates with necrotizing edema and is associated with BBB disruption and therefore contrast enhancement in the MRI. Surrounding these two areas, there is an area of perilesional edema that contains viable nervous tissue. Over time the central core of the ablated area will eventually be replaced with granulation tissue that in turn will cause lesion shrinkage [Citation9–11]. This is correlated with the T1 hyperintense area begin to decrease in size and the T1 peripheral hypointense area increases concentrically. At the same time the ring-enhancement at the periphery could decrease with some residual enhancement that can be present years after the ablation. Finally, the perilesional edema usually resolves between 2 weeks and 2 months after ablation [Citation12–14].
LITT in gliomas
High grade gliomas (HGG), WHO grade III and IV, are the most frequent primary malignant brain tumors with an annual incidence of 3–4 per 100,000 population [Citation15]. Glioblastoma (GBM) has a dismal prognosis with a 14–16 month median survival after gross-total resection, chemotherapy, and fractionated radiation [Citation16]. Anaplastic astrocytoma, on the other hand, has a slightly better prognosis than GBM with a 3.5 year median survival [Citation16]. Overall survival (OS) will depend on several factors including Karnofsky performance status (KPS), age, tumor extension, neurological deficit, molecular or genetic markers, extent of resection (EOR), and chemoradiation treatment [Citation17,Citation18]. It is established that one of the modifiable factors for survival in HGG is the EOR but also the residual tumor volume left after surgery [Citation19–21]. Therefore, the current standard of care involves a multidisciplinary approach including maximal safe tumor cyto-reduction with subsequent chemotherapy and radiation [Citation22]. There are several surgical techniques that would improve the EOR while making the surgery safer by minimizing morbidity secondary to neurological deficits when eloquent or difficult-to-reach areas are involved [Citation23–25]. These techniques include awake mapping, intraoperative MRI, functional MRI and/or tractography, parafasicular technique, and use of 5-aminolevulenic acid, among others. However, in some cases, HGG are located in brain regions that subject the patient to an unacceptable risk of morbidity if open surgical resection is performed even with the abovementioned strategies. Even with maximal resections and secondary treatments, according to the current standard-of-care, HGG will eventually recur and at that point the prognosis is even poorer with a 5–15% 6-month progression-free survival (PFS) [Citation26,Citation27]. LITT has gained popularity in these instances because of its minimally invasive nature and other advantages including small skin incision, shorter hospital stay, and the ability to receive adjuvant shortly after surgery instead of several weeks as required after open surgical resection. Several reports have demonstrated the usefulness of LITT in the treatment of newly-diagnosed and recurrent gliomas in adults and also for pediatric populations ().
Table 1. Summary of the studies evaluating the use of LITT in patients with gliomas [Citation13,Citation28–53].
LITT in recurrent high-grade gliomas
HGG will eventually recur and not all patients will be eligible for surgical treatment at that time. It has been suggested that the median survival for GBM at first progression is between 30 and 39 weeks [Citation54]. There are different treatment strategies for patients with recurrent HGG including chemotherapy (lomustine, bevacizumab, temozolomide, carmustine wafers), radiotherapy, stereotactic radiosurgery, immunotherapy, high-dose brachytherapy, and reoperation [Citation55]. Some studies provide evidence that EOR is also correlated with survival in patients with recurrent HGG [Citation56]. Surgical resection for recurrence may provide benefit in terms of survival but the risk of complications is higher than for newly-diagnosed patients and is even higher for resection at second recurrence [Citation28,Citation57]. Several factors have to be taken into account when considering surgical resection such as whether the tumor is in or near eloquent areas, performance status, and subependymal infiltration among others. These factors can potentially prevent the patient from undergoing surgical treatment or other oncological treatments. Therefore, as a minimally invasive treatment, LITT can provide a safe and effective cytoreductive option for patients with recurrent HGG [Citation39]. Furthermore, LITT can also offer the ability to treat recurrent tumors in deep or eloquent areas that would be considered inoperable for open surgery [Citation47]. As previously stated, there are unique advantages of LITT in the setting of recurrent HGG in comparison with open surgery. These include smaller incisions that would lead to fewer wound complications, shorter recovery time and therefore possibility of oncological treatment in the early postoperative period. All these and other advantages make LITT particularly useful for patients with recurrent HGG. Despite this, there are some limitations or disadvantages of LITT including the potential of post-procedure edema, thermal injury of eloquent areas or major blood vessels, and also larger tumors may require multiple trajectories.
Schwarzmaier et al. reported two patients with recurrent GBM treated with LITT with OS of 13 and 15 months [Citation58]. Later on these authors reported 16 patients with recurrent GBM treated with LITT and a median OS of 11.6 months was achieved after LITT [Citation48]. Moreover, Carpentier et al. reported median PFS and OS times of 30 days and 10 months respectively in 4 patients with recurrent GBM treated with LITT [Citation49]. Sloan et al. published the first human phase I study that used escalating dose of hyperthermia to assess the safety and efficacy of the procedure in patients with recurrent HGG [Citation39]. This multi-institutional study included 11 patients and was carried out using the NeuroBlate System (Monteris Medical, Inc., Plymouth, MN) with a follow-up for a minimum of six months or until death. These authors assessed three escalating thermal damage thresholds (TDT) as follows: First, the yellow TDT line (equivalent to heating of tissues to 43 °C for 2 min); second, the blue TDT line (43 °C for 10 min); and third, the white TDT line (43 °C for 60 min). Initially three patients were treated with the first threshold and followed for 14 days to assess for signs of toxicity. If two out of three patients developed signs of toxicity, no further dose escalation was performed. If no toxicity was observed, the dose of treatment was escalated to the second and third TDT sequentially. The median OS was 10.5 months after LITT which was increased compared to historic controls by 3–9 months [Citation59,Citation60]. These findings demonstrated that LITT was a feasible and safe treatment modality for recurrent HGG. Later, Mohammadi et al. published a retrospective multicenter study including 34 patients with recurrent and newly-diagnosed HGG treated with the NeuroBlate System. They reported a one-year survival rate of 68% and a median PFS of 5.1 months. In this study, it was found that the extent of tumor coverage is as important as the EOR in open surgery with the caveat that this may not be the case in recurrent HGG. The recurrence pattern of the ablated tumors is similar of what we have seen in open resection with most recurrences within the treatment field or in the immediate periphery [Citation47].
LITT in newly-diagnosed high-grade gliomas
Traditionally, the standard treatment for patients with newly-diagnosed HGG is maximal safe resection followed by chemoradiation. In some cases, this standard strategy, cannot be carried out when eloquent or difficult-to-access areas are involved by the tumor because of an unacceptable risk of morbidity. In those cases, the standard of care include biopsy followed by chemoradiation and this is associated with a clear disadvantage in regards of tumor cytoreduction that would be provided by surgical resection. LITT has shown to be a safe and feasible cytoreductive therapy in patients with recurrent HGG. Furthermore, in newly-diagnosed HGG, LITT may provide comparable outcomes to open surgery. A recent metanalysis assessed the use of LITT for newly-diagnosed HGG [Citation2]. Four articles were identified where 25 patients were treated with LITT for newly diagnosed HGG. The results of this meta-analysis were comparable to open surgery in terms of OS for selected patients. The rate of complications was also similar to open surgery [Citation2,Citation61,Citation62]. Therefore, LITT was demonstrated to be a safe and effective strategy for newly diagnosed HGG achieving outcomes similar to cases with open surgical resection. Thomas et al. reported the outcome of eight patients with newly diagnosed HGG located in the insula, deep nuclei, and other difficult-to-access locations. These patients did not achieve tumor response in imaging surveillance after LITT procedure and therefore, the findings of this study did not clarify the role of LITT in newly-diagnosed difficult-to-access HGG [Citation28]. Conversely, Mohammadi et al. demonstrated an improved disease-specific OS and PFS in patients treated with upfront LITT followed by chemoradiation compared with a propensity score-matched control group based on age, gender, tumor location, and tumor volume. This group was treated with biopsy-only followed by chemoradiation. These authors also demonstrated that the extent of ablation is an independent predictor of disease-specific OS and PFS. In this study, tumor progression was defined as per the response assessment in neurooncology (RANO) criterion for HGG. Also, disease-specific OS was analyzed using competing risks methods where death from reasons other than tumor progression was considered a competing risk [Citation50]. An illustrative case from our experience demonstrating a newly diagnosed GBM treated by LITT and its 7 years follow up is demonstrated in and .
Figure 1. Illustrative case: 48 years old male admitted with complaints of cognitive decline and dizziness. Intracranial imaging showed a left paracentral lobule lesion where frozen section biopsy demonstrated glioblastoma and the patient underwent concurrent LITT. The patient received adjuvant treatment with radiation and temozolomide. Subsequently, the patient received monthly temozolomide maintenance doses. After 6 cycles, the patient returned back to work and reported no seizures or side effects. Overall, the patient completed 22 cycles of temozolomide and then treatment was discontinued. At the end of 6 years, MRI showed near total resolution of the lesion with minimal residual edema. The figure shows MRI images with T1 axial contrast-enhanced, T1 coronal contrast-enhanced and flair axial images from preoperative period to 36th month.
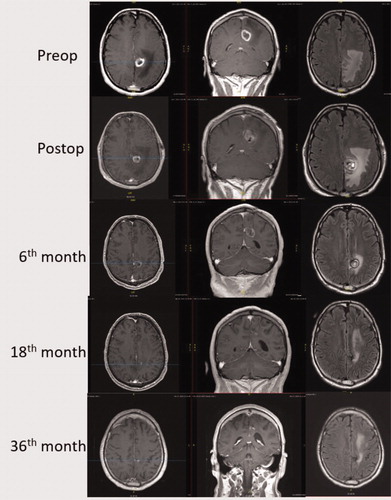
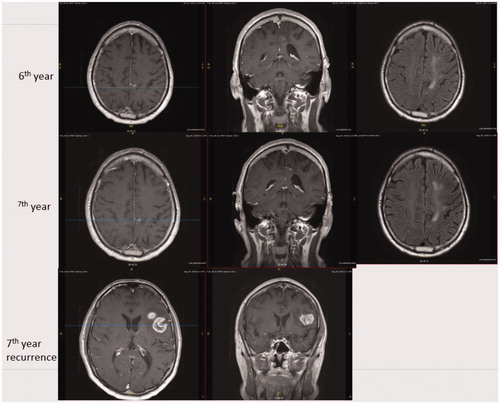
Figure 2. 40 months after completing temozolomide, a heterogeneously contrast-enhanced lesion was found at the left operculum. The patient was right-handed and his left hemisphere was determined to be dominant for speech using functional MRI. A stereotactic biopsy was performed and GBM was confirmed. The patient was enrolled in one of our clinical trials “A Randomized Phase 2 Open Label Study of Nivolumab plus Standard Dose Bevacizumab versus Nivolumab plus Low Dose Bevacizumab”. He since has had disease progression and placed back on temozolomide. The figure shows MRI images with T1 axial contrast enhanced, T1 coronal contrast enhanced and flair axial images from 6th year to recurrence.
LITT in difficult to access high-grade gliomas
LITT is a particularly useful technique for difficult-to-access HGG since these tumors are usually not amenable for open surgery. These tumors include corpus callosum, thalamus, basal ganglia, limbic lobes, or small tumors located deep in the white matter; this can also include tumors located in eloquent areas [Citation13]. In order to consider open surgery for these tumors, the surgical plan would include long and narrow surgical corridors that could pose a significant risk of injury to a great deal of normal brain tissue. This would in turn be associated with unacceptable neurological complications that would worsens the performance status and quality of life of these patients. Therefore, in the majority of these cases, patients would only be considered for biopsy and subsequent chemoradiotherapy. With the advent of LITT, these kind of tumors can be cytoreduced while minimizing the amount of normal brain tissue manipulation and with a rate of complications comparable to open surgery for accessible HGG [Citation1]. At the same time, chemoradiotherapy treatment (CRT) can be administered in the early postoperative period as it is usually done in biopsy cases. Mohammadi et al. showed that upfront treatment with LITT followed by CRT is associated with a better disease-specific OS and PFS than biopsy followed by CRT [Citation50]. These authors also demonstrated that extent of ablation, defined by the TDT lines, is an independent predictor of improved disease-specific OS and PFS. In an earlier study, the same group investigated the use of laser for difficult to access HGG. This study included patients with HGG treated with LITT as upfront or as salvage therapy [Citation47]. It was again demonstrated that complete coverage of the lesion by the TDT lines correlated with better PFS. As expected, smaller tumors were more likely to have complete coverage with the TDT lines. This evidence demonstrates that LITT is an effective treatment modality for newly diagnosed and recurrent HGG and that complete coverage of the tumor is as important as gross total resection in open surgery.
HGGs of the corpus callosum are associated with poor survival because of the inability to obtain a meaningful extent of resection. In a recent study, Beaumont et al. demonstrated that treatment with LITT in patients with HGG in the corpus callosum has comparable median survival of 7 months after LITT when compared with surgical series with ∼65% extent of resection. In this study, patients with larger tumors (>15 cm3) were 6 times more likely to experience a complication and based on this, the authors recommended to consider LITT for patients with 15 cm3 as a volume threshold [Citation51]. As previously demonstrated by others, this study also highlights the importance of tumor coverage for maximizing the survival benefit. Shah et al. showed, in a small case series, that LITT could equal the results of open surgical resection for selected patients with deep inaccessible gliomas that would otherwise be offered a stereotactic biopsy [Citation52]. Based on this pooled evidence, LITT seems to be a reasonably effective option for patients with deep-inaccessible or eloquent regions tumors, ideally less than 2.5–3 cm diameter. Similar to surgical series, neurosurgeons should be able to ablate at least 78–80% of the tumor in order to achieve a meaningful survival benefit.
Special imaging modalities in LITT
When HGG are located in eloquent areas the risk for significant neurological deficits in most cases is unacceptably high. Awake craniotomy, or surgical resection coupled with navigation, functional MRI, or tractography can minimize the risk of postoperative deficits. Furthermore, the use of intraoperative imaging can enhance the safety of the procedure by providing an updated information on the locations of residual tumor and the structures at risk. Still, there are some instance where the tumor is located in or near eloquent regions and the surgical resection or even the surgical corridor to those regions would pose significant risk such as deep white matter, thalamic, or basal ganglia tumors, among others. In these cases, LITT is particularly useful for its minimally invasive nature and the flexibility of the system for complex and multiple trajectories. However, the most common complication for LITT is temporary or permanent neurological deficit ranging from 0% to 29.4% for transient and 0% to 10% for permanent deficits. In many cases these deficits are related to white matter damage from hyperthermia. Also, another serious, albeit rare, event after LITT is pseudoaneurysm formation and rupture and this seems to be associated with thermal damage of the blood vessels [Citation53]. Careful preoperative planning using MRI angiography or tractography should increase the safety of the procedure. Tractography is a technique that models the trajectory of the major white matter pathways in the brain. This technique is based on the DWI sequence that maps the movement of water molecules in the brain. Tractography has been used with LITT in order to avoid the damage of the white matter pathways around the tumor. Sharma et al. study the extent of involvement of the corticospinal tract by the threshold lines defined in the NeuroBlate System [Citation63]. They showed that even minimal involvement of the CST by the ablation area can cause motor deficit after ablation. This study showed similar results as has been demonstrated in the use of tractography in open surgical resections. Therefore, tractography of the eloquent white matter pathways is a useful adjunct in the planning and treatment of HGG with LITT. This way, it is possible to avoid overlap of the critical TDT lines with the white matter tracts and thereby minimize the risk of neurological deficit. Another advantage of LITT compared to craniotomy, is that brain shift is minimal (if at all) and the visualization of the locations of critical structures are maintained. DWI has also been evaluated in regards to its prognostic significance after LITT. Mohammadi et al. suggested that areas of DWI signal decrease and an increase in apparent diffusion coefficient along the peritumoral areas in the 24 h post-LITT MRI could harbor residual tumor. These findings could potentially be used to identify areas of future progression and recurrence [Citation29].
Chemotherapy and LITT
One of the main difficulties for chemotherapy to reach appropriate concentrations in brain tissue is the presence of the blood brain barrier (BBB). Several methods have been devised with the aim of improving the delivery of chemotherapeutic agents to the tumor by either direct contact with the tumor or temporary disruption of the BBB. These methods include focused ultrasound, convection-enhanced delivery, intratumoral delivery, or intrarterial mannitol injections. LITT has been implicated in the transient disruption of the BBB at the periphery of the ablation region which is in turn correlated with the zone of contrast-enhancement. This has been demonstrated in animal models but also was demonstrated using advance MRI imaging in human subjects [Citation6,Citation30]. It was suggested that the BBB is disrupted for the first few weeks after ablation. Therefore, LITT could potentially enhance chemotherapy delivery to brain tumor tissue in the perioperative period. The ability of LITT to temporarily disrupt the BBB has also been demonstrated in animal models where increased infusion of dye and/or a chemotherapeutic drug in the perlesional tissue has been observed after local hyperthermia [Citation6,Citation54,Citation64]. Leuthardt et al reported a cohort of recurrent HGGs calculating the vascular transfer coefficients using dynamic contrast-enhancement MRI after treatment with LITT. They demonstrated that disruption of the BBB effect extends outwards 1–2 cm from the tumor ring and persisted for up to 4 weeks after LITT. The authors state that this effect can potentially enhance the delivery of chemotherapeutic agents that would be otherwise hampered by the BBB [Citation30].
Radiation therapy and LITT
Radiotherapy is part of the standard of care for newly-diagnosed glioblastoma and has also been used as a salvage therapy, along with stereotactic radiosurgery, for recurrent GBM [Citation56]. Currently there are some studies based on animal experiments demonstrating the synergistic effect of hyperthermia and ionizing radiation [Citation65,Citation66]. It has been suggested that hyperthermia would sensitize tumor tissue to radiation therapy and therefore enhancing the cytotoxic effects of the radiation on the tumor tissue. One of these studies demonstrated that glioma stem cells are more significantly damaged when hyperthermia is used in conjunction with radiation therapy. This synergistic effect produced impairment in the mechanisms of cell repair which translated into a consistent reduction in tumor size and improved survival in the animals exposed to both therapies compared with animals exposed to one of them [Citation7]. Furthermore, clinical evidence has also shown benefit of simultaneous hyperthermia and radiotherapy in terms of OS for patients with HGG [Citation56,Citation67].
Current perspectives
For newly-diagnosed HGG, LITT would be indicated mostly for smaller deep seated brain tumors that craniotomy may have higher complication risk. For smaller tumors close to surface, using LITT or craniotomy depends on the surgeon and patient discretion given the minimally invasive nature of the LITT. For larger tumors, LITT would need to be combined with a surgical debulking technique such as craniotomy or tubular retraction devices. Given the longer surgical time to do combined procedures, there is limited advantage of doing LITT for large tumors with mass effect compared with craniotomy. On the other hand, for recurrent HGG, LITT is useful for nodular recurrence. Even for larger recurrences, LITT might have advantage over craniotomy due to minimally invasive nature and small size of incision in a post-radiation scalp. For both newly-diagnosed or recurrent HGG, LITT would be indicated if a total or near-total ablation is feasible with one or two trajectories. Partial ablation seems to have limited benefit in terms of overall or progression-free survival.
Because of the minimal postoperative care needed after uneventful LITT, a reasonable question would be if reducing the time between LITT and chemoradiation would be a safe intervention associated with increase survival. An ongoing clinical trial was devised to answer this specific question. The main aim of this study is to determine the safety and feasibility of reducing the time interval between LITT and the start of chemoradiation to ≤ 7 days. The primary endpoint is determined by the number of patients who experience pre-specified adverse events including wound dehiscence, seizures, cerebral edema, or failure to complete a course of 60 Gy radiation (clinicaltrials.gov, NCT02970448). Another clinical trial is aims to evaluate the initiation of chemotherapy with lomustine on postoperative day 1 followed by six cycles. The primary outcomes include different measures of disease control, OS and PFS as well as toxicity (clinicaltrials.gov, NCT03022578). In the same line, a clinical trial has also been created to evaluate the use of chemotherapy in the perioperative period of LITT. In this case, pembrolizumab will be administered 7 days before, and 14 and 35 days after LITT in order to evaluate optimal timing of LITT/pembrolizumab as well as tumor response, OS and PFS (clinicaltrials.gov, NCT03277638). In regards to the potential synergistic effect of hyperthermia and ionizing radiation, another clinical trial will evaluate the treatment regimen of using LITT and hypo-fractionated radiation therapy in patients with recurrent HGG. The primary endpoint of this study is completion of the treatment protocol without undue treatment-related acute. The secondary endpoints include different measures of OS and PFS, response rate, and quality of life (clinicaltrials.gov, NCT04181684).
To date, most all clinical reports have repeatedly highlighted the importance of the extent of ablation. In order to maximize the extent of ablation while minimizing thermal damage of the surrounding brain tissue, the near real-time temperature monitoring must correlate with the depiction of the dose delivery provided by the laser workstation. In this sense, there are several tissue factors that will influence the extent of ablation such as histological type of the tumor or blood vessels [Citation68]. The development of more accurate methods to determine the extent of ablation will be needed since this is an important predictor of OS and PFS. In terms of image guidance, improvements in MRI coil design and developments in laser probe design will not only improve the flexibility of the treatment but also would expand the possibility to treat lesions with even more complex shapes and locations. For instance, novel designs in the laser tip could provide the possibility to treat tumors in sensitive structures such as the brainstem [Citation69]. Furthermore, Hafez et al reported the first case with a staged ablation of a large left insular tumor in order to minimize the postoperative edema or vascular complications in this highly eloquent area [Citation70]. Moreover, Laurent et al. demonstrated the successful application of LITT without general anesthesia in a small cohort of patients (mostly HGG) without any major complication [Citation71].
Conclusions
LITT is a minimally invasive technique that is safe and well-tolerated in the treatment of brain tumors. This technique is particularly useful for HGGs in difficult-to-access or eloquent regions because of its ability to cytoreduce tumor while having direct visualization of nearby critical brain structures throughout the procedure due to minimal brain shift during the intervention. Established evidence has demonstrated that LITT confers improved OS and PFS in patients with newly-diagnosed and recurrent HGG undergoing a near-complete ablation. It remains to be seen if LITT’s ability to improve the delivery of chemotherapy by disrupting the BBB will be associated with improved OS. Similarly, the synergistic effect of hyperthermia and ionizing radiation will need to be evaluated by well-designed clinical trials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Barnett GH, Voigt JD, Alhuwalia MS . A systematic review and meta-analysis of studies examining the use of brain laser interstitial thermal therapy versus craniotomy for the treatment of high-grade tumors in or near areas of eloquence: an examination of the extent of resection and major complication rates associated with each type of surgery. Stereotact Funct Neurosurg. 2016;94(3):164–173.
- Ivan ME, Mohammadi AM, De Deugd N, et al. Laser ablation of newly diagnosed malignant gliomas. Neurosurgery. 2016;79(suppl_1):S17–S23.
- Chiu JC, Clifford T, Greenspan M, et al. Percutaneous microdecompressive endoscopic cervical discectomy with recently added application of non-ablative lower laser energy (laser thermodiskoplasty)-140 cases, 1997. Neurosurgery. 1997;41(3):724–725.
- Hale AT, Sen S, Haider AS, et al. Open resection versus laser interstitial thermal therapy for the treatment of pediatric insular epilepsy. Neurosurgery. 2019;85(4):E730–E736.
- Kuroda K. Non-invasive MR thermography using the water proton chemical shift. Int J Hyperth. 2005;21(6):547–560.
- Sabel M, Rommel F, Kondakci M, et al. Locoregional opening of the rodent blood-brain barrier for paclitaxel using Nd:YAG laser-induced thermo therapy: a new concept of adjuvant glioma therapy? Lasers Surg Med. 2003;33(2):75–80.
- Man J, Shoemake JD, Ma T, et al. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res. 2015;75(8):1760–1769.
- Overgaard J, Bichel P. The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiology. 1977;123(2):511–514.
- Rahmathulla G, Recinos PF, Kamian K, et al. MRI-guided laser interstitial thermal therapy in neuro-oncology: a review of its current clinical applications. Oncology (Switzerland). 2014;87(2):67–82.
- Elder JB, Huntoon K, Otero J, et al. Histologic findings associated with laser interstitial thermotherapy for glioblastoma multiforme. Diagn Pathol. 2019;14(1):19.
- Schulze PC, Schober R. Fine structural analysis of laser induced tissue alterations in the central nervous system and in brain tumors. Med Laser Appl. 2002;17:159–163.
- Silva D, Sharma M, Barnett GH. Laser ablation vs open resection for deep-seated tumors: evidence for laser ablation. Neurosurgery. 2016;63 Suppl 1(1):15–26.
- Schroeder JL, Missios S, Barnett GH, et al. Laser interstitial thermal therapy as a novel treatment modality for brain tumors in the thalamus and basal ganglia. Photonics Lasers Med. 2014;3(2):151–158.
- Patel NV, Mian M, Stafford RJ, et al. Laser interstitial thermal therapy technology, physics of magnetic resonance imaging thermometry, and technical considerations for proper catheter placement during magnetic resonance imaging–guided laser interstitial thermal therapy. Neurosurgery. 2016;79(suppl_1):S8–S16.
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344(2):114–123.
- Stupp R, Brada M, van den Bent MJ, et al. High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii93–101.
- Filippini G, Falcone C, Boiardi A, et al. Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro Oncol. 2008;10(1):79–87.
- Nuño M, Birch K, Mukherjee D, et al. Survival and prognostic factors of anaplastic gliomas. Neurosurgery. 2013;73(3):458–465.
- Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123.
- Mohammadi AM, Sullivan TB, Barnett GH, et al. Use of high-field intraoperative magnetic resonance imaging to enhance the extent of resection of enhancing and nonenhancing gliomas. Neurosurgery. 2014;74(4):339–350.
- Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198.
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996.
- Rivera-Rivera PA, Rios-Lago M, Sanchez-Casarrubios S, et al. Cortical plasticity catalyzed by prehabilitation enables extensive resection of brain tumors in eloquent areas. J Neurosurg. 2016;126(4):1323–1333.
- Picart T, Herbet G, Moritz-Gasser S, et al. Iterative surgical resections of diffuse glioma with awake mapping: how to deal with cortical plasticity and connectomal constraints? Neurosurgery. 2019;85(1):105–116.
- Bush NAO, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. 2017;40(1):1–14.
- Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro-oncology. 2007;9(1):29–38.
- Hervey-Jumper SL, Berger MS . Reoperation for Recurrent High-Grade glioma: a current perspective of the literature. Neurosurgery. 2014;75(5):491–499.
- Thomas JG, Rao G, Kew Y, et al. Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg Focus. 2016;41(4):E12.
- Mahammedi A, Bachir S, Escott EJ, et al. Prediction of recurrent glioblastoma after laser interstitial thermal therapy: the role of diffusion imaging. Neuro-Oncology Adv. 2019;1(1):11.
- Leuthardt EC, Duan C, Kim MJ, et al. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):e0148613.
- Sugiyama K, Sakai T, Fujishima I, et al. Stereotactic interstitial laser-hyperthermia using Nd-YAG laser. Stereotact Funct Neurosurg. 1990;54(1–8):501–505.
- Bettag M, Ulrich F, Schober R, et al. Stereotactic laser therapy in cerebral gliomas. Acta Neurochir Suppl. 1991;52:81–83.
- Ascher PW, Justich E, Schröttner O. A new surgical but less invasive treatment of central brain tumours preliminary report. Acta Neurochir Suppl. 1991;52:78–80.
- Roux FX, Merienne L, Leriche B, et al. Laser interstitial thermotherapy in stereotactical neurosurgery. Laser Med Sci. 1992;7(1–4):121–126.
- Kahn T, Bettag M, Ulrich F, et al. MRI-guided laser-induced interstitial thermotherapy of cerebral neoplasms. J Comput Assist Tomogr. 1994;18(4):519–532.
- Schwabe B, Kahn T, Harth T, et al. Laser-induced thermal lesions in the human brain: short- and long-term appearance on MRI. J Comput Assist Tomogr. 1997;21(5):818–825.
- Reimer P, Bremer C, Horch C, et al. MR-monitored LITT as a palliative concept in patients with high grade gliomas: preliminary clinical experience. J Magn Reson Imaging. 1998;8:240–244.
- Leonardi MA, Lumenta CB. Stereotactic Guided Laser-Induced Interstitial Thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR: clinical expierence. Minim Invasive Neurosurg. 2002;45(4):201–207.
- Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al . Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: clinical article. J Neurosurg. 2013;118(6):1202–1219.
- Schulze PC, Vitzthum HE, Goldammer A, et al . Laser-induced thermotherapy of neoplastic lesions in the brain-underlying tissue alterations, MRI-monitoring and clinical applicability. Acta Neurochir (Wien). 2004;146(8):803–812.
- Jethwa PR, Barrese JC, Gowda A, et al . Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery. 2012;71(1 Suppl Operative):133–145.
- Borghei-Razavi H, Koech H, Sharma M, et al. Laser interstitial thermal therapy for posterior fossa lesions: an initial experience. World Neurosurg. 2018;117:e146–e153.
- Tovar-Spinoza Z, Choi H. Magnetic resonance-guided laser interstitial thermal therapy: report of a series of pediatric brain tumors. J Neurosurg Pediatr. 2016;17(6):723–733.
- Shah AH, Semonche A, Eichberg DG, et al. The role of laser interstitial thermal therapy in surgical neuro-oncology: series of 100 consecutive patients. Neurosurgery. 2019:1–10. DOI:10.1093/neuros/nyz424
- Tovar-Spinoza Z, Choi H. MRI-guided laser interstitial thermal therapy for the treatment of low-grade gliomas in children: a case-series review, description of the current technologies and perspectives. Childs Nerv Syst. 2016;32(10):1947–1956.
- Kamath AA, Friedman DD, Akbari SHA, et al. Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: safety, efficacy, and outcomes. Neurosurgery. 2019;84(4):836–843.
- Mohammadi AM, Hawasli AH, Rodriguez A, et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med. 2014;3(4):971–979.
- Schwarzmaier H-J, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: preliminary results in 16 patients. Eur J Radiol. 2006;59(2):208–215.
- Carpentier A, Chauvet D, Reina V, et al. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012;44(5):361–368.
- Mohammadi AM, Sharma M, Beaumont TL, et al. Upfront magnetic resonance imaging-guided stereotactic laser-ablation in newly diagnosed glioblastoma: a multicenter review of survival outcomes compared to a matched cohort of biopsy-only patients. Neurosurgery. 2019;85(6):762–772.
- Beaumont TL, Mohammadi AM, Kim AH, et al. Magnetic resonance imaging-guided laser interstitial thermal therapy for glioblastoma of the corpus callosum. Neurosurgery. 2018;83(3):556–565.
- Shah AH, Burks JD, Buttrick SS, et al. Laser interstitial thermal therapy as a primary treatment for deep inaccessible gliomas. Neurosurgery. 2019;84(3):768–777.
- Hawasli AH, Bagade S, Shimony JS, et al . Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: single-institution series. Neurosurgery. 2013;73(6):1007–1017.
- Lee I, Kalkanis S, Hadjipanayis CG. Stereotactic laser interstitial thermal therapy for recurrent high-grade gliomas. Neurosurgery. 2016;79(suppl_1):S24–S34.
- Banerjee C, Snelling B, Berger MH, et al. The role of magnetic resonance-guided laser ablation in neurooncology. Br J Neurosurg. 2015;29(2):192–196.
- Rodriguez A, Tatter SB. Laser ablation of recurrent malignant gliomas. Neurosurgery. 2016;79(suppl_1):S35–S39.
- McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. JNS. 2009;110(1):156–162.
- Schwarzmaier H-J, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser irradiation of recurrent glioblastomas. J Magn Reson Imaging. 2005;22(6):799–803.
- Barker FG, Chang SM, Gutin PH, et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42(4):709–719.
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740.
- Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6(3):227–235.
- Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467–473.
- Sharma M, Habboub G, Behbahani M, et al. Thermal injury to corticospinal tracts and postoperative motor deficits after laser interstitial thermal therapy. Neurosurg Focus. 2016;41(4):E6.
- Nakagawa M, Matsumoto K, Higashi H, et al . Acute Effects of Interstitial Hyperthermia on Normal Monkey brain-magnetic resonance imaging appearance and effects on blood-brain barrier. Neurol Med Chir (Tokyo). 1994;34(10):668–675.
- Man J, Shoemake J, Zhou W, et al. Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation. Cell Rep. 2014;9(5):1812–1826.
- Overgaard J, Bentzen SM, Overgaard J, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet. 1995;345(8949):540–543.
- Sneed PK, Stauffer PR, McDermott MW, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost ± hyperthermia for glioblastoma multiforme. Int J Radiat Oncol. 1998;40(2):287–295.
- Schwarzmaier HJ, Eickmeyer F, Fiedler VU, et al. Basic principles of laser induced interstitial thermotherapy in brain tumors. Med Laser Appl. 2002;17(2):147–158.
- Xu DS, Rosenfeld A, Ponce FA, et al. Cerebral peduncle tumor ablated by novel 3-mm laser tip. Stereotact Funct Neurosurg. 2015;93(1):38–41.
- Hafez DM, Liekweg C, Leuthardt EC. Staged Laser Interstitial Thermal Therapy (LITT) treatments to left insular low-grade glioma. Neurosurgery. 2020;86(3):E337–E342.
- Laurent D, Oliveria SF, Shang M, et al. Techniques to ensure accurate targeting for delivery of awake laser interstitial thermotherapy. Oper Neurosurg (Hagerstown). 2018;15(4):454–460.
