Abstract
Introduction
Pseudomyxoma peritonei (PMP) is a rare disease characterized by the progressive accumulation of mucinous ascites and peritoneal implants. The optimal treatment for PMP includes the association of complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). For patients with a large burdensome disease, the completeness of cytoreduction sometimes requires maximal effort surgery. The aim of this article is to provide proof of concept for two stage cytoreductive surgery (CRS) in this category of patients.
Methods and materials
A two stage CRS and HIPEC with oxaliplatin was proposed for patients with bulky PMP including important involvement of the serosal surfaces of the bowel or colon who had an impaired nutritional status. The residual disease at the end of the first stage was less than 5 mm of thickness on several implants. Clinical, surgical and histopathological variables were analyzed.
Results
All eight patients completed the two-stage strategy. Mortality was nil. One Clavien Dindo grade 3 event occurred in each stage. After a median follow up of 29.5 months, all patients were alive and free of recurrence. All of the patients had histopathological complete response on the specimens obtained from the residual sites during the second stage surgery.
Conclusions
Two-stage surgical strategy is feasible for bulky PMP patients and it is associated with little high-grade morbidity and enhanced visceral sparing.
1. Introduction
Pseudomyxoma peritonei (PMP) is a rare disease characterized by the progressive accumulation of mucinous ascites and peritoneal implants, generally originating from a perforated mucinous tumor of the appendix. This perforation is often due to the obstruction of the lumen due to tumor growth, and leads to the peritoneal spread of mucin-containing epithelial cells [Citation1]. Other origins for PMP such as the ovary, the urachus, the colon, and the pancreas, were also identified but their frequency is even rarer [Citation2].
The optimal treatment for PMP includes the association of complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) [Citation1]. Long-term survival data were obtained on large cohort studies and was significantly superior to historical treatment (debulking surgery) making phase III trials ethically unfeasible [Citation3]. Overall survival rates after optimal treatment reach 55% to 63% at 10 years and 50% to 59% at 15 years [Citation3,Citation4]. By contrast, the lack of treatment leads to mucin accumulation in the peritoneal cavity with progressive growth of the abdominal girth, nutritional impairment and eventually occlusion and death [Citation1].
As it is the case for most peritoneal surface malignancies, completeness of cytoreduction represents a major prognostic factor for overall survival whereas non-definitive treatment consisting of debulking surgery is detrimental to the outcome [Citation4]. Another major impact factor is disease differentiation. The most recent consensus classification identifies acellular mucin, low-grade mucinous carcinoma peritonei, high-grade mucinous carcinoma peritonei and high-grade mucinous carcinoma peritonei with signet ring cells [Citation5]. The first two groups have a highly better prognosis than the latter two.
Nevertheless, for patients with a large burdensome disease, with a peritoneal cancer index (PCI) higher than 30 or many implants on the digestive tract (small bowel and colon) making a complete cytoreduction (CCR 0/1) impossible, debulking surgery is the only alternative. Although multiple studies have shown that debulking surgery is inferior to complete resection in terms of long term outcome, for patients with a very large disease, any attempt to diminish the tumoral burden may be beneficial [Citation6]. Extensive cytoreductive surgery is associated to important postoperative morbidity especially in patients with impaired nutritional status which may further impact on overall survival [Citation7]. New strategies have to be defined in order to give patients with bulky disease and impaired status access to the prognostic of curatively treated patients.
The present study aims to present a proof of concept of a two-stage organ preserving cytoreductive surgery associated with HIPEC for these patients as to grant them access to a final CCR0-1 status due to the complete pathologic response after the first stage.
2. Patients and methods
2.1. Patients
Between June 2014 and June 2018, 52 patients were treated for pseudomyxoma peritonei in the Department of Surgical oncology in Montpellier Cancer Institute. Among them, eight patients with bulky disease were eligible for treatment with two-stage cytoreductive surgery plus HIPEC. The selection was performed during surgery taking into account the extension of the disease as well as the necessary extent of visceral resections. The inclusion criteria were as follows: adult patients considered fitted for surgery who gave their informed consent; PMP with a PCI superior to 20; macroscopic and pathologic aspect suggesting acellular mucin or low grade mucinous neoplasia (LAMN) with mucinous peritoneal implants of gelatinous consistency, easily detachable of the serosal surface except for a thin tissular base superior to 5 mm; involvement of the digestive tract with several implants present on the serosal surface of the small bowel or/and of the colon requiring at least three resections with anastomoses or four long running sutures. An impaired nutritional status was not considered as an exclusion criterion. Patients who were easily resectable under organ-preserving strategies as well as those presenting with infiltrative lesions suggesting a high-grade disease were not eligible for this treatment. The retrospective study was approved by the Institutional Review Board of the investigating center in accordance with the good clinical practice criteria and the ethical standards of the Helsinki declaration of 1975.
2.2. Surgery
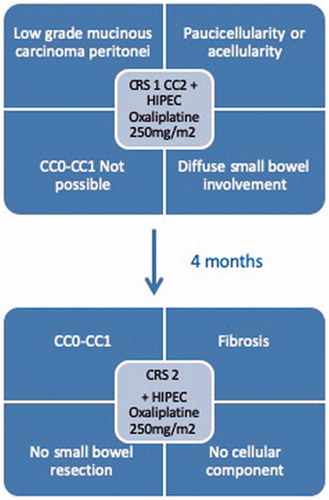
The preoperative workup included a contrast enhanced CT-scan and a peritoneal diffusion MRI before operative planning as well as between the two stages. The surgical procedure consisted of a two-stage cytoreductive surgery; each stage associated with HIPEC with oxaliplatin 250 mg/m2 in a glucose solution 0.5% at 2 l/m2. The final objective of this therapeutic strategy was to reach a CC-R0/1 resection for these patients at the end of the therapeutic management. The first surgical stage included resection of the whole peritoneal surface and/or of the lesions at risk of obstruction, but carefully excluded all other digestive tract resections. The residual tumor thickness was defined as the maximal thickness of the residual tumor and the cutoff was 5 mm [Citation8–10]. These residual implants were not unique and, usually, more than four, located on the serosal surface of the digestive tube. They would have imposed at least three resections with anastomoses or four long running sutures in the eventuality of a CCR0/1. The second stage included a complete exploration, resection of the macroscopic disease, and resection or biopsy of previously described lesions not performed in the initial surgery, although not always macroscopically visible ().
The histologic evaluation was performed for each step by two pathologists (KL, FB) with conventional Hematoxylin-eosin-saffron stained microscopy using the current pathologic criteria. One of the two pathologist (FB) in an expert in this disease in the National Network of Rare Peritoneal Disease (RENAPE) [Citation11].
All personal, clinical, histological and operative variables were recorded for this pilot cohort. A regular follow-up with clinical examination and contrast-enhanced CT-scan was performed every 4 months during the first two years and every 6 months for the following 3 years then once yearly thereafter. All patients have at least 17 months of postoperative follow-up.
3. Results
Eight patients were included in the study (seven female patients) with a median age of 66.5 years (range 57-76). They had a median PCI of 25 (range 20-39) and an impaired nutritional status linked to their oncologic disease (). All patients were ASA III. The treatment flowchart applied to this small series of patients is detailed in .
Table 1. Patients’ characteristics, medical history, and peritoneal disease evaluation.
Cytoreductive surgery during the two stages consisted of various resections (). Median operative time and length of stay for each stage are presented in . In all cases, the residual disease after the first surgical stage had the planned maximum thickness of 5 mm and concerned the serosal surface of the small bowel. One patient had residual mucin on the serosal surface of the right colon and another patient on the serosal surface of the sigmoid colon. The placement and spreading of the mucinous lesions on the serosal surface of the digestive tract did not allow electrofulguration for these patients.
Table 2. Surgical description of the two stages.
Table 3. Operative and postoperative outcomes.
No macroscopically-visible residual disease was found in the second stage of CRS (). Fibrotic scars were sometimes present on the peritoneal surface. They were all resected and sent for pathological examination. The sites of the residual disease described at the end of the first stage were also either resected or sampled during the second stage, depending on their report with the digestive serosa. All biopsies and specimens were free of residual disease at the pathology examination and presented signs of fibrosis suggesting a complete histologic response to HIPEC (). The median time interval between the two stages was 4 months (range 2,5-4).
Figure 2. Radiological evaluation before surgery. (a) Axial contrast-enhanced CT images prior first stage surgery shows ascites (a1) and a large ill-defined multicystic mass within the abdominal cavity (a2). This complex cystic mass is seen repressing the intraperitoneal organ such as the right colon (a2 arrow). (b) Axial contrast-enhanced CT images performed just before the second time surgery show in comparison to images a1 and a2 the complete disappearance of ascites and of the complex cystic mass with the intraperitoneal organ back in their proper position in the abdominal cavity. Those results are in keeping with a complete radiological response of the first stage CRS/HIPEC oxaliplatin 250mg/m2
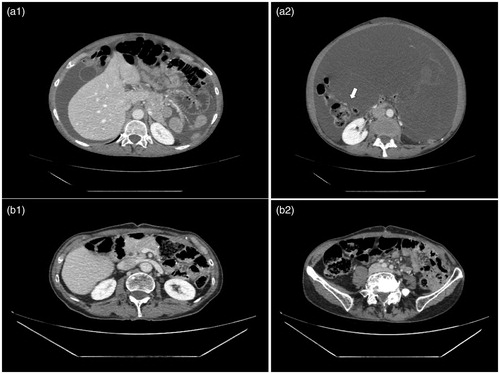
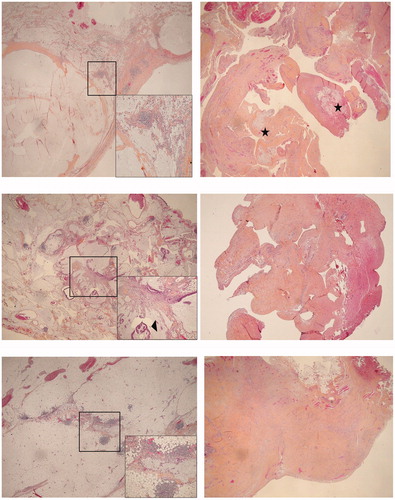
Figure 3. Example of histological samples from the two-stages.. (a) Patient 5 (a1) Stage I, parietal peritoneum involved by pools of acellular mucin, assessed as low-grade mucinous carcinoma peritonei (pseudomyxoma peritonei). HES, magnification × 20. Insert: evidence of acellular mucin with a reactive lymphocytic infiltrate. HES, × 100. (a2) Stage II, mesenteric peritoneum sampling showing a fibrous scar tissue embedding inconspicuous residual acellular mucin (asterisks). HES, × 20. (b) Patient 6 (b1) Stage I, parietal peritoneum involved by pools of extracellular mucin along with low grade neoplastic epithelium, assessed as low-grade mucinous carcinoma peritonei. HES, × 20. Insert: neoplastic epithelium highlighted by arrowheads. HES, × 100. (b2) Stage II, mesenteric peritoneum sampling showing merely fibrous scar tissue. HES, × 40. (b) Patient 8 (c1) Stage I, parietal peritoneum involved by pools of acellular mucin (low-grade mucinous carcinoma peritonei). HES, × 20. Insert: evidence of acellular mucin with a reactive lymphocytic infiltrate. HES, × 100. (c2) Stage II, mesenteric peritoneum sampling showing merely fibrous scar tissue. HES, × 40. HES: Hematoxylin eosin saffron.
One patient had bowel resection during the first stage and two patients had isolated bowel resections in the second stage. Morbidity of the two stages consisted of one grade 3 event under the Clavien-Dindo classification [Citation12] in each stage (). Mortality was nil. All patients are presently alive, without any evidence of recurrent disease. The median follow-up was of 31.5 months (range 17–65) at the time of analysis (November 2019).
4. Discussion
The present study is the first to show a proof of concept for two stage surgical treatment of bulky PMP on a small series of patients.
Most patients with pseudomyxoma peritonei are currently eligible for a complete cytoreductive surgery associated with HIPEC. This treatment offers the best chance of survival when compared to serial debulking surgery or medical treatment [Citation3,Citation4,Citation13]. However, some of these patients present with a very extensive disease or with an impaired general status, which does not allow extensive surgery [Citation14]. In a standard situation, these patients are thus usually treated in a palliative setting.
In a retrospective comparative study of debulking versus cytoreductive surgery [Citation6], the benefit in overall survival rate of complete cytoreductive surgery (CC-R0/1) over debulking surgery (CC-R2) was 75% at 5 years and 70% at 10 years. Debulking surgery had initial benefits, but this has rapidly decreased in time as a very high recurrence rate was reported. However, in this study, little information was delivered on the residual disease in the R2 resections and on the interval between the different successive debulking surgeries.
Even for teams specialized in the treatment of large pseudomyxoma, achieving a CC-R0/1 resection is conditioned by the limited involvement of the small bowel, the capacity of conservation of the upper part of the stomach, the resectability of the hepatic pedicle involvement and the general status of the patient [Citation15]. In expert centers, 29% of all large pseudomyxoma remain unresectable despite advanced multidisciplinary approaches; this percentage is irrespective of the grade [Citation15].
Under the current definitions, CCR0 indicates the absence of any macroscopic residual disease while CCR1 indicates that the maximum diameter of any remaining nodule is 2.5 mm [Citation4]. These two terms characterize complete cytoreductive surgery. CCR2 indicates that nodules between 2.5 mm and 2.5 cm in diameter remained while for patients with CCR3 resections, nodules larger than 2.5 cm in diameter remained. These two terms characterize debulking surgery and have a significantly less favorable prognosis when compared with the first two for many peritoneal diseases including PMP [Citation4]. While there is a high discrepancy in prognosis between CCR1 and CCR2, the range of size for CCR2 is very large justifying further research. For the present study, we chose a cutoff point of 5 mm which is a CCR2 status but within the initial range. In our technique, this threshold represents the residual thickness, not the maximal diameter of the remaining disease at the end of stage 1. This parameter was arbitrarily chosen based on the fact that previous studies pointed out a tissue penetration rate of 3-5 mm during the hyperthermic intraperitoneal chemotherapy [Citation16].
Our strategy, the two-stage cytoreductive surgery associated with HIPEC for PMP, targets patients with an important tumoral burden and a too fragile condition for very extensive surgery. The CC-R0/1 resection may theoretically be obtained at the end of the second stage but, in this experience, a complete pathologic response to HIPEC was already observed during the second surgical stage, as a result of the treatment applied in the first stage. This finding opens the door for future refining of the CCR2 category and of the real necessity for the second stage.
This two-stage surgical strategy had already been tested without the use of HIPEC in the first stage but the initial results in three patients were not encouraging [Citation17]. Our improved results are probably due to the acquired knowledge concerning LAMN, the limited residual tumoral thickness of less than 5 mm, the response to the hyperthermic intraperitoneal chemotherapy at the end of the first surgical stage and, eventually, the administration of local treatment also during the second surgical stage.
Several cytotoxic drugs are used for HIPEC in pseudomyxoma peritonei with interesting results [Citation13,Citation18]. A recent short-term ex vivo assay aimed to evaluate the tumor cell sensitivity to different local chemotherapy drugs in samples from patients with PMP. The study found that IC50 values for cisplatin and oxaliplatin were almost identical in PMP subgroups [Citation19]. Given that a higher dose of oxaliplatin compared to cisplatin can be administered intraperitoneally, oxaliplatin was preferred to achieve a maximum effect in this setting. That was consistent with the choice of oxaliplatin in our two-stage surgical strategy. However, we favored a less aggressive dose of 250 mg/m2 [Citation20] over our usual high dose of 460 mg/m2 [Citation21] as to reduce the risk of morbidity at the end of the first stage, thus ensuring completion of the two stages for the selected patients. This choice translated into a 100% completion rate of the two-stage strategy with an important visceral sparing.
Although there is little clinical consensus about the use of oxaliplatin over mitomycin C for HIPEC in the PMP setting, both the quality of the surgery and the drug choice remain important. In the ex vivo study pre-cited [Citation19], drug sensitivity had a prognostic impact on progression-free survival but not on overall survival suggesting that qualified surgery was crucial for good long-term outcomes. A recent systematic review in the setting of peritoneal metastases of colorectal origin failed to show any superiority of one drug over the other in terms of both disease-free and overall survival [Citation22].
The patients included in this proof of concept cohort had a reasonable morbidity when compared to other extensive disease cohorts [Citation7] with only one Clavien Dindo grade 3 event in each stage. Small or large bowel resections were avoided in seven cases in the first stage and in six cases in the second stage which probably contributes extensively to the diminished morbidity. Even more so, the two resections performed in the second-stage concerned specimens with complete response to the first stage HIPEC and they were performed in order to secure CCR0 at the end of the treatment given the nature of our innovative strategy.
The interval between the two surgical stages was planned to be of 3 to 4 months. The rationale for this interval was based on the association of estimated postoperative recovery (1 month), the kinetics of the intraperitoneal adhesions [Citation23] and the preparation for the iterative surgery.
A complete pathologic response was found, during the second CRS stage in all patients included in our study. This result is essential for the future management of large PMP especially in frail patients [Citation24]. Also, our results only concern low-grade carcinoma PMP and may not be applicable to peritoneal diseases of higher grades. Finally, a score for histopathologic response assessment was recently proposed but it is difficult to use it in the setting of low-grade PMP as acellular mucin is a marker of the presence of the disease and not of the response to therapy in this clinical entity [Citation25].
The limitations of the current study are related to the low number of patients and to the lack of comparative strategy. Large number of patients are difficult to assemble for testing new strategies in a relatively rare disease. However, these results are very encouraging for the future treatment of patients with voluminous tumoral burden of low-grade PMP and an impaired general status. We show that two-stage strategy is feasible and safe in these patients as it has a low morbidity, enhanced visceral sparing and promising long-term survival outcome. Confirmation of these preliminary results is needed in a future clinical trial and other de-escalating strategies will be consequently developed.
Acknowledgements
The authors would like to thank Mrs Hélène de Forges for English formatting.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mittal R, Chandramohan A, Moran B. Pseudomyxoma peritonei: natural history and treatment. Int J Hyperthermia. 2017;33(5):511–519.
- Delhorme J-B, French National Network of Peritoneal Surface Malignancies (RENAPE), Severac F, Averous G, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei of appendicular and extra-appendicular origin. Br J Surg. 2018;105(6):668–676.
- Chua TC, Yan TD, Smigielski ME, et al. Long-term survival in patients with pseudomyxoma peritonei treated with cytoreductive surgery and perioperative intraperitoneal chemotherapy: 10 years of experience from a single institution. Ann Surg Oncol. 2009;16(7):1903–1911.
- Chua TC, Al-Zahrani A, Saxena A, et al. Secondary cytoreduction and perioperative intraperitoneal chemotherapy after initial debulking of pseudomyxoma peritonei: a study of timing and the impact of malignant dedifferentiation. J Am Coll Surg. 2010;211(4):526–535.
- Carr NJ, Peritoneal Surface Oncology Group International, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of Pseudomyxoma Peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) modified Delphi process. Am J Surg Pathol. 2016;40(1):14–26.
- Andréasson H, Graf W, Nygren P, et al. Outcome differences between debulking surgery and cytoreductive surgery in patients with pseudomyxoma peritonei. Eur J Surg Oncol. 2012;38(10):962–968.
- Benhaim L, Faron M, Gelli M, et al. Survival after complete cytoreductive surgery and HIPEC for extensive pseudomyxoma peritonei. Surg Oncol. 2019;29:78–83.
- Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother. Pharmacol. 1999;43(7):S15–S25.
- Goéré D, Souadka A, Faron M, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. 2015;22(9):2958–2964.
- Bakrin N, FROGHI (FRench Oncologic and Gynecologic HIPEC) Group, Bereder JM, Decullier E, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013;39(12):1435–1443.
- Villeneuve L, RENAPE Network, Passot G, Glehen O, et al. The RENAPE observational registry: rationale and framework of the rare peritoneal tumors French patient registry. Orphanet J Rare Dis. 2017;12(1):37.
- Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Ann Surg. 2004;240(2):205–213.
- Moran B, Baratti D, Yan TD, et al. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei). J Surg Oncol. 2008;98(4):277–282.
- Saxena A, Yan TD, Chua TC, et al. Critical assessment of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2010;17(5):1291–1301.
- Benhaim L, Honoré C, Goéré D, et al. Huge pseudomyxoma peritonei: surgical strategies and procedures to employ to optimize the rate of complete cytoreductive surgery. Eur J Surg Oncol. 2016;42(4):552–557.
- Ceelen WP, Flessner MF. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence. Nat Rev Clin Oncol. 2010;7(2):108–115.
- De Simone M, Scuderi S, Vaira M, et al. Treatment of pseudomyxoma peritonei with two times- cytoreduction and hypertermic antiblastic peritoneal perfusion (HAPP). J Exp Clin Cancer Res. 2003;22(4 suppl):25–28.
- McBride K, McFadden D, Osler T. Improved survival of patients with pseudomyxoma peritonei receiving intraperitoneal chemotherapy with cytoreductive surgery: a systematic review and meta-analysis. J Surg Res. 2013;183(1):246–252.
- Bjersand K, Mahteme H, Sundström Poromaa I, et al. Drug sensitivity testing in cytoreductive surgery and intraperitoneal chemotherapy of pseudomyxoma peritonei. Ann Surg Oncol. 2015;22(S3):810–816.
- Stewart JH, Shen P, Russell G, et al. A phase I trial of oxaliplatin for intraperitoneal hyperthermic chemoperfusion for the treatment of peritoneal surface dissemination from colorectal and appendiceal cancers. Ann Surg Oncol. 2008;15(8): 2137–2145.
- Elias D, Bonnay M, Puizillou JM, et al. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol. 2002;13(2):267–272.
- Wisselink DD, Braakhuis LLF, Gallo G, et al. Systematic review of published literature on oxaliplatin and mitomycin C as chemotherapeutic agents for hyperthermic intraperitoneal chemotherapy in patients with peritoneal metastases from colorectal cancer. Crit Rev Oncol Hematol. 2019;142:119–129.
- Duron J-J. Postoperative intraperitoneal adhesion pathophysiology. Colorect Dis. 2007;9(s2):14–24.
- Gagnière J, Veziant J, Pereira B, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the elderly: is it reasonable? A meta-analysis. Ann Surg Oncol. 2018;25(3):709–719.
- Solass W, Sempoux C, Carr NJ, et al. Reproducibility of the peritoneal regression grading score for assessment of response to therapy in peritoneal metastasis. Histopathology. 2019;74(7):1014–1024.