Abstract
Objective
The study aimed to compare effectiveness and safety of thermal ablation and hepatic resection in patients with liver metastases of gastrointestinal stromal tumors (GISTs).
Method
A total of 55 patients (27 in the ablation group and 28 in the surgery group) with liver metastases were included. Overall survival (OS) and progression-free survival (PFS) were assessed with Kaplan–Meier’s survival estimate curves. Univariate and multivariate regression analyses were carried out to identify potential prognostic factors.
Results
The median OS was 102.0 months in the ablation group and 117.0 months in the surgery group (p = .875). The 1-, 3- and 5-year OS rates were 100%, 88.9% and 74.1% in the ablation group and 92.8%, 82.1% and 78.6% in the surgery group, respectively. The 1-, 3- and 5-year PFS rates were 48.1%, 25.9% and 18.5% in the ablation group and 67.8%, 64.3% and 64.3% in the surgery group, respectively. Multivariate analysis showed that preoperative tyrosine kinase inhibitor (TKI) treatment (progressive disease, PD) (HR, 13.985; 95% CI, 1.791–109.187; p = .012) was the only significant independent prognostic factor for OS. Tumor number (HR, 1.318; 95% CI, 1.021–1.702; p = .034) was identified as an independent predictor for PFS in multivariate analysis. There were fewer postoperative complications (18.5% vs. 78.6%, p = .001) and shorter lengths of hospital stay (8.0 vs. 16.5 days, p = .001) in the ablation group.
Conclusion
Compared with resection, thermal ablation offered comparable OS for liver metastases of GISTs. Furthermore, thermal ablation had the advantages of fewer complications and shorter lengths of hospital stay.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the digestive tract arising from the interstitial cells of Cajal [Citation1]. Approximately, 20–60% of GIST patients develop liver metastasis [Citation2,Citation3], and metastatic liver disease is a major determinant of patient survival [Citation4]. Tyrosine kinase inhibitors (TKIs) have been regarded as the first-line therapy for metastatic GISTs [Citation5]. Although up to 80% of patients exhibit an initial response to TKI treatment [Citation5], secondary resistance may occur even after years of treatment [Citation6,Citation7]. Several studies have shown that the number of remaining tumor cells might increase the risk of acquiring secondary mutations following TKI treatment [Citation8,Citation9]. This concept has led to various treatment strategies, including surgical resection and thermal ablation. Surgical resection is now widely used in clinical practice for progressive liver metastasis following TKI treatment [Citation10]. The treatment protocol is referenced in current practical guidelines on GIST management [Citation11]. However, tumor multiplicity and the general condition of the patient limit surgical indications. Furthermore, potential complications of surgery may outweigh the theoretical benefit in terms of survival rates.
Compared to resection, thermal ablation, such as radiofrequency ablation (RFA), has been shown to achieve effective and reproducible local tumor control and overall survival (OS) in patients with small hepatocellular carcinoma (HCC) and metastases, with lower morbidity and mortality rates [Citation12,Citation13]. There are various reports of hepatic resection with or without thermal ablation for liver metastases from GIST [Citation14–16]. However, despite the increasing use of thermal ablation, few prospective or retrospective studies have selected hepatic resection as a control group, limiting the level of evidence regarding the potential benefit of thermal ablation. Therefore, the aim of this study was to compare the outcomes of thermal ablation and hepatic resection in patients with liver metastases of GIST.
Materials and methods
Patients
This retrospective study was approved by the institutional review board of the First Affiliated Hospital, Sun Yat-sen University. Written informed consent was obtained from every patient prior to treatment. Patients who had undergone thermal ablation for GIST liver metastases from 11 November 2005 to 28 January 2019 were assessed in this study. Moreover, patients treated with hepatic resection between 15 April 2009 and 6 January 2019 were included in the study as a control group. The inclusion criteria were as follows: (1) patients were diagnosed with liver metastasis from GISTs based on (a) a pathological diagnosis of tissue specimens obtained from surgery or biopsy or (b) the presence of recurrent lesions with typically enhanced imaging manifestations of GIST liver metastases in clinical follow-up; and (2) liver metastases were resectable. The choice of thermal ablation or surgery was decided by the MDT meeting, which was organized by radiologists, hepatologists and gastroenterologists. Then, the final choice of treatment protocol was made by the patient. The exclusion criterion was a lack of sufficient clinical data, such as being lost to follow-up or having insufficient imaging studies. A total of 65 patients met the inclusion criteria, but 10 patients were excluded because of insufficient clinical data. A final cohort of 55 patients was included in the study, of whom 27 with 54 lesions had undergone thermal ablation, and 28 with 42 lesions had received treatment with hepatic resection. The ablation group comprised 21 (77.8%) males and six (22.2%) females, while the surgery group comprised 18 (64.3%) males and 10 (35.7%) females. The baseline characteristics of the GIST patients in the ablation and surgery groups are described in .
Table 1. Patient and tumor characteristics.
Characteristics of the primary tumor and liver metastases
For both groups, the primary GIST was located in the stomach in 18 (32.1%) patients, in the small intestine in 34 (60.7%) patients, and in other sites in four (7.1%) patients. Furthermore, 19 (33.9%) patients were found to have synchronous liver metastases, and 36 (64.2%) patients had metachronous liver metastases. Synchronous metastasis was defined as the detection of a liver metastasis during the diagnosis of the primary tumor or within the first six months [Citation17]. The median time to liver metastases from the primary tumor diagnosis was 36 months. Before local treatment, 18 patients had extrahepatic metastases: five in the ablation group and 13 in the surgery group (p = .394). All extrahepatic metastases were responsive to TKI treatment. Characteristics of the primary tumor and liver metastases are summarized in .
TKI treatment
The GIST liver metastasis patients received imatinib therapy as a first-line treatment. These patients were initially treated with imatinib at a dose of 400 mg once daily. If the disease progressed following treatment with 400 mg of imatinib in either group, the patients were sequentially treated with increasing doses of imatinib to 600 or 800 mg per day. If the patients were intolerant or refractory to imatinib, the treatment was switched to sunitinib at a dose of 37.5 mg/d. The response to TKI treatment was assessed according to the RECIST guidelines [Citation18]. The radiologic responses included progressive disease (PD), partial response (PR), stable disease (SD) and complete response (CR). Postoperative TKI treatment was recommended if the patients could tolerate it.
Thirty-eight (69.1%) patients received imatinib as the initial TKI therapy prior to local treatment. Sixteen patients were administered increasing doses of imatinib or switched to second-line sunitinib because of PD or side effects. Before ablation or surgery, preoperative TKI treatment was administered for a median of 19 (range 1–89) months. Nine patients demonstrated a response (PR or SD) with preoperative TKI treatment. Twenty-nine patients exhibited an initial response to TKI, but secondary resistance occurred (PD) after years of treatment. The remaining patients were not treated with TKIs, or data on treatment were not available. After ablation or surgery, 51 (91.1%) patients received postoperative TKI treatment ().
Thermal ablation
Thermal ablation included RFA and microwave ablation (MWA). RFA or MWA procedures were performed with real-time ultrasonography guidance. We used the Kangyou MWA system (KY2000; Nanjing Kangyou Biological Energy Co. Ltd., Nanjing, China), which consists of an MTC-3 microwave generator (frequency 2.45 GHz, power output 0–100 W), a flexible low-loss cable, a 15-gauge cooled-shaft antenna and a steady-flow pump (BT01-100 LanGe-Pump, LanGe Steady Flow Pump, Baoding, China). The radiofrequency needle electrodes were a Cool-tipTM system (a unipolar needle 15–20 cm in length and a tip exposed 2–3 cm). MWA has a comparable efficacy on local tumor progression (LTP) and OS compared to RFA [Citation19]. Our therapeutic strategy for thermal ablation was to create an ablative margin of at least 0.5 cm in the normal hepatic parenchyma surrounding the tumor. If the tumors were adjacent to the major structure (e.g., portal vein, hepatic artery or bile duct), combined percutaneous ethanol ablation (PEI) and RFA was performed [Citation20].
Before thermal ablation, the primary site was removed. For the ablation group, RFA was performed in 20 (74.1%) patients, RFA + PEI was performed in two (7.4%) patients, MWA alone was performed in three (11.1%) patients, and MWA + PEI was performed in two (7.4%) patients. All of the lesions achieved complete ablation, of which 53 lesions were subjected to a single ablation procedure, and only one lesion had to undergo three sessions. When disease progression occurred after thermal ablation, 14 (51.9%) patients underwent increasing doses of imatinib or accepted second-line chemotherapy if possible. Following TKI treatment, five (18.5%) patients underwent repeat ablation for liver lesions, two (7.4%) patients successively underwent repeat ablation and transcatheter hepatic arterial chemoembolization (TACE), four (14.8%) patients underwent surgery for the progressive lesion and one (3.7%) patient underwent surgery and TAE early or late. Only one (3.7%) patient refused to receive any treatment because of tumor progression ().
Table 2. Details of ablation and surgery.
Hepatic resection
Before hepatic resection, the extent of surgery was determined based on the locations of the metastases and the estimated hepatic functional reserve [Citation14]. Resection was classified as hemihepatectomy, sectionectomy and segmentectomy or bisegmentectomy [Citation21]. Completeness of resection was defined according to the International Union Against Cancer (UICC) criteria: R0: the entire gross tumor was negative < 1 mm from the resection margin; R1: microscopic tumor < 1 mm from the resection margin; R2: macroscopic residual hepatic or extrahepatic disease.
For the surgery group, hemihepatectomy was performed in six (21.4%) patients, sectionectomy was performed in 13 (46.4%) patients, and segmentectomy or bisegmentectomy was performed in nine (32.1%) patients. Simultaneous resection of the primary tumor was performed in seven (25.0%) patients. R0 resection was accomplished in 24 (85.7%) patients, and R1/R2 resection was achieved in four (14.3%) patients. When disease progression occurred after surgery, 17 (60.7%) patients continued to receive TKI treatment only. At the same time of TKI treatment, eight (28.6%) patients underwent thermal ablation for liver lesions, while two (7.1%) patients did not receive any treatment. None of the patients underwent repeat hepatic resection ().
Follow-up, endpoints and definitions
After local treatment (ablation or surgery), all patients underwent dynamic contrast-enhanced computed tomography (CT) one month later. The ablation technique was considered effective when the complete ablation of the macroscopic tumor with surrounding areas (ablative margin) of at least 5 mm was non-enhanced on the CT scan. To evaluate the target tumor or recurrence, contrast-enhanced CT was performed at 3, 6 and 12 months and annually thereafter. Follow-up was continued until death or the patient’s last visit until 31 January 2020. OS was defined as the interval, in months, between the initial treatment and the patient’s death from any cause or from cancer. Progression-free survival (PFS) was defined as the time from initial treatment to tumor progression of GIST liver metastases or to the date of the last follow-up visit before 31 January 2020. LTP describes any new peripheral or nodular enhancement within 1 cm or an enlargement of the baseline ablation defect on contrast-enhanced CT [Citation22]. The LTP-free survival period was defined as the interval between the initial local treatment and any follow-up imaging showing LTP. The complications of ablation and surgery were recorded. A major complication is defined as an event that increases morbidity and disability, which requires an increased level of care, hospital admission or a lengthened hospital stay. All other were regarded as minor complications [Citation2,Citation23].
Statistical analysis
To compare the patient characteristics, t-test was used for continuous and normally distributed variables. Fisher’s exact test was used for categorical variables, and the nonparametric Mann–Whitney’s U test was used for nonnormally distributed variables. PFS and OS were analyzed using the Kaplan–Meier method and compared using the log-rank test. To assess significant prognostic factor on OS and PFS, univariate and multivariate analyses were performed using stepwise Cox proportional hazards regression modeling. Factors with a p value of <.05 in univariate analysis were included in multivariate analysis. A p value <.05 was considered to be statistically significant. Statistical analysis was performed using R software, version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Therapeutic response and complications
The median follow-up period was 63.0 months (95% CI, 44.1–81.9 months) in the ablation group and 42.0 months (95% CI, 13.0–71.0 months) in the surgery group. At the end of the follow-up period, nine (33.3%) patients in the ablation group died, and seven (25%) patients in the surgery group died. For the ablation group, 22 (81.5%) patients had confirmed disease progression; one of them (1.9%) had LTP at 2 months, seven (25.9%) developed new liver disease, two (7.4%) developed extrahepatic disease and 12 (44.4%) had intra- and extrahepatic disease. For the surgery group, nine (32.1%) patients experienced progressive tumors, of which three (10.7%) had new liver disease, three (10.7%) developed extrahepatic disease and three (10.7%) had both intra- and extrahepatic lesions (). The length of hospital stay was significantly reduced in the ablation group at eight (5–15) days vs. 16.5 (14–22.2) days in the surgery group. In the ablation group, the complications only included five (18.5%) minor complications with endurable pain. However, in the surgery group, 19 (67.8%) patients had minor complications with persistent pain in 10 cases, fever in three cases and ascites in six cases. Major complications occurred in three (10.7%) patients (septic shock, hepatic failure, hemothorax). The incidence of complications increased significantly in the surgery group (p=.001).
Table 3. Patient outcomes following thermal ablation and surgery.
PFS
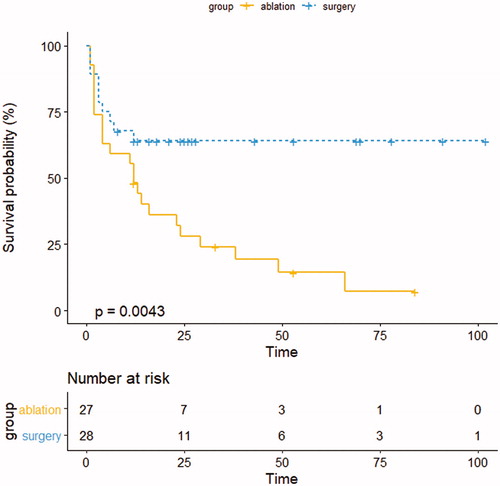
The median time to PFS was 12.0 (95% CI: 8.7–15.3) months in the ablation group. The median time to PFS of the surgery group could not be calculated because the progression curve did not decrease to 50%. shows that there was a difference between the two groups in terms of PFS in the Kaplan–Meier analysis (p=.004). The cumulative PFS rates at 1, 3 and 5 years were 48.1%, 25.9% and 18.5% in the ablation group and 67.8%, 64.3% and 64.3% in the surgery group, respectively. In the univariate analysis, a number of significant factors for PFS were identified: age >60 years (p=.009), treatment group (surgery) (p=.007), preoperative TKI treatment (yes) (p=.025), preoperative TKI treatment (PD) (p=.001) and tumor number (p=.001). Multivariate Cox regression analysis revealed that tumor number (HR, 1.318; 95% CI, 1.021–1.702; p=.034) was an independent predictor ().
Table 4. Univariate and multivariate analyses using Cox proportional hazards regression modeling.
OS
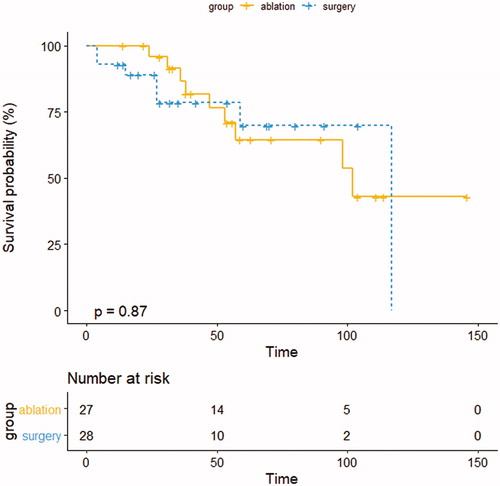
The 1-, 3- and 5-year OS rates were 100%, 88.9% and 74.1% in the ablation group and 92.8%, 82.1% and 78.6% in the surgery group, respectively. The median OS was 102.0 months in the ablation group and 117.0 months in the surgery group (p=.875) (). Univariate analysis revealed that preoperative TKI treatment (PD) (p=.007) and tumor number (p=.018) were significantly associated with reduced survival. In the multivariate analysis, preoperative TKI treatment (PD) (HR, 13.985; 95% CI, 1.791–109.187; p=.012) was an independent predictor of poorer survival ().
Discussion
Our results indicated that GIST patients with liver metastases undergoing thermal ablation may show comparable outcomes in terms of OS compared with those who undergo hepatic resection. Moreover, thermal ablation showed advantages in fewer complications and shorter lengths of hospital stay. The result indicates that thermal ablation is a safe therapy for liver metastases from GIST.
There was a difference between thermal ablation and surgery in terms of PFS in the Kaplan–Meier analysis. However, though thermal ablation had more tumor recurrence after treatment, it is seemed that improved local control rates achieved with resection do not necessarily transport into superior survival. Thermal ablation was found to be equivalent to resection regarding the median OS in our study. The study of Chen et al. [Citation24] reported a similar result, but the sample size of their study was even smaller. On the one hand, treatment modality was not chose as an independent predictor in the multivariate analysis. On the other hand, long-term survival is affected by many factors. For example, subsequent treatment such as repeat ablation after tumor recurrence can greatly improve survival.
Repeat ablation may be the result contributing to comparable outcomes for thermal ablation compared to surgery. Thermal ablation can retain as much liver parenchyma as possible, which enables the repeat ablation of recurrent lesions after the first treatment. In this study, 15 (27.3%) patients underwent repeat ablation for new liver lesions in both groups. A previous study revealed that even for recurrent liver metastases, repeat ablation has good oncological outcomes [Citation25]. Moreover, preserving as much liver parenchyma as possible improves the chance of performing repeat curative treatments such as surgery or TACE in the case of tumor recurrence. In the present study, there were five (18.5%) patients who underwent postoperative surgery in the ablation group, but none of the patients could undergo repeat hepatic resection in the surgery group.
Unsurprisingly, thermal ablation has advantages in shorter hospital stays and a low rate of complications compared with liver resection [Citation26]. In the present study, the length of hospital stay was significantly reduced in the ablation group at eight (5–15) days vs. 16.5 (14–22.2) days in the surgery group. Furthermore, another important advantage for ablation is that the clinical benefit of survival far outweighs its complications. None of the patients in the ablation group developed any major complications, with only five (18.5%) cases of minor complications. The low rate of complications was also confirmed in a previous study, with a rate of 8.3–20% [Citation3,Citation14,Citation24]. However, in the surgery group, 19 (67.9%) patients had minor complications, and three (10.7%) patients had major complications (septic shock, hepatic failure, hemothorax). There is no doubt that a lower rate of complications and a shorter length of hospital stay may improve the quality of patients’ lives.
The technical success rate of thermal ablation was 100% in the present study. Previous studies have also reported similar technical effectiveness rates of 92–100% [Citation2,Citation27,Citation28]. Only one patient in the present study showed LTP after thermal ablation, and the LTP rate was 1.9% (one of 54 lesions). The LTP rates were lower than those in previous studies, which reported LTP rates of 4.8–6.0% [Citation2,Citation3]. This LTP rate seems to be lower than that after thermal ablation for colorectal liver metastasis, which reported LTP rates ranging from 9.6% to 38% [Citation29,Citation30]. It is well known that GIST liver metastases show very low echogenicity compared with other liver metastases. Additionally, GIST liver metastases tend to have a clear margin that can be distinguished from the normal liver parenchyma [Citation31]. Both of these reasons may facilitate the accurate sonographic targeting of index tumors and high technical effectiveness rates. Lower LTP rates were believed to be related to longer PFS and OS.
The median OS (102 months) in our study was longer than that in previous studies, of which Chen et al. reported that the median OS was 73 months following RFA [Citation24] and Jung et al. reported that the median OS after RFA was 90.2 months [Citation3]. This finding may be the result of the lower tumor number included in our study. The median tumor number in the previous two studies was two, while the median tumor number in our study was one. In the multivariate analysis, multiple metastases were an independent factor for poor PFS in our study. A previous study confirmed this finding [Citation32].
In this study, it was found that GIST liver metastasis with PD following preoperative TKI treatment was an independent poor prognostic factor for OS. Machairas et al. pointed out that clinical response to TKIs was a predictive factor for survival [Citation33]. Thermal ablation can be a potential curative option for patients exhibiting a PR to TKI treatment with focal liver lesions [Citation28,Citation34]. Furthermore, thermal ablation can delay a change in systemic therapy by achieving local control at the site of single disease progression in GIST liver metastases [Citation35]. As a result, patients with progressive liver metastases of GIST were also included in this study.
There are a few limitations to the present study. First, this was a retrospective study from a single institution. Non-randomization regarding choices of treatment inevitably introduced selection bias. Second, because of the low incidence of GIST, the sample size was small. A prospective randomized study with a larger sample size is needed to confirm our findings. Third, the tumor size of the largest metastasis was larger in the surgery group. Although there were no statistically significant differences due to the small sample size, the factor may have an impact on the outcome. Fourth, important prognostic factors for primary GISTs, such as mitotic rate, were not available for all patients.
In conclusion, the present study indicated that thermal ablation produces comparable outcomes compared to hepatic resection for GIST liver metastases in selected patients. Thermal ablation is feasible, tolerable and effective in the treatment of GIST liver metastases with a low rate of complications, shorter length hospital stay and repeatable application. The results of this study add weight to the current clinical decision of performing thermal ablation in patients with recurrent or metastatic GISTs localized to the liver.
Acknowledgements
The authors thank all the participating clinicians for their support.
Informed consent: This study was conducted with the approval of the hospital review boards of the First Affiliated Hospital, Sun Yat-sen University, and was performed according to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all of the patients for the US-guided percutaneous RFA procedure. Additional informed consent was not required for this retrospective study.
Consent for publication: Consent for publication was obtained for every individual person’s data included in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Correa-Cote J, Morales-Uribe C, Sanabria A. Laparoscopic management of gastric gastrointestinal stromal tumors. World J Gastrointest Endosc. 2014;6(7):296–303.
- Yamanaka T, Takaki H, Nakatsuka A, et al. Radiofrequency ablation for liver metastasis from gastrointestinal stromal tumor. J Vasc Interv Radiol. 2013;24(3):341–346.
- Jung JH, Won HJ, Shin YM, et al. Safety and efficacy of radiofrequency ablation for hepatic metastases from gastrointestinal stromal tumor. J Vasc Interv Radiol. 2015;26(12):1797–1802.
- Bayraktar UD, Bayraktar S, Rocha-Lima CM. Molecular basis and management of gastrointestinal stromal tumors. World J Gastroenterol. 2010;16(22):2726–2734.
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–1134.
- Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11(11):4182–4190.
- Chen LL, Trent JC, Wu EF, et al. A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Res. 2004;64(17):5913–5919.
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342–4349.
- Debiec-Rychter M, Dumez H, Judson I, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2004;40(5):689–695.
- Mussi C, Ronellenfitsch U, Jakob J, et al. Post-imatinib surgery in advanced/metastatic GIST: is it worthwhile in all patients? Ann Oncol. 2010;21(2):403–408.
- Casali PG, Abecassis N, Bauer S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:68–78.
- Peng ZW, Lin XJ, Zhang YJ, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262(3):1022–1033.
- McDermott S, Gervais DA. Radiofrequency ablation of liver tumors. Semin Intervent Radiol. 2013;30(1):49–55.
- Yoon IS, Shin JH, Han K, et al. Ultrasound-guided intraoperative radiofrequency ablation and surgical resection for liver metastasis from malignant gastrointestinal stromal tumors. Korean J Radiol. 2018;19(1):54–62.
- Ye YJ, Gao ZD, Poston GJ, et al. Diagnosis and multi-disciplinary management of hepatic metastases from gastrointestinal stromal tumour (GIST). Eur J Surg Oncol. 2009;35(8):787–792.
- Park SJ, Ryu MH, Ryoo BY, et al. The role of surgical resection following imatinib treatment in patients with recurrent or metastatic gastrointestinal stromal tumors: results of propensity score analyses. Ann Surg Oncol. 2014;21(13):4211–4217.
- Moreno P, de la Quintana Basarrate A, Musholt TJ, et al. Adrenalectomy for solid tumor metastases: results of a multicenter European study. Surgery. 2013;154(6):1215–1222.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3(5):317–325.
- Huang G, Lin M, Xie X, et al. Combined radiofrequency ablation and ethanol injection with a multipronged needle for the treatment of medium and large hepatocellular carcinoma. Eur Radiol. 2014;24(7):1565–1571.
- Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12(5):351–355.
- Adam R, Bhangui P, Poston G, et al. Is perioperative chemotherapy useful for solitary, metachronous, colorectal liver metastases? Ann Surg. 2010;252(5):774–787.
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7 Suppl.):S377–S390.
- Chen Q, Li C, Yang H, et al. Radiofrequency ablation versus resection for resectable liver metastases of gastrointestinal stromal tumours: results from three national centres in China. Clin Res Hepatol Gastroenterol. 2019;43(3):317–323.
- Hof J, Wertenbroek MW, Peeters PM, et al. Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg. 2016;103(8):1055–1062.
- Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières Meeting 2013. Eur Radiol. 2015;25(12):3438–3454.
- Hakimé A, Le Cesne A, Deschamps F, et al. A role for adjuvant RFA in managing hepatic metastases from gastrointestinal stromal tumors (GIST) after treatment with targeted systemic therapy using kinase inhibitors. Cardiovasc Intervent Radiol. 2014;37(1):132–139.
- Jones RL, McCall J, Adam A, et al. Radiofrequency ablation is a feasible therapeutic option in the multi modality management of sarcoma. Eur J Surg Oncol. 2010;36(5):477–482.
- Lorentzen T, Skjoldbye BO, Nolsoe CP. Microwave ablation of liver metastases guided by contrast-enhanced ultrasound: experience with 125 metastases in 39 patients. Ultraschall Med. 2011;32(5):492–496.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275 e261.
- Casali PG, Abecassis N, Aro HT, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl. 4):iv267.
- Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am. 2003;12(1):165–192.
- Machairas N, Prodromidou A, Molmenti E, et al. Management of liver metastases from gastrointestinal stromal tumors: where do we stand? J Gastrointest Oncol. 2017;8(6):1100–1108.
- Pawlik TM, Vauthey J-N, Abdalla EK, et al. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg. 2006;141(6):537–544.
- Vassos N, Agaimy A, Hohenberger W, et al. Management of liver metastases of gastrointestinal stromal tumors (GIST). Ann Hepatol. 2015;14(4):531–539.