Abstract
The concept of thermal therapy toward the treatment of brain tumors has gained traction in recent years. Traditionally, thermal therapy has been subdivided into hyperthermia, with mild elevation of temperature in treated tissue above the physiologic baseline; and thermal ablation, where even higher temperatures are achieved. The recent surge in interest has been driven by the use of novel thermal ablation technologies, including laser interstitial thermal therapy (LITT), that are implemented in brain tumor treatment. Here, we review previous scientific literature on the biologic effects of thermal therapy on brain tumors, with an emphasis on glioblastoma (GBM), an aggressive brain malignancy. In addition, we present in vitro evidence from our laboratory that even moderate elevations in temperature achieved in the penumbra around laser-ablated coagulum may also produce GBM cell death. While much remains to be elucidated in terms of the biology of thermal therapy, we propose that it is a welcome addition to the neuro-oncology armamentarium, in particular with regard to GBM, which is generally resistant to current chemoradiotherapeutic regimens.
Introduction
Brain tumors have always been considered a challenge in the field of oncology due to limited efficacy of conventional chemoradiotherapeutic approaches. Our biggest challenge in neuro-oncology remains the treatment of malignant primary brain tumors. The most common primary brain malignancy, glioma, is in dire need of new treatments. In glioblastoma (GBM) in particular, the most aggressive form of glioma, median survival remains only ∼16 months after surgery, and chemoradiotherapy [Citation1]. The high propensity of GBM tumor cells to infiltrate brain tissue eliminates a curative role for the surgery. Instead, surgery is used as a key cytoreductive step in therapy, which is almost always followed by chemoradiotherapy. However, GBM utilizes tumor-intrinsic and microenvironment-mediated mechanisms to resist conventional cytotoxic chemotherapy and high doses of radiotherapy. In addition, recent large-scale randomized trials testing anti-angiogenic therapy, gene therapy and antibody-drug conjugates targeting the epidermal growth factor receptor (EGFR), a receptor tyrosine kinase commonly amplified in GBM, have shown minimal efficacy [Citation2–6]. There is therefore a critical need to identify novel therapies or potentiate current treatments.
Thermal therapy for brain tumors, and GBM in particular, is a treatment approach aimed at raising intratumoral temperatures in order to directly or indirectly facilitate tumor cell kill. In general, thermal therapy can be subdivided into two modalities that differ by the amount of heat delivered and the change in tissue temperature [Citation7,Citation8]. First, hyperthermia usually refers to mild elevations in tumor temperature (43–45 °C), which may be sub-lethal but induce sufficient biological changes to facilitate therapy in conjunction with other modalities [Citation9]. Hyperthermia has long been known to radiosensitize tumors, including glioma, in part by limiting activation of AKT [Citation10], a kinase central to glioma biology.
A second type of thermal therapy involves thermal ablation of tumor cells, by raising intratumoral temperatures to lethal ranges. Thermal ablation can be achieved through a variety of technologies, including laser interstitial thermal therapy (LITT), high-intensity focused ultrasound (HIFU), magnetic hyperthermia, as well as radiofrequency and microwave ablation [Citation7]. These approaches are minimally- or noninvasive and usually require live MR thermography to monitor heat spread. Previous work has indicated that within the zone of ablation, temperatures exceed 50 °C and may reach up to 80–90 °C, while in the penumbra immediately around the ablation temperatures typically reach the 40–50 °C range [Citation11].
The efficacy of these treatments against GBM is still being evaluated [Citation12–22]. From the safety point of view, there are two major considerations. First, heat spread should be titrated, so it reaches ablative levels within the tumor bulk without damaging the tumor-infiltrated but potentially still functional surrounding brain tissue, in order to reduce the risk of neurologic deficits [Citation20]. Second, the fact that ablated tumor tissue is not removed, as opposed to conventional surgery, raises concerns about post-procedure increases in intracranial pressure (ICP), especially because of anticipated increases in inflammation and edema in an innate response to cell death. The size and location of the tumor are, therefore, important parameters in determining whether thermal ablation is an appropriate therapy.
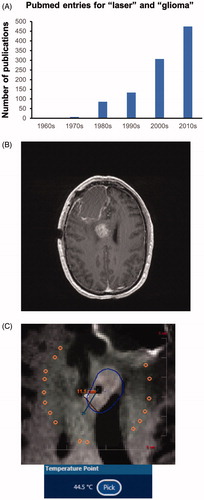
LITT has received considerable attention in recent years as a treatment option [Citation23,Citation24]. Indeed, a survey of Pubmed, using ‘laser’ and ‘glioma’ as search terms, shows a dramatic increase in publications over the past few decades (). At our institution, we have employed LITT to treat recurrent () and even newly diagnosed GBMs that we deem poor candidates for conventional surgical excision. While we view LITT as a salvage procedure in a carefully selected subset of patients, we and others postulate that it may be exploited toward the development of novel GBM therapies due to two of its biological effects. First, several groups have observed a break-down of the blood-brain barrier (BBB) around the area of LITT ablation, lasting up to one month post-procedure [Citation25,Citation26]. This phenomenon may be exploited to treat tumor cells infiltrating brain tissue around the tumor bulk with small molecules or biologics that would otherwise not cross the BBB. Indeed, there are current clinical trials evaluating cytotoxic agents in post-LITT GBM patients. Second, we and others postulate that cell death secondary to LITT ablation of GBM may incite activation of the immune response [Citation27–30] in an otherwise immunologically ‘cold’ tumor. The combination of LITT with immune checkpoint inhibitor therapy for recurrent GBM is current being evaluated in clinical trials in GBM.
Figure 1. Laser ablation of glioma. (A) A Pubmed search for ‘laser’ and ‘glioma’ shows a striking increase in the number of publications over the past two decades. (B) Example of recurrent GBM in the corpus callosum. (C) This callosal tumor was treated with LITT at our institution. This image was captured with live MR thermography during the ablation. The white target is located on the expanding border of the predicted cell kill zone (blue contour line) and has reached a temperature of 44.5 °C. Higher temperatures were generated within the area outlined by the contour line.
The mechanisms of LITT-induced cell death remain unclear. The temperatures reached within the core of laser-ablated GBM tumors exceed 60 °C normally and are, therefore, presumed to generate primary necrosis [Citation31–33]. In primary necrosis, an acute insult, such as heat, induces severe enough damage to cellular constituents at the molecular and organellar level to generate breakdown of the plasma membrane [Citation33]. When the plasma membrane is disrupted, the cell’s DNA is accessible to DNA-binding fluorophores that are otherwise membrane impermeant [Citation34]. This can be exploited to visualize dying cells with flow cytometry.
Regulated or programed cell death differs from primary necrosis in that the cell itself or extrinsic stimuli trigger a cellular program that leads to death [Citation33]. Conventional apoptosis is the most common mechanism of programed cell death, but certainly not the only one. In apoptosis, changes in the plasma membrane lead to phosphatidylserine being exposed on the extracellular side of the plasma membrane. This moiety is recognized by annexin V and this interaction can also be exploited to detect programed cell death with flow cytometry [Citation34]. Programed cell death ultimately leads to secondary necrosis, which is again detectable with DNA-binding fluorophores [Citation34]. A fundamental difference between primary necrosis and apoptosis is that the former is highly immunogenic. In contrast, most, but not all, mechanisms of programed cell death evade immune recognition [Citation33].
While it is established that a necrotic coagulum forms within the core of laser-ablated GBM tumors, there is less known about whether the milder elevations in temperature in the penumbra of the ablation may also result in cell death [Citation35]. This is an important question because GBM is notorious for brain infiltration beyond the tumor bulk that is laser-ablated. As shown in , tissue along the margins of ablation reaches temperature elevations in the 40–50 °C range. Here, we use patient-derived GBM cultures and flow cytometry to show that temperatures found in the penumbra of laser-ablated tissue increase the rate of cell death in vitro [Citation36]. We believe that our preliminary findings can lay the foundation for more detailed characterization of the tumor cell-intrinsic and microenvironment-mediated effects of thermal ablation in GBM.
Materials and methods
Patient biospecimens and primary tumor cultures
Experiments were performed with two patient-derived IDH wild-type GBM tumorsphere cultures, GBML20 and GBML61. To establish the cultures, operative specimens were minced in Hank’s Balanced Salt Solution (HBSS, Life Technologies, Bengaluru, India) and enzymatically dissociated into single cells (Accutase, Innovative Cell Technologies, San Diego, CA). Upon dissociation, cells were cultured in suspension on non-adherent plates in Neurobasal media (Life Technologies), supplemented with N2 (Life Technologies), B27 (without vitamin A; Life Technologies), 20 ng/mL of human recombinant EGF (Life Technologies) and 20 ng/ml bFGF (R&D Systems, Minneapolis, MN) [Citation37,Citation38]. This protocol was approved by the institution’s IRB (IRB#12-01130) and required informed consent by patients. Molecular subtyping of parental tumors was performed with DNA methylation 450 K arrays, as previously described [Citation37–39].
Heating GBM cells
GBM tumorsphere cultures were placed in an incubator set at specific temperatures for defined periods. Oxygen levels were kept at 21%. Each condition was tested 3–5 times.
Flow cytometry
Cells were dissociated with Accutase (Innovative Cell Technologies). To identify cells with impaired membrane integrity, we incubated cells with 100 nM 4′,6-diamidino-2-phenylindole (DAPI, Thermo Fisher, Waltham, MA), a fluorescent DNA-binding stain. We also incubated cells with annexin V conjugated to phycoerythrin (annexin V − PE, Thermo Fisher), according to the manufacturer’s instructions, to identify cells undergoing apoptosis [Citation34]. The LSRII analyzer (BD Biosciences) was used for flow cytometry analysis.
Statistical analysis
Statistical comparisons included one-way and 2-way ANOVA with post-hoc Tukey tests. Statistical significance was set at p < 0.05. Prism (GraphPad) was used for statistical analyses. Population statistics are represented as mean ± standard error (SE).
Results
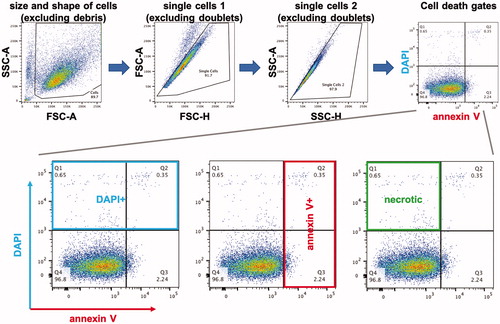
We used two previously established proneural IDH wild-type patient-derived GBM cultures, GBML20 and GBML61, for our experiments [Citation37]. Tumor cells were grown as tumorspheres. To analyze the effects of heat on cell viability, we incubated cells at either 43, 46 or 49 °C, for 30, 60 or 180 min. At the end of the incubation period, tumorspheres were dissociated and subjected to flow cytometric analysis with the DNA-binding fluorescent stain DAPI, indicating breakdown of the plasma membrane, and annexin V − PE, a marker of programed cell death/apoptosis [Citation34]. shows the flow cytometry protocol used for identifying single cells that qualified for the DAPI/annexin V analysis. Because apoptotic annexin V + cells can also show DAPI staining during late phases of apoptosis (or secondary necrosis), we assumed that DAPI + but annexin V − cells represented the cellular population that underwent acute primary necrosis.
Figure 2. Flow cytometry protocol. We used a series of gates based on side scatter (SSC) and forward scatter (FSC) to isolate single dissociated cells for analysis of their DAPI and annexin V − PE fluorescence. The far right dot plot in the top row is used as an example in the bottom row to illustrate the populations of cells we analyzed: DAPI+, annexin V+, and DAPI+/annexin V−.
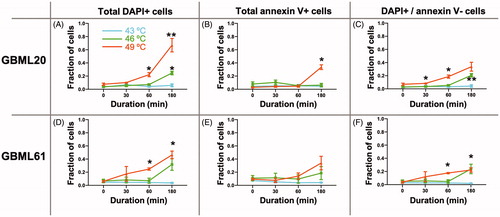
As shown in , heating the cells to 49 °C caused an increase in both DAPI and annexin V staining in a time-dependent manner, relative to the baseline at 37 °C (0 min). To quantify the effects of different temperatures and the duration of heating, we plotted the fraction of DAPI+, annexin V + and DAPI+/annexin V − cells as a function of temperature and time (). Two-way ANOVA indicated no effect of temperature on annexin V staining. However, the DAPI signal showed significant increases in both patient-derived cultures at 60 and 180 min at 49 °C, and in one of the cultures after 180 min at 46 °C. When we focused in on the DAPI+/annexin V − population, the presumably acutely necrotic cells, the two cultures showed significant increases after as little as 60 min of 49 °C, and in the case of GBML20, after as little as 30 min. In GBML61, heating the cells to 46 °C for 180 min also produced a statistically significant increase in the DAPI+/annexin V − cell fraction. These findings suggested that heating GBM cells generates cell death via primary necrosis predominantly, rather than programed cell death/apoptosis mechanisms.
Figure 3. Heating GBM cells to 49 °C increases DAPI and annexin V staining. Representative flow cytometric analysis of GBML20 and GBML61 cells for DAPI and annexin V staining after heating to 49 °C for 30, 60 or 180 min.
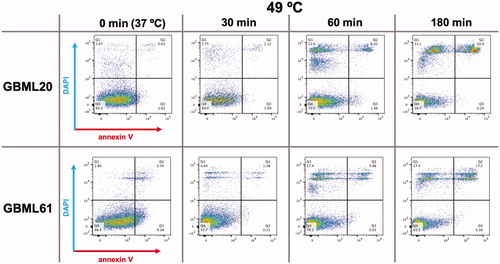
Figure 4. Heat induces a predominantly non-apoptotic death in GBM cells in vitro. Summary graphs of effects of three different temperatures applied for 30, 60 or 180 min to two patient-derived GBM cultures on DAPI and annexin V staining (A–F). The DAPI + and DAPI+/annexin V − cell populations show the most statistically significant increases in response to heat in a temperature and time-dependent manner. Two-way ANOVA was used for statistical comparisons. The effect of temperature was significant in all conditions with the exception of annexin V staining for GBML20 (B) (F2,10 = 2.160, p = 0.1661) and GBML61 (E) (F2,10 = 1.291, p = 0.3170). Time, and the interaction of temperature–time were significant in all conditions. Asterisks indicate post-hoc significant differences by Tukey test. *p < 0.05; **p < 0.01.
Discussion
Our findings suggest that heating GBM cells to temperatures comparable to those found in the penumbra of LITT ablations produces cell death in a temperature- and time-dependent manner. The fact that the increase in the DAPI + cell population precedes and exceeds that in the annexin V + fraction suggests that cell death may not be predominantly programed, but rather primary necrosis. The time and temperature dependence of cell kill in our study is generally consistent with the Arrhenius equation and models for heat-induced cell death [Citation40–42]. While our findings are not surprising, given that heat has pronounced effects on all biomolecules relevant to cells, they raise the possibility that such heat-induced acute necrosis in the penumbra of laser ablations can be exploited toward immunologic therapies. Indeed, the sudden unregulated death associated with heat causes the release of cellular antigens, as well as pro-inflammatory molecules [Citation36]. Most mechanisms of programed cell death, on the other hand, are considered less immunogenic, because they evade immune system activation via a regulated death program that leads to dying cells being engulfed by phagocytes without the sudden release of cellular antigens and pro-inflammatory signals [Citation33]. In the future, we intend to test the hypothesis that LITT-induced cell death may invite the immune system to clear cellular debris, and that immune infiltrates may trigger an additional wave of pro-inflammatory cytokine release.
The concept that thermal therapy of tumors may incite an immune response has gained traction in recent years [Citation43–45]. In this context, thermal ablation serves as a ‘vaccination’ mechanism, which presents tumor antigens to immune cells infiltrating the ablated area. Although the current study does not provide direct support for our theory, we postulate that the breakdown of the BBB in the penumbra of ablated GBM, along with the tumor cell death that occurs as our data indicate, allows immune infiltration to clear necrotic debris. We envision pairing GBM thermal therapy with immune checkpoint inhibition, which is based on unleashing the immune system against tumor antigens. This is particularly relevant in GBM tumors, which are immunologically ‘cold’ through a number of mechanisms, and are therefore generally resistant to conventional immune checkpoint inhibitors by themselves, as shown in recent clinical trials [Citation4,Citation46,Citation47].
The importance of studying the effects of heat in the penumbra of laser ablations is justified by the fact that this penumbra is the region where immune infiltration may occur. As mentioned above, the core of each ablation represents coagulum, which is devoid of vascular perfusion. However, the penumbra around the ablation is not only vascularized, but, as suggested by radiographic data, develops breakdown of the BBB [Citation26]. One may speculate that it is this penumbra that uniquely combines immunogenic cell death with vascular access to immune cells that may trigger an immune response against tumor antigens. Our study supports this hypothesis by providing evidence for cell death triggered by moderate temperatures in this penumbra.
Our in vitro study will need to be further validated in in vivo rodent models of LITT. Our in vitro model, while informative, is artificial. For example, it is clear that LITT procedures do not usually last 3 h. Instead, live MRI thermography shows temperature elevations within minutes after activating the laser (). The time discrepancy between our model and the actual clinical scenario is most likely explained by delayed heating of the cells due to two parameters: first, the high heat capacity of the aqueous medium in which our tumorspheres are suspended for the in vitro experiments; and second, the fact that heating in our in vitro system is achieved via convection, rather than the infrared laser-induced heating obtained during laser ablation procedures. Nonetheless, our findings are consistent with the Arrhenius equations and models for heat-induced cell death [Citation40–42], including the temperature and time dependence.
The potential opportunity to combine LITT for GBM with immune checkpoint inhibitors is currently being tested in a few clinical trials. We hope that this study will serve as a springboard for further studies on the biologic effects of laser ablation on GBM and brain tumors in general. We also hope that our preliminary studies will convince the pharmaceutical industry to take a closer look at immune-oncology opportunities generated by LITT in the context of GBM, a malignancy with sadly very few treatment options.
Disclosure statement
DGP has received consultant fees from Tocagen, Synaptive Medical, Monteris and Robeaute. DGP and NYU Grossman School of Medicine have filed a patent application titled “Method for treating high grade glioma” on the use of GPR133 as a treatment target in glioma.
Additional information
Funding
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996.
- Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722.
- Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708.
- Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro-oncology. 2018;20(5):674–686.
- van den Bent M, Eoli M, Sepulveda JM, et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFRamplified glioblastoma. Neuro Oncol. 2019;22(5):684–693.
- Lassman AB, van den Bent MJ, Gan HK, et al. Safety and efficacy of depatuxizumab mafodotin + temozolomide in patients with EGFR-amplified, recurrent glioblastoma: results from an international phase I multicenter trial. Neuro Oncol. 2019;21(1):106–114.
- Bredlau AL, McCrackin MA, Motamarry A, et al. Thermal therapy approaches for treatment of brain tumors in animals and humans. Crit Rev Biomed Eng. 2016;44(6):443–457.
- Dewhirst MW, Vujaskovic Z, Jones E, et al. Re-setting the biologic rationale for thermal therapy. Int J Hypertherm. 2005;21(8):779–790.
- Ahmed K, Tabuchi Y, Kondo T. Hyperthermia: an effective strategy to induce apoptosis in cancer cells. Apoptosis. 2015;20(11):1411–1419.
- Man J, Shoemake JD, Ma T, et al. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res. 2015;75(8):1760–1769.
- Munier SM, Hargreaves EL, Patel NV, et al. Ablation dynamics of subsequent thermal doses delivered to previously heat-damaged tissue during magnetic resonance-guided laser-induced thermal therapy. J Neurosurg. 2019;131(6):1958–1965.
- Mohammadi AM, Sharma M, Beaumont TL, et al. Upfront magnetic resonance imaging-guided stereotactic laser-ablation in newly diagnosed glioblastoma: a multicenter review of survival outcomes compared to a matched cohort of biopsy-only patients. Neurosurgery. 2019;85(6):762–772.
- Rahmathulla G, Recinos PF, Kamian K, et al. MRI-guided laser interstitial thermal therapy in neuro-oncology: a review of its current clinical applications. Oncology. 2014;87(2):67–82.
- Silva D, Sharma M, Juthani R, et al. Magnetic resonance thermometry and laser interstitial thermal therapy for brain tumors. Neurosurg Clin N Am. 2017;28(4):525–533.
- Voigt JD, Barnett G. The value of using a brain laser interstitial thermal therapy (LITT) system in patients presenting with high grade gliomas where maximal safe resection may not be feasible. Cost Eff Resour Alloc. 2016;14(1):6.
- Hafez DM, Liekweg C, Leuthardt EC. Staged laser interstitial thermal therapy (LITT) treatments to left insular low-grade glioma. Neurosurgery. 2020;86(3):E337–E342.
- Kamath AA, Friedman DD, Akbari SHA, et al. Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: safety, efficacy, and outcomes. Neurosurgery. 2019;84(4):836–843.
- Beaumont TL, Mohammadi AM, Kim AH, et al. Magnetic resonance imaging-guided laser interstitial thermal therapy for glioblastoma of the corpus callosum. Neurosurgery. 2018;83(3):556–565.
- Hawasli AH, Kim AH, Dunn GP, et al. Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus. 2014;37(6):E1.
- Sharma M, Habboub G, Behbahani M, et al. Thermal injury to corticospinal tracts and postoperative motor deficits after laser interstitial thermal therapy. Neurosurg Focus. 2016;41(4):E6.
- Lee I, Kalkanis S, Hadjipanayis CG. Stereotactic laser interstitial thermal therapy for recurrent high-grade gliomas. Neurosurgery. 2016;79:S24–S34.
- Karampelas I, Sloan AE. Laser-induced interstitial thermotherapy of gliomas. Prog Neurol Surg. 2018;32:14–26.
- Kim AH, Tatter S, Rao G, et al. Laser ablation of abnormal neurological tissue using robotic neuroblate system (LAANTERN): 12-month outcomes and quality of life after brain tumor ablation. Neurosurgery. 2020. DOI:10.1093/neuros/nyaa071. [Online ahead of print].
- Rennert RC, Khan U, Bartek J, et al. Laser ablation of abnormal neurological tissue using robotic neuroblate system (LAANTERN): procedural safety and hospitalization. Neurosurgery. 2020;86(4):538–547.
- Morris S-A, Rollo M, Rollo P, et al. Prolonged blood-brain barrier disruption following laser interstitial ablation in epilepsy: a case series with a case report of postablation optic neuritis. World Neurosurg. 2017;104:467–475.
- Leuthardt EC, Duan C, Kim MJ, et al. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):e0148613.
- Bull JMC. A review of immune therapy in cancer and a question: can thermal therapy increase tumor response? Int J Hyperthermia. 2018;34(6):840–852.
- He K, Liu P, Xu LX . The cryo-thermal therapy eradicated melanoma in mice by eliciting CD4+ T-cell-mediated antitumor memory immune response. Cell Death Dis. 2017;8(3):e2703.
- Takaki H, Cornelis F, Kako Y, et al. Thermal ablation and immunomodulation: from preclinical experiments to clinical trials. Diagn Interv Imaging. 2017;98(9):651–659.
- Hersh DS, Kim AJ, Winkles JA, et al. Emerging applications of therapeutic ultrasound in neuro-oncology: moving beyond tumor ablation. Neurosurgery. 2016;79(5):643–654.
- Gu ZT, Wang H, Li L, et al. Heat stress induces apoptosis through transcription-independent p53-mediated mitochondrial pathways in human umbilical vein endothelial cell. Sci Rep. 2014;4:4469.
- Thompson SM, Callstrom MR, Butters KA, et al. Heat stress induced cell death mechanisms in hepatocytes and hepatocellular carcinoma: in vitro and in vivo study. Lasers Surg Med. 2014;46(4):290–301.
- Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2018;25(3):486–541.
- Janko C, Munoz L, Chaurio R, et al. Navigation to the graveyard-induction of various pathways of necrosis and their classification by flow cytometry. Methods Mol Biol. 2013;1004:3–15.
- Song AS, Najjar AM, Diller KR. Thermally induced apoptosis, necrosis, and heat shock protein expression in 3D culture. J Biomech Eng. 2014;7:136.
- Sachet M, Liang YY, Oehler R. The immune response to secondary necrotic cells. Apoptosis. 2017;22(10):1189–1204.
- Bayin NS, Frenster JD, Kane JR, et al. GPR133 (ADGRD1), an adhesion G-protein-coupled receptor, is necessary for glioblastoma growth. Oncogenesis. 2016;5(10):e263.
- Bayin NS, Frenster JD, Sen R, et al. Notch signaling regulates metabolic heterogeneity in glioblastoma stem cells. Oncotarget. 2017;8(39):64932–64953.
- Bayin NS, Ma L, Thomas C, et al . Patient-specific screening using high-grade glioma explants to determine potential radiosensitization by a TGF-β small molecule inhibitor. Neoplasia. 2016;18(12):795–805.
- Feng Y, Tinsley Oden J, Rylander MN. A two-state cell damage model under hyperthermic conditions: theory and in vitro experiments. J Biomech Eng. 2008;130(4):041016.
- Qin Z, Balasubramanian SK, Wolkers WF, et al. Correlated parameter fit of Arrhenius model for thermal denaturation of proteins and cells. Ann Biomed Eng. 2014;42(12):2392–2404.
- Rofstad EK, Brustad T. Arrhenius analysis of the heat response in vivo and in vitro of human melanoma xenografts. Int J Hyperthermia. 1986;2(4):359–368.
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia. 2014;30(8):531–539.
- Toraya-Brown S, Sheen MR, Zhang P, et al. Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors. Nanomedicine. 2014;10(6):1273–1285.
- Chen Q, Xu L, Liang C, et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193.
- Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486.
- Zhao J, Chen AX, Gartrell RD, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25(3):462–469.
