Abstract
Objective
To explore various microwave (MW) time/power combinations to achieve maximum single-probe system performance in a live pig liver model.
Methods
Fifty-one microwave ablations performed in 12 female pigs using the following time/power combinations: 65 W for 10 min (65W 10MIN), ramped from 20 to 65 W (RAMPED), 95 W pulses with cooling periods (95W PULSED), 40 W for 16 min 15 s (LOW POWER), 1 min 95 W pulse then 8 min 65 W then a second 1 min 95 W pulse (BOOKEND 95W) and 65 W for 15 min (65W 15MIN). Temperatures 1.5 cm from the antenna were measured. Livers were excised, and ablations were measured and compared.
Results
At fixed overall energy, LOW POWER produced ablation zones with the smallest volume compared to 65W 10MIN, RAMPED and 95W PULSED. At a fixed time of 10-min, BOOKEND 95W protocol achieved wider and larger ablation zones than 65W 10MIN (p = 0.038, p = 0.008) and 95W PULSED (p = 0.049, p = 0.004). The 65W 15MIN combination had significantly larger diameters (p = 0.026), larger lengths (p = 0.014) and larger volumes (p = 0.005) versus 65W 10MIN. Maximum temperatures were highest with BOOKEND 95W (62.9 °C) and 65 W 15 MIN (63.0 °C) and lowest with LOW POWER (45.9 °C), p = 0.009.
Conclusions
Low power ablations, even if controlled for total energy delivery, create small ablation zones. High peak powers are associated with larger ablation zones and high margin temperatures if cooling pauses are avoided. Ramping and pulsing protocols with interleaved cooling appear to be of no benefit versus continuous 65 W for creating large ablation zones.
Introduction
Percutaneous thermal ablation with radiofrequency (RF) and microwave (MW) is accepted for the treatment of small, unresectable liver tumors [Citation1]. The overall goal of thermal ablation procedures is to deliver lethal temperatures to both the tumor and an ablative margin. The creation of an adequate (10 mm) circumferential ablation margin is known to decrease local tumor progression (LTP) and improve survival for patients with both hepatocellular carcinoma and liver metastases [Citation2–6]. However, despite recent improvements in ablation technology, high reported rates of LTP (5–48%) suggest that ablation zones and margins are often inadequate [Citation5,Citation7,Citation8].
With a particular system and antenna, the proceduralist has some ability to adjust the size and shape of MW ablation zones by varying generator power and time. Still, the vast array of time and power combinations makes choosing a specific protocol to maximize system performance unclear. While increasing time and power are known to create larger ablation zones, the relationship between these variables is complex and non-linear [Citation9]. For example, doubling either the time or power does not necessarily double the volume of the ablation zone [Citation10]. In practice, ablation zone sizes and shapes are heavily influenced by physiologic factors such as blood flow, lipid content, cirrhosis, prior chemotherapy, tumor properties, and physical factors such as mechanical tissue deformation (shrinkage) and tissue property changes in response to ablative heating [Citation9,Citation11–13]. High peak powers are known to help drive maximum ablation zone size, but the exact time of peak power for maximum effect is unknown [Citation9]. Questions also remain as to the precise relationship between total delivered energy (Energy = Power × Time) and the impact of various power/time combinations. To date, these uncertainties have led to a lack of standardization, resulting in a wide range of parameters, technical success, local tumor progression and procedural-related complications amongst different users, manufacturers and centers [Citation5,Citation14–22].
The purpose of this study is to determine the effect of varying time, peak power, and total delivered energy on ablation zone size and shape in a live animal model using six representative power and time protocols.
Materials and methods
Live pig model and ablation
This protocol was approved by the Institutional Animal Care and Use Committee and included a total of twelve female domestic swine (mean weight 42.5 kg, range 40–44 kg). The animals were initially sedated with a pre-mixed cocktail of 2.5 mg/kg of ketamine (Ceva Animal Health, Glenorie, NSW, Australia), 2.5 mg/kg of xylazine (Ilium/Troy Laboratories, NSW, Australia), 5.0 mg/kg of tiletamine HCl and zolazepam HCl (Zoletil 50, Virbac Animal Health, Carros Cedex, France) and 1 ml of atropine via an intra-muscular injection. Isoflurane was then used for inhalational anesthesia throughout the procedure.
The liver was exposed through a bilateral subcostal incision. Using ultrasound, a 3-dimensional region of liver parenchyma approximately 5.0 × 5.0 × 5.0 cm devoid of large vasculature (>4 mm in diameter) was identified. A 6.0 × 1.2 × 1.2 cm solid acrylic block with laser-cut parallel holes 1.5 cm apart was applied to the liver surface, and a MW antenna (PR 15; NeuWave Medical, Madison Wisconsin, USA) was advanced 4.5–5.0 cm through the center hole. All MW antennas used for this study were 17-gauge, ‘modified triaxial’ design operating at 2.45 GHz. The antenna’s radiating segment length is 15 mm, and the system uses carbon dioxide gas to ensure the antenna shaft never exceeds 41 °C. [Citation23] A second microwave 17 G antenna was then advanced through an adjacent hole to equal depth and used passively as a thermocouple to measure maximum temperature 1.5 cm from the heating antenna. Power was never applied to this second antenna. Lastly, an 8 Fr flexible latex catheter was advanced over a Chiba needle through the last hole of the acrylic block as a fixed position marker to account for the known tissue contraction during MW () [Citation12]. The Chiba needle was removed before the start of ablation. MW power was applied using one of the six protocols described in .
Figure 1. Diagrammatic illustration of a microwave ablation cross-section (left). Actual photograph of a microwave ablation performed in an in-vivo pig liver model (right).
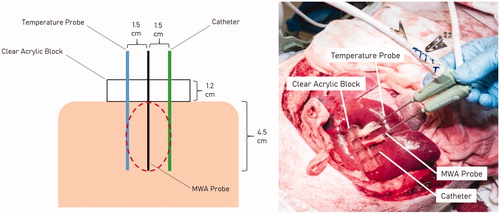
Table 1. The 6 protocols.
The various protocols are summarized in . The power refers to the power setting at the generator; the actual power delivered is estimated to be 65% of the power setting at the generator. The first 4 protocols were designed to each deliver a fixed total energy of 650 W-min (39 kJ): (1) 65 W continuous for 10 min (65W 10MIN), (2) 25 W-65W ramped protocol (RAMPED), (3) high-power pulses with 31–32 s cooling pauses (95W PULSED), and (4) low-power 40 W continuous for 16 min 15 s (LOW POWER). Protocol 3 (95W PULSED) was designed to deliver total energy of 650 W-min (39 kJ) in 10 min, six 95 W/1-min pulses were performed with 31–32 s cooling pauses (570 W-min) followed by a shorter 51 s 95 W pulse (total 650 W-min). The rationale for including RAMPED and 95W PULSED protocols was to evaluate if techniques used in RFA to reduce charring are also advantageous in MWA. Protocols 5 and 6 were designed to explore power levels nearing the system limit (BOOKEND 95W), and prolonged high-power ablations (65 W 15 MIN), respectively. The BOOKEND 95W protocol consisted of a 95 W pulse for 1 min, followed by continuous 65 W for 8 min, then a second 1 min 95 W pulse with no interleaved cooling pauses (710 W-min, 42.6 kJ). The 65 W 15 MIN protocol was the longest and highest total energy protocol (975 W-min, 58.5 kJ) employed in this study. The different protocols were randomly distributed throughout the various lobes of the pig liver to minimize bias based on the assigned lobe. After ablations were completed, pigs were euthanized using an intravenous barbiturate overdose (300 mg/ml pentobarbitone sodium). Completing all ablations in 1 pig takes approximately 1 h–1 h 15 min. The liver was then harvested en-bloc and the ablations were sectioned in sequence.
Ablation zone measurements
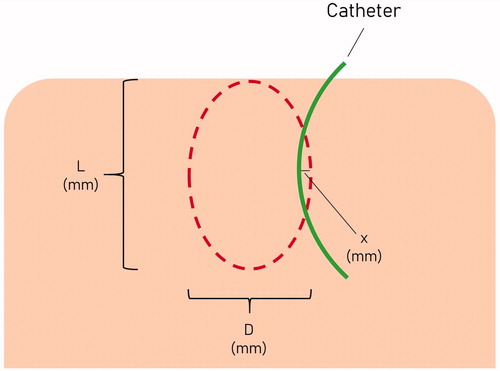
All ablation zones were sliced, measured, and recorded by a single radiology resident with three years of research and radiology experience using previously described methods [Citation12]. The ablation zones were sectioned sequentially starting from the first ablation performed in that pig. The ablation zones were cut into 5-mm-thick sections in the axial plane parallel to the insertion tracts of the microwave antennas and catheter across the largest diameter of the ablation zone. [Citation24] The ablation zones were then stained with triphenyl tetrazolium chloride (TTC, 2,3,5-triphenyl-2H-tetrazolium chloride) to help distinguish metabolically active (alive) versus non-viable ablated tissue [Citation25,Citation26]. The ablation zone dimensions were measured to the nearest 1 mm with a Vernier caliper. illustrates how the ablation zones were measured. The diameter (D) was defined as the maximal diameter of the ablation zone measured in the plane perpendicular to the antenna. The length (L) was defined as the distance between the proximal and the distal edges of the ablation zone, in the axis of the antenna. When L of the ablation extended beyond the liver surface, the distance from the deep edge of the ablation zone to the widest part of the ablation along the antenna track was measured and multiplied by 2 to obtain an estimated L for that ablation. To account for tissue contraction, the maximum distance the ablation zone extended beyond (or failed to reach) the known fixed distance catheter (15 mm from the MW antenna) was measured as ‘x’ mm. Effective D (ED) was the calculated diameter that accounted for contraction, and determined using the formula: Effective D = 30 mm + 2x. If the ablation zone did not reach the catheter, x was negative, resulting in effective D being less than 30 mm. Ablation volumes (assuming an ellipsoidal shape) were calculated using both ‘diameter’ and ‘effective diameter’ and denoted as ‘volume’ and ‘effective volume’, respectively, using the formula (4/3πabc). Ellipticity index (EI) for both ‘diameter’ and ‘effective diameter’ were calculated and denoted as ‘ellipticity index’ and ‘effective ellipticity index’, respectively. Ellipticity index was calculated using the formula: diameter/length [Citation24,Citation27].
Statistical analysis
Treatments were distributed randomly amongst lobes which were considered as independent subjects. A one-way ANOVA was performed on all variables as all variables have passed Shapiro-Wilk’s Normality test. Based on the ANOVA results, a Bonferroni’s post-hoc test was applied to determine comparisons between any two groups. Statistical analysis was performed using Stata (version 13.1, College Station, TX: StataCorp LP), significance tests were 2-sided at the 5% significance level.
Results
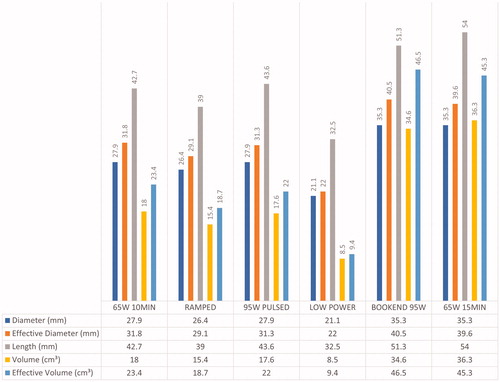
The overall results are summarized in and and . A total of 56 ablations were created in 12 pigs (mean 4.7 ablations per pig, range: 4–5 ablations per pig). Five ablation zones were excluded as they were distorted by the adjacent blood vessel. Fifty-one ablation zones were included in our analysis: 65W 10MIN (n = 9), RAMPED (n = 9), 95W PULSED (n = 8), LOW POWER (n = 8), BOOKEND 95W (n = 8), 65W 15MIN (n = 9).
Figure 3. Representative images of the ablation zones after TTC staining illustrate differences between the various protocols. The photographs are to scale. (A) 65 W continuous for 10 min (65W 10MIN), (B) 25 W–65W ramped protocol (RAMPED), (C) high-power pulses with 31–32 s cooling pauses (95W PULSED), (D) low-power 40 W continuous for 16 min 15 s (LOW POWER), (E) 95 W pulse for 1 min, followed by continuous 65 W for 8 min, then a second 1 min 95 W pulse with no interleaved cooling pauses (BOOKEND 95W), (F) 65 W continuous for 15 min (65W 15MIN).
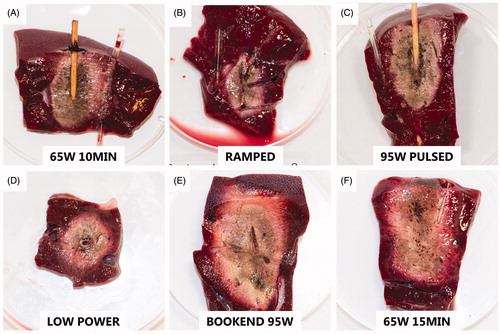
Table 2. Summary of all 6 protocols.
Comparison of protocols with equivalent overall energy
Among the four protocols with equivalent delivered energy (65W 10MIN, RAMPED, 95W PULSED, LOW POWER), the smallest ablation zone was created by LOW POWER (volume = 2.9 cm³) while the largest ablation zone was created by 65W 10MIN (volume = 26.7 cm³). LOW POWER produced ablation zones with significantly smaller effective D compared to 65W 10MIN (p = 0.019) and 95W PULSED (p = 0.042). LOW POWER also produced ablation zones with the smallest D (mean D = 21.1 mm), smallest L (mean L = 32.5 mm) and smallest volume (mean measured volume = 8.5 cm³; mean effective volume= 9.4 cm³) amongst all protocols, though no statistical significance was found.
Comparison of protocols with fixed time (10-min)
At a fixed time of 10-min, BOOKEND 95W made ablation zones with a larger D than 65W 10MIN (p = 0.038) and 95W PULSED (p = 0.049) protocols, and a larger effective D than 95W PULSED (p = 0.042). BOOKEND 95W ablations were also significantly larger in volume when compared to 65W 10MIN (volume, p = 0.008; effective volume, p = 0.008) and 95W PULSED (volume, p = 0.004; effective volume, p = 0.002), respectively. BOOKEND 95W also produced larger L (mean length = 51.3 mm) though no statistically significant difference was found.
Effect of extending ablation time from 10 to 15 min
Ablation zones created by 65W 15MIN had significantly larger D (p = 0.026), larger L (p = 0.014) and larger volumes compared to 65 W 10 MIN (volume, p = 0.002; effective volume, p = 0.005). While the 15-min ablations had a larger effective D than at 10 min (39.6 mm versus 31.8 mm), this difference was not statistically significant.
Comparison of protocols with high delivered energy
The two protocols with the highest amount of delivered energy were 65W 15MIN and BOOKEND 95W (58.5 kJ and 42.6 kJ, respectively). Despite the difference in total energy delivery, there was no statistically significant difference found between D, L, effective D, volume and effective volume for both of these protocols.
Ellipticity index
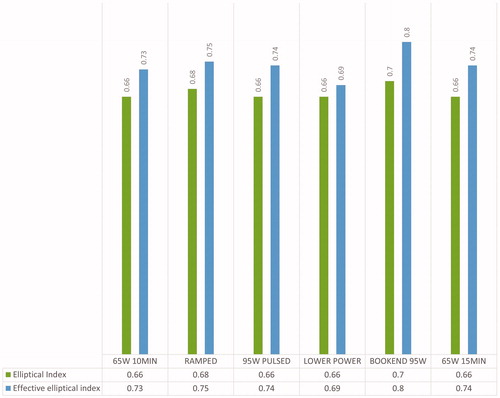
Among the protocols, the ablation zones made by BOOKEND 95W were the roundest (mean EI = 0.7, mean effective EI = 0.8), though no statistically significant difference was found ().
Temperatures
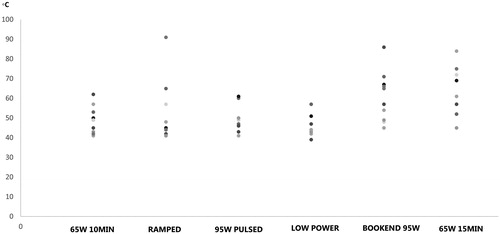
illustrates the maximum temperatures at 1.5 cm from the heating antenna for each of the protocols in the scatter plot diagram. The highest temperatures were recorded with 65W 15MIN (mean maximum temperature = 63.0 °C). The lowest temperatures were recorded with LOW POWER (mean maximum temperature = 45.9 °C). Statistically significant differences were only found between these 2 protocols (p = 0.045).
Discussion
The results of this study demonstrate the complex nature of tissue and microwave energy interactions and underscore the importance of how energy is applied. To maximize ablation zone size with a single antenna, there appear to be several important considerations. First, the application of more total energy appears to increase ablation zone size, but only when ablations are performed above a certain power threshold. Exploring this exact threshold was beyond the scope of this study, but 65 W appeared to outperform a lower power protocol (40 W) even when controlled for total energy applied. Increasing ablation time also increased ablation zone size when performed at high power (65 W). Peak power also appears to play a role in ablation zone size with some of the largest ablations created using 95 W pulses at the beginning and end of a 65 W ablation.
Lower applied powers created smaller ablation zones, even when an identical amount of energy was delivered to tissue. In an earlier study at a fixed input energy of 30 kJ, Bedoya et al. found that ablations produced at 25 W were significantly smaller in volume compared to 50 W or 100 W [Citation9]. The etiology of the smaller ablations created using low power in this and the prior study is likely due to in vivo perfusion mediated cooling. At low powers, blood vessels divert the applied heat away from the ablation zone, limiting ablation zone size. However, at higher powers and temperatures, blood vessels are overpowered and more likely to thrombose, thus limiting the primary source of tissue cooling [Citation9]. This has implications for clinical practice, but also likely explains the over-estimation of ablation zone sizes by manufacturer’s charts in ex vivo non-perfused tissue, particularly at low powers [Citation28]. The rate of power application may also have critical biologic effects. Velez et al. showed that low power ablations may have undesirable pro-oncogenic peri-ablation inflammatory and off-target effects that are not seen at high powers in an animal model [Citation29].
Sequential increases in power application (ramping) is a vestige of RF ablation in which early use of large amounts of power can result in impedance spikes, tissue charring, and ultimately small ablations [Citation30]. In this study, there appeared to be no particular benefit of ramping MW power in terms of ultimate ablation size. Continuous application of 65 W produced slightly larger ablations than ramped in a slightly shorter time (18.0 cm³ versus 15.4 cm³, 10 min versus 10 min 46 s, respectively). In fact, the reverse strategy of rapidly heating tissue (BOOKEND 95W) created the largest ablations of all the strategies examined.
The system and probes that were used for this study are rated for a maximum of 65 W in continuous power (ablation) mode. However, short 95 W pulses of up to one minute are possible in “surgical mode.” Thus, we explored two different methods of applying 95 W pulses: (1) one-minute 95 W pulses with interleaved 32 s cooling periods, and (2) bookend 95W pulses with no cooling pauses. BOOKEND 95W achieved ablation zones equivalent with those made by 65W 15MIN despite less total energy delivered (42.6 kJ versus 58.5 kJ) and a shorter duration (10 versus 15 min). This implies that high peak powers can contribute to larger ablation zones if applied with no cooling pauses, suggesting that systems with the ability to add short high-power pulses can increase ablation size and/or reduce procedure time without creating unpredictable shapes. The 95 W pulses with interleaved cooling periods (95W PULSED) appear to have no advantages versus 65W 10MIN, a finding similar to that of Bedoya et al. [Citation9]. In that study, there was no difference between continuous power (50 W, 600 s) and pulsed high power with cooling (100 W, 75 s time-on, 75 s time-off, 50% duty-cycle) equating for total energy (30 kJ) and average power (50 W). This suggests that the cooling pauses in this and the Bedoya study caused significant peripheral heat dissipation; as a result, much of the following pulse was used to reheat previously ablated tissue before further expansion of the ablation zone. When cooling pauses were avoided (BOOKEND 95W), the ablation zones expanded in size. Based on this finding, even larger ablation zones may be possible with an increased number of 95 W pulses interleaved with 65 W and no cooling pauses. However, testing all the potential combinations of 95 W pulses, cooling pauses, and 65 W power was beyond the scope of this study.
The highest temperatures at a point 1.5 cm radially from the heating antenna were found with BOOKEND 95W and 65W 15MIN. However, the only statistically significant difference was between LOW POWER and 65W 15MIN protocols. Higher maximum temperatures appeared to correlate with the largest ablation zones, an expected result. High temperatures at the periphery of ablation zones are a desired result as local recurrences are almost exclusively located at the periphery due to the exponential decrease in temperatures away from the applicator [Citation14]. Our study suggests that using a continuous delivery of high-energy achieves higher peripheral temperatures and has the potential to decrease the rate of local tumor progression, primarily by expanding the ablation zone at the periphery and increasing ablation margins.
There were certain limitations to this study, the first being the use of a healthy porcine liver model. Ablation zone propagation is likely to differ in a heterogeneous tumor with a pseudocapsule in cirrhotic liver versus metastatic tumors in healthy or steatotic livers. Additionally, the swine model has liver lobes which are flatter and more anatomically separated by deep fissures and a separate blood supply when compared to the human liver. While there is no perfect surrogate for human clinical tumors in a diseased background liver, pigs are widely used for pre-clinical studies of human thermal ablation devices due to the scale of the model [Citation12,Citation27,Citation31,Citation32]. Secondly, the protocols were randomized across liver lobes, and each liver lobe in each pig was considered to be an independent subject. It can be argued that early ablations might alter the pig’s hemodynamic status and hepatic blood flow in an animal. Nevertheless, this potential effect is at least minimized by randomization. Thirdly, the size and shape of the ablation zone are potentially altered by the passive antenna and the latex catheter. To our knowledge, there is no prior in vivo MWA study using the same probe with similar protocols in a porcine model for comparison; such a study would help account for these effects. An additional limitation was the limitation in resources, resulting in a limited number of power and time combinations that could be tested. The results of this study demonstrate that several other power and time combinations may be of interest, including multiple 95 W pulses interleaved with 65 W, 65 W for 20 min, etc. Likewise, this study focused on achieving the largest ablation zones feasible with a single antenna, a situation that might not apply to specific clinical scenarios where narrower or shorter ablation zones are desired. Lastly, in this particular MW system, the power is measured at the generator (applied power), not at the probe tip (delivered power). Therefore, systems with different probe and system designs could have different delivered power and the results obtained in this study may not be directly applicable to all manufacturers.
In conclusion, this study demonstrates that the method of power application is highly impactful on the size of the ultimate ablation zone. Low powers, even if applied for prolonged times, are associated with smaller ablation zones and lower temperatures. High peak powers are associated with larger ablation zones if cooling pauses are avoided. Longer ablation times and higher total energy delivered also create large ablations if a power threshold is met. Ramping and pulsing protocols with interleaved cooling (similar to those used in RFA to minimize charring) appear to be of no benefit versus continuous 65 W for creating large ablation zones with MWA.
Ethical approval
Approval from the institutional animal care committee was obtained prior to conduct of this study.
Guarantor
The scientific guarantor of this publication is Dr. Uei Pua.
Statistics and biometry
We seeked the expertise of our in-house statisticians, Aruni Seneviratna MBBS, MPH and Xin Tang BSc, MSc (Tan Tock Seng Hospital, Singapore).
Acknowledgments
The authors thank Fred Chua, BVSc, MSc, MRCVS, Jean Chor BVMSc, Yvonne Ng MBBS, FRCS, Marjorie DiMaggio MD, Daniel Choo MBBS, Spencer Tan for their assistance in the animal laboratory, and Aruni Seneviratna MBBS, MPH and Xin Tang BSc, MSc for their help with biostatistics.
Disclosure statement
Dr. Brace reports personal fees from Ethicon, Inc (paid consultant).
Dr. Hinshaw reports personal fees from NeuWave Medical (paid consultant) and stock holder of Accure Medical, Histosonics, Elucent, Cellectar Biosciences outside the submitted work.
Dr. Lee, Jr. reports grants from Singapore Radiological Society Trust Research Grant, grants from Johnson and Johnson Inc. (Ethicon, Inc.) during the conduct of the study; personal fees from Ethicon, Inc. (paid consultant), personal fees from Histonics, Inc. (Board of Directors, Stock Holder, Research Support), outside the submitted work; In addition, Dr. Lee, Jr. has a patent Medtronics, Inc with royalties paid.
Additional information
Funding
References
- [email protected] EAftSotLEa, Liver EAftSot. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275.e1.
- Zytoon AA, Ishii H, Murakami K, et al. Recurrence-free survival after radiofrequency ablation of hepatocellular carcinoma. A registry report of the impact of risk factors on outcome. Jpn J Clin Oncol. 2007;37(9):658–672.
- Liao M, Zhong X, Zhang J, et al. Radiofrequency ablation using a 10-mm target margin for small hepatocellular carcinoma in patients with liver cirrhosis: a prospective randomized trial. J Surg Oncol. 2017;115(8):971–979.
- Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology. 2016;278(2):601–611.
- Bai XM, Yang W, Zhang ZY, et al. Long-term outcomes and prognostic analysis of percutaneous radiofrequency ablation in liver metastasis from breast cancer. Int J Hyperthermia. 2018;35(1):183–193.
- Meloni MF, Chiang J, Laeseke PF, et al. Microwave ablation in primary and secondary liver tumours: technical and clinical approaches. Int J Hyperthermia. 2017;33(1):15–24.
- Leung U, Kuk D, D'Angelica MI, et al. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg. 2015;102(1):85–91.
- Bedoya M, del Rio AM, Chiang J, et al. Microwave ablation energy delivery: influence of power pulsing on ablation results in an ex vivo and in vivo liver model. Med Phys. 2014;41(12):123301
- Laeseke PF, Lee FT, van der Weide DW, et al. Multiple-antenna microwave ablation: spatially distributing power improves thermal profiles and reduces invasiveness. J Interv Oncol. 2009;2(2):65–72.
- Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng. 2010;38(1):65–78.
- Brace CL, Diaz TA, Hinshaw JL, et al. Tissue contraction caused by radiofrequency and microwave ablation: a laboratory study in liver and lung. J Vasc Interv Radiol. 2010;21(8):1280–1286.
- Farina L, Weiss N, Nissenbaum Y, et al. Characterisation of tissue shrinkage during microwave thermal ablation. Int J Hyperthermia. 2014;30(7):419–428.
- Swan RZ, Sindram D, Martinie JB, et al. Operative microwave ablation for hepatocellular carcinoma: complications, recurrence, and long-term outcomes. J Gastrointest Surg. 2013;17(4):719–729.
- Shi J, Sun Q, Wang Y, et al. Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. J Gastroenterol Hepatol. 2014;29(7):1500–1507.
- Groeschl RT, Pilgrim CH, Hanna EM, et al. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg. 2014;259(6):1195–1200.
- Zhang NN, Lu W, Cheng XJ, et al. High-powered microwave ablation of larger hepatocellular carcinoma: evaluation of recurrence rate and factors related to recurrence. Clin Radiol. 2015;70(11):1237–1243.
- Sun AX, Cheng ZL, Wu PP, et al. Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. WJG. 2015;21(10):2997–3004.
- Liu Y, Li S, Wan X, et al. Efficacy and safety of thermal ablation in patients with liver metastases. Eur J Gastroenterol Hepatol. 2013;25(4):442–446.
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S192–S203.
- Iannitti DA, Martin RC, Simon CJ, et al. Hepatic tumor ablation with clustered microwave antennae: the US Phase II trial. HPB (Oxford). 2007;9(2):120–124.
- Xu HX, Lu MD, Xie XY, et al. Prognostic factors for long-term outcome after percutaneous thermal ablation for hepatocellular carcinoma: a survival analysis of 137 consecutive patients. Clin Radiol. 2005;60(9):1018–1025.
- Lubner MG, Hinshaw JL, Andreano A, et al. High-powered microwave ablation with a small-gauge, gas-cooled antenna: initial ex vivo and in vivo results. J Vasc Interv Radiol. 2012;23(3):405–411.
- Mulier S, Ni Y, Frich L, et al. Experimental and clinical radiofrequency ablation: proposal for standardized description of coagulation size and geometry. Ann Surg Oncol. 2007;14(4):1381–1396.
- Mattson AM, Jensen CO, Dutcher RA. Triphenyltetrazolium chloride as a dye for vital tissues. Science. 1947;106(2752):294–295.
- Fishbein MC, Meerbaum S, Rit J, et al. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101(5):593–600.
- Lubner MG, Ziemlewicz TJ, Hinshaw JL, et al. Creation of short microwave ablation zones: in vivo characterization of single and paired modified triaxial antennas. J Vasc Interv Radiol. 2014;25(10):1633–1640.
- Winokur RS, Du JY, Pua BB, et al. Characterization of in vivo ablation zones following percutaneous microwave ablation of the liver with two commercially available devices: are manufacturer published reference values useful? J Vasc Interv Radiol. 2014;25(12):1939–1946.e1.
- Velez E, Goldberg SN, Kumar G, et al. Hepatic thermal ablation: effect of device and heating parameters on local tissue reactions and distant tumor growth. Radiology. 2016;281(3):782–792.
- Appelbaum L, Sosna J, Pearson R, et al. Algorithm optimization for multitined radiofrequency ablation: comparative study in ex vivo and in vivo bovine liver. Radiology. 2010;254(2):430–440.
- Ruiter SJS, Heerink WJ, de Jong KP. Liver microwave ablation: a systematic review of various FDA-approved systems. Eur Radiol. 2019;29(8):4026–4035.
- Yu NC, Raman SS, Kim YJ, et al. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19(7):1087–1092.