Abstract
Background
Transcatheter arterial chemoembolization (TACE), radiofrequency ablation (RFA), and microwave ablation (MWA) are regarded as effective therapies for treating unresectable hepatocellular carcinoma (HCC). We conducted this study to compare the efficiency and safety of TACE combined with RFA (TR group) or MWA (TM group).
Method
PubMed, the Cochrane Library, Ovid Medline, Web of Science, Scopus, Embase, ScienceDirect, and Google Scholar were searched. The primary endpoints were overall survival (OS), progression-free survival (PFS), response rates, and complications.
Result
Eight cohort studies and one randomized controlled trial were included. The TM group had better OS (Hazard ratio [HR]: 1.55; 95% confidence interval [CI]: 1.09–2.21, p = 0.01) and a better 2- and 3-year OS rate, 24-month PFS rate (Risk ratio [RR]: 0.67; 95% CI: 0.46–0.96, p = 0.03), and complete response rate (RR: 0.87; 95% CI: 0.79–0.96, p = 0.003) than the TR group. Furthermore, the TM and TR groups did not show significant differences in PFS, the disease control rate or complications. The advantage of TM was mainly reflected in younger patients (50–60 years old) compared with patients aged 60–70 years, as well as in patients with larger tumors (≥3 cm) compared with patients with tumors <3 cm. Moreover, patients treated with conventional TACE (cTACE) in the TM group showed longer OS, while patients treated with drug-eluting bead transarterial chemoembolization (DEB-TACE) in the TR group showed a higher overall response rate.
Conclusion
TM seems to be a more effective therapy than TR for unresectable HCC, with better survival and similar safety.
Introduction
With 841,080 diagnosed cases and 781,631 deaths in 2018 worldwide, hepatocellular carcinoma (HCC) is regarded as the fifth most malignant tumor worldwide [Citation1, Citation2]. Although surgery and liver transplantation are effective treatments [Citation3], fewer than 30% of patients can receive these therapies because of late tumor stages, unsuitable tumor locations, extensive disease, limited liver functional reserves, and high operative risks [Citation4].
Transcatheter arterial chemoembolization (TACE) is the mainstream therapy for treating unresectable HCC with good toleration and rare complications [Citation5]. According to many clinical practice guidelines, ablative techniques, especially radiofrequency ablation (RFA) and microwave ablation (MWA), are considered curative therapies for unresectable HCC [Citation6, Citation7]. Currently, combination therapies, such as TACE combined with ablative techniques or systemic therapies, are effective treatments for unresectable HCC [Citation8]. Although many scholars have compared the efficiency and safety of RFA and MWA, the efficiency and safety of TACE combined with RFA (TR) compared with those of TACE combined with MWA (TM) in treating HCC remain unclear. Wang et al. suggested that the short-term local efficiency, complications, and one-year survival rates of TR and TM were not significantly different. However, TR causes slighter injury than TM regarding postoperative liver damage [Citation9]. Yuan et al. and Shen et al. reported that TM is more efficient than TR for tumors larger than 5 cm but that the postoperative liver damage resulting from TR is less than that resulting from TM [Citation10, Citation11]. Ginsburg et al. showed that both combination therapies, TR and TM, are effective therapies for HCC [Citation12].
The purpose of this study was to compare the survival, disease control, and safety outcomes between MWA and RFA in combination with TACE for the treatment of unresectable HCC.
Materials and methods
This meta-analysis and systemic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Table S1) [Citation13].
Search strategy
Related articles published before August 9, 2019 were searched in PubMed, Scopus, Web of Science, Ovid Medline, Embase, ScienceDirect, Google Scholar, and the Cochrane Library. The main search words were as follows: “hepatocellular carcinoma”, “radiofrequency ablation”, “microwave ablation”, and “transcatheter arterial chemoembolization”. The search strategy is detailed in Appendix A1. In this study, no language restriction was set, and additional eligible articles were also included.
Selection criteria
The inclusion criteria were as follows:
P (patients): patients with unresectable HCC were identified as patients who had a late tumor stage, an unsuitable tumor location, extensive disease, limited liver functional reserve, and high operative risk [Citation4].
I (intervention) and C (comparison): TR versus TM.
O (outcomes): progression-free survival (PFS), overall survival (OS), progression-free survival rate (PFSR), overall survival rate (OSR), response rates (complete response rate [CRR], overall response rate [ORR], partial response rate [PRR], and disease control rate [DCR]), aspartate transaminase [AST] level, alanine transaminase [ALT] level, and complications.
S (studies): randomized controlled trials (RCTs) or cohort studies.
The exclusion criteria were as follows: (1) articles lacking the original data; (2) meta-analyses, animal experiments or meeting articles; (3) articles with abstracts only or duplicated data; and (4) articles comparing only RFA and MWA or RFA and MWA combined with other therapies.
Data extraction
The following data were extracted by two investigators: article name, first author, publication year, type of study, nation, number of participants, characteristics of participants (age, sex, tumor type, tumor stage, tumor size, number of tumors, and etiology), type of TACE, treatment order, OS, OSR, PFS, PFSR, response rates (ORR, DCR, CRR, and PRR), AST and ALT levels, and complications. We used the modified Response Evaluation Criteria in Solid Tumors (mRECIST) to assess the response rates [Citation14]. To fully assess survival, we also evaluated the 1-, 2-, 3-, 4-, and 5-year OSRs and the 6-, 12-, 18-, 24-, 30-, and 36-month PFSRs. When any disagreements arose between the two investigators, the outcomes were assessed by the third investigator.
Quality assessment
The two investigators used the five-point Jadad scale to assess the quality of the RCTs. The randomization, masking, and accountability of all patients are the three items of the scale, with scores ≥3 points indicating high-quality studies [Citation15]. Comparability, exposure, and selection are the three main items of the Newcastle-Ottawa Scale (NOS, 9 points), which was used to evaluate the quality of the cohort studies. Articles that scored 8–9 points were regarded as high-quality articles, and those that scored 6–7 points were regarded as medium-quality articles [Citation16]. To evaluate the level of evidence, we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system, which includes the following five evaluation items: inconsistency, risk of bias, imprecision, indirectness, and publication bias. The level of evidence was considered high, moderate, low, or very low [Citation17].
Statistical analysis
We used STATA 12.0 (Stata Corp, TX, USA) and Review Manager 5.3 (Nordic Cochrane Center, Oxford, UK) to evaluate the pooled data and used hazard ratios (HRs) with 95% confidence intervals (CIs) to analyze and present the OS and PFS data. When HR < 1, the results supported the TR group. We extracted data from Kaplan–Meier curves according to the method described by Tierney et al. [Citation18] or directly from the included studies. Risk ratios (RRs) with 95% CIs were used to analyze the dichotomous variables (response rates and complications). When RR > 1, the results supported the TR group in the analysis of response rates or the TM group in the analysis of complications. The mean difference was used to evaluate continuous variables (ALT and AST levels). To examine whether nation, tumor size, treatment order, or Barcelona Clinic Liver Cancer (BCLC) stage influenced the results, we performed a subgroup analysis of OS, PFS, and the ORR. We used the I2 statistic and χ2 test to evaluate heterogeneity. If I2 < 50% or p > 0.1, indicating no significant heterogeneity, we used a fixed-effects model; otherwise, we used a random-effects model. The publication bias and sensitivity analysis were conducted in STATA. We used Egger’s linear regression test [Citation19] and Begg’s rank correlation test [Citation20] to evaluate publication bias. Statistical significance was indicated when p < 0.05.
Results
Search results and study evaluation
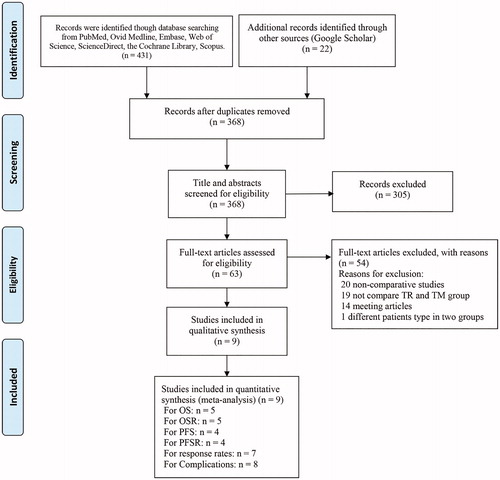
Of 453 initially screened articles, only 9 articles with 238 patients in the TR group and 295 patients in the TM group were included [Citation9, Citation10, Citation12, Citation21–26]. The details of how articles were screened and selected are shown in . We summarized the baseline characteristics of the groups in the included articles (), which were equivalent in both cohorts. Regarding article quality, there were six high-quality articles and three medium-quality articles (Table S2). All levels of evidence were low or very low, as presented in Table S3.
Table 1. Characteristics of the included studies.
Tumor response
Five articles included 142 patients in the TR group and 174 patients in the TM group to assess OS. The TM group showed longer OS than the TR group (HR: 1.55; 95% CI: 1.09–2.21, p = 0.01) (). The TM group also had a higher 2-year (RR: 0.68; 95% CI: 0.53–0.88, p = 0.003) and 3-year (RR: 0.46; 95% CI: 0.32–0.66, p < 0.0001) OSR. Moreover, the TM group tended to have a higher 1-year (RR: 0.94; 95% CI: 0.85–1.05, p = 0.29), 4-year (RR: 0.49; 95% CI: 0.18–1.35, p = 0.17), and 5-year (RR: 0.47; 95% CI: 0.19–1.18, p = 0.11) OSR (Figure S1).
Figure 2. Forest plot of the HR or RR of OS (A) and PFS (B) associated with the TR group versus the TM group.
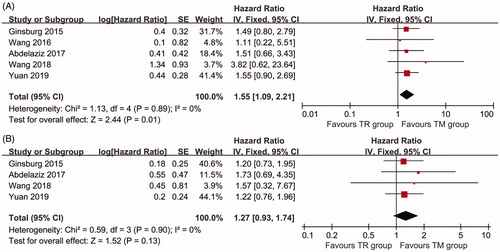
Three studies were used to compare PFS between the two groups. There was no significant difference between TM and TR group for PFS (HR: 1.27; 95% CI: 0.93–1.74, p = 0.13) (). The TM group had a higher 24-month PFSR than the TR group (RR: 0.67; 95% CI: 0.46–0.96, p = 0.03) and tended to have a higher 12-month (RR: 0.87; 95% CI: 0.70–1.09, p = 0.22), 18-month (RR: 0.75; 95% CI: 0.53–1.08, p = 0.12), 30-month (RR: 0.39; 95% CI: 0.12–1.24, p = 0.11), and 36-month (RR: 0.71; 95% CI: 0.39–1.29, p = 0.26) PFSR (Figure S2).
The results showed no significant difference in the ORR (RR: 1.02; 95% CI: 0.95–1.10, p = 0.52) (94.18% vs 87.68%) or DCR (RR: 1.03; 95% CI: 0.96–1.11, p = 0.46) (95.24% vs 88.41%), while the TM group had a higher CR rate (RR: 0.87; 95% CI: 0.79–0.96, p = 0.003) (75.66% vs 86.43%) and a lower PR rate (RR: 2.16; 95% CI: 1.30–3.57, p = 0.003) (21.47% vs 8.8%) than the TR group (, ).
Figure 3. Forest plot of the RR of the subgroup analysis of response rates associated with the TR group versus the TM group.
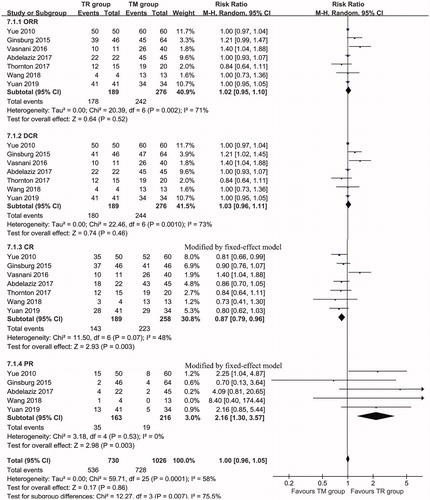
Table 2. Response rates of the TR group and the TM group.
Complications
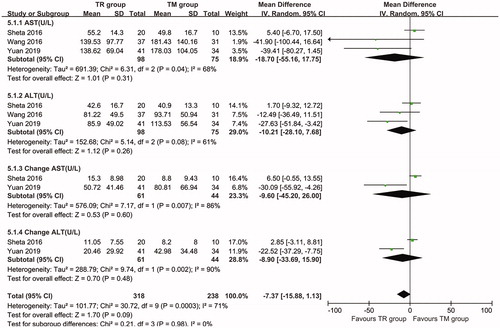
Four articles were used to evaluate the liver function of the patients. The results show that there was no significant difference in the AST (Mean difference [MD]: −18.70; 95% CI: −55.16 to 17.75, p = 0.31) or ALT (MD: −10.21; 95% CI: −28.10 to 7.68, p = 0.26) level after treatment. In addition, there was no significant difference in the increment of the AST (MD: −9.60; 95% CI: −45.2 to 26.00, p = 0.60) or ALT (MD: −8.90; 95% CI: −33.69 to 15.90, p = 0.48) level ().
Figure 4. Forest plot of the RR of ASL (A), AST (B), the change in AST (C), and the change in ALT (D) associated with the TR group versus the TM group.
There were no significant differences in total complications between the TM and TR groups (RR: 0.98; 95% CI: 0.63–1.55, p = 0.95) (Figure S3). The top 10 complications were as follows: recurrence, ascites, pain, decompensation, bleeding, shortness of breath, nausea/vomiting, portal vein thrombosis, bone metastasis, and local skin burns. The safety was similar in the two groups (). We also evaluated the top 5 complications in the TR and TM groups (Tables S4 and S5).
Table 3. Top 10 complications associated with the TR group and the TM group.
Subgroup analysis
We conducted a subgroup analysis to evaluate whether the efficiency of TR versus TM was consistent across the subgroups. The outcomes of the subgroup analysis of PFS were stable (). In the subgroup analysis, the advantage of TM was mainly reflected in younger patients (50–60 years old; HR: 1.58; 95% CI: 1.03–2.24, p = 0.04) compared with patients aged 60–70 years, as well as in patients with larger tumors (≥3 cm; HR: 1.62; 95% CI: 1.04–2.53, p = 0.03) compared with patients with tumors <3 cm. Moreover, patients treated with cTACE in the TM group had longer OS (HR: 1.58; 95% CI: 1.03–2.24, p = 0.04), while patients treated with drug-eluting bead transarterial chemoembolization (DEB-TACE) in the TR group had a higher ORR (RR: 1.25; 95% CI: 1.06–1.48, p = 0.009).
Table 4. Subgroup analysis for overall survival, progress free survival, and objective response rate.
Sensitivity analysis
We performed sensitivity analyses to assess the consistency of the ORR and the DCR. The results of the sensitivity analyses were consistent with the results of the main analysis (Figure S4).
Publication bias
We assessed the publication bias, and the results were as follows: OS (Begg’s test p = 1.000; Egger’s test p = 0.551; Figure S5(A)); PFS (Begg’s test p = 0.602; Egger’s test p = 0.496; Figure S5(B)); CR (Begg’s test p = 0.548; Egger’s test p = 0.99; Figure S5(C)); and PR (Begg’s test p = 0.462; Egger’s test p = 0.76; Figure S5(D)).
Discussion
As one of the most common malignant tumors, HCC is the leading cause of cancer-related death worldwide, and curative therapies are considered limited to a restricted number of cases [Citation27, Citation28]. Many studies have reported that TACE combined with thermal ablative techniques has a clear benefit for both small and large lesions [Citation29]. However, the clinical efficiency and safety of TR compared with those of TM for treating HCC remain unclear. This article is the first meta-analysis to evaluate the efficiency and safety of TR and TM. The TM group had better OS and a better 2- and 3-year OSR, 24-month PFSR, and CRR than the TR group. Furthermore, the TM and TR groups did not show significant differences in PFS, the DCR or complications. In the subgroup analysis, the advantage of TM was mainly reflected in younger patients (50–60 years old) compared with patients 60–70 years old, as well as in patients with larger tumors (≥3 cm) compared with patients with tumors <3 cm. Moreover, patients treated with cTACE in the TM group had longer OS, while patients treated with DEB-TACE in the TR group had a higher ORR.
In the survival analysis, we found that the TM group had better OS and tended to have better PFS than the TR group. Moreover, the TM group also tended to have a higher OSR and PFSR than the TR group. Shen et al. reported no significant difference in the 1-year OSR (RFA: 83.64%; MWA: 91.52%) but a significant difference in the 2-year OSR (RFA: 53.85%; MWA: 68.52%); moreover, when the tumor size was between 5 and 10 cm, the recurrence rates were not significantly different between the two groups [Citation11]. However, a meta-analysis reported that percutaneous MWA (p-MWA) had efficiency similar to that of percutaneous RFA (p-RFA) [Citation30]. As mentioned in previous studies, RFA uses an electric current to destroy the tumor, and MWA uses electromagnetic energy, which can destroy tumor cells more completely by accelerating water molecules in the surrounding tissue, producing heat and friction. Due to this consistent high temperature, MWA can be used to ablate larger areas faster, thus exceeding the limitations of RFA [Citation31]. Moreover, the higher CR in the TM group also led to better OS than that in the TR group [Citation21]. To examine whether nation, age, tumor size, or BCLC stage influenced the results, we performed a subgroup analysis. The TM group had better OS regardless of the subgroup; however, because of the small sample size, statistical significance was found only for younger patients (50–60 years old) and larger tumors (≥ 3 cm). Abdelaziz et al. also showed that TM has better efficiency than TR for tumors 3–5 cm [Citation24]. The above results suggest better survival of HCC with TM than TR.
To fully assess the efficiency of TM and TR, we also evaluated the responses rates of the two groups. The TM and TR groups both had high response rates, but the TM group appeared to have a higher CRR and lower PRR than the TR group. With the high ORRs (94.18% vs. 87.68%) in the two groups the significance of a higher CRR and lower PRR in TM group is consistent with the advantage observed in the TM group [Citation24]. Tan et al. suggested that RFA and MWA yielded no significant difference in the CRR [Citation30]. Yue et al. reported TM has a higher CRR than TR [Citation21], while a study by Vasnani et al. showed the opposite result [Citation22]. As mentioned above, the mechanisms of MWA allow it to cover larger zones with higher temperatures than RFA, and this may have led to the higher CRR and lower PRR in the TM group [Citation31].
The major complications of TR and TM remain controversial. We compared the top 10 and total complications between the two groups. Both therapies are safe, with similar incidence rates of total complications. The major complications were recurrence (17.91%), ascites (12.37%), and pain (7.36%). To evaluate the safety of the therapies, we also assessed the AST and ALT levels after treatment and the increment in the AST and ALT levels. No significant differences were found in the AST and ALT levels between the two groups. Wang et al. and Yuan et al. also reported that the postoperative liver damage was milder in the TR group than in the TM group [Citation11, Citation12]. In addition, Liang et al. showed that MWA has a larger ablation range than RFA, which may damage neighboring organs and cause MWA to have a greater effect on liver function [Citation32]. However, our study found similar safety for both therapies with minimal morbidity.
Our study has several limitations that may call for caution in interpreting the results. First, this meta-analysis included only 9 articles with a total of 533 patients, and only 1 article was an RCT, limiting the reliability of the results. Second, most of the included studies were homogeneous in terms of the study design, but different clinical centers, periods, devices, tumor locations, and tumor sizes may increase the heterogeneity of the results. Although we used a random-effects model to analyze data with high heterogeneity, we should be conservative when considering the results. Third, the follow-up times of the included trials were different; some were short, leading to insufficient long-term follow-up data and thus limiting the assessment of long-term effects. Fourth, because the sample size was relatively small, the results of subgroup analysis were under-powered and needed to be interpreted with caution.
Conclusion
TM seems to be a more effective therapy than TR, with better survival and similar safety. Furthermore, the advantage of TM was mainly reflected in younger patients (50–60 years old) compared with patients 60–70 years old, as well as in patients with larger tumors (≥3 cm) compared with patients with tumors <3 cm. However, more high-quality studies are needed to evaluate the superiority of TR to TM.
Ethics approval and consent to participate
All analyses were based on previously published studies, and hence no ethical approval and patient consent were required.
Supplemental Material
Download PDF (410.8 KB)Supplemental Material
Download PDF (211.9 KB)Supplemental Material
Download PDF (195.7 KB)Supplemental Material
Download PDF (180.3 KB)Supplemental Material
Download PDF (180.1 KB)Supplemental Material
Download TIFF Image (2 MB)Supplemental Material
Download TIFF Image (2.1 MB)Supplemental Material
Download TIFF Image (226.2 KB)Supplemental Material
Download TIFF Image (543.2 KB)Supplemental Material
Download TIFF Image (497.4 KB)Supplemental Material
Download PDF (127.2 KB)Acknowledgments
The authors thank Professor Wenbin Ma, MD (Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College) for his statistical advice and Professor Jichun Liu, MD, PhD (The second affiliated hospital of Nanchang University) for his data collection. The authors also thank the support from National Natural Science Foundation of China (NSFC). All authors designed and conducted this review. Jiani Zhao and Wenxiong Zhang wrote the paper. Xinmin Zeng and Qinghua Zeng helped the study design. Yiping Wei revised the statistical methodology. Jiani Zhao and Wenxiong Zhang had primary responsibility for the final content. All authors read and approved the final manuscript.
Disclosure statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Data availability statement
All data generated or analyzed during this study are included in this manuscript and its additional files.
Additional information
Funding
Reference
- Bray F, Ferlay J, Soerjomataram I, et al. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
- Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855.
- Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616.
- Benson A, D’Angelica MI, Abbott DE, et al. National Comprehensive Cancer Network. Hepatobiliary cancers (version 2). Fort Washington (PA): National Comprehensive Cancer Network®; 2018. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750.
- Yu JJ, Yan WT, Quan B. Recommendations for EASL clinical practice guidelines: management of hepatocellular carcinoma. J Clin Hepatol. 2018;34(6):1183–1186.
- Tinkle CL, Haas-Kogan D. Hepatocellular carcinoma: natural history, current management, and emerging tools. Biologics. 2012;6:207–219.
- Wang XW, Fu SZ, Dai F, et al. The curative effect and safety in treating primary hepatocellular carcinoma: comparison between TACE plus radiofrequency ablation and TACE plus microwave ablation. J Intervent Radiol. 2016;25(8):673–676.
- Yuan P, Zhang ZG. K J. Analysis of efficacy and safety of TACE in combination with RFA and MWA in the treatment of middle and large primary hepatic carcinoma. J Buon. 2019;24(1):164–170.
- Shen Z, Yan H. Efficacy and safety of high-power microwave ablation combined with transcatheter arterial chemoembolization in interventional treatment of large liver cancer. Med Pharm J Chin PLA. 2016;28(10):31–35.
- Ginsburg M, Zivin SP, Wroblewski K, et al. Comparison of combination therapies in the management of hepatocellular carcinoma: transarterial chemoembolization with radiofrequency ablation versus microwave ablation. J Vasc Interv Radiol. 2015;26(3):330–341.
- Moher D, Liberati A, Tetzlaff J, et al.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. Br Med J. 2009;339:b2535–b2535.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
- Wells GA, Shea BJ, O’Connell D, et al. The Newcastle–Ottawa Scale (nos) for assessing the quality of non-randomized studies in meta-analysis. Appl Eng Agric. 2014;18(6):727–734.
- Atkins D, Best D, Briss PA, et al.; GRADE Working Group. Grading quality of evidence and strength of recommendations. Br Med J. 2004;328(7454):1490.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
- Yue JY, Li YX, Wang QH, et al. Comparison on therapeutic effect between TACE combined with radiofrequency ablation and TACE combined with psychro-circulation percutaneous microwave coagulation therapy for primary hepatocellular carcinoma. Chin J Terv Imaging Ther. 2010;7(6):656–659.
- Vasnani RJ, Ginsburg M, Ahmed O, et al. Radiofrequency and microwave ablation in combination with transarterial chemoembolization induce equivalent histopathologic coagulation necrosis in hepatocellular carcinoma patients bridged to liver transplantation. Hepatobiliary Surg Nutr. 2016;5(3):225–233.
- Sheta E, El-Kalla F, El-Gharib M, et al. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled study. Eur J Gastroenterol Hepatol. 2016;28(10):1198–1203.
- Abdelaziz AO, Abdelmaksoud AH, Nabeel MM, et al. Transarterial chemoembolization combined with either radiofrequency or microwave ablation in management of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2017;18(1):189–194.
- Thornton LM, Cabrera R, Kapp M. Radiofrequency vs microwave ablation after neoadjuvant transarterial bland and drug-eluting microsphere chembolization for the treatment of hepatocellular carcinoma. Curr Probl Diagn Radiol. 2017;46(6):402–409.
- Wang Y, Ma L, Sheng SP, et al. Combination therapy of TACE and CT-guided partial hepatic segment ablation for liver cancer. Minim Invasive Ther Allied Technol. 2018;27(6):355–364.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314.
- Habib A, Desai K, Hickey R, et al. Transarterial approaches to primary and secondary hepatic malignancies. Nat Rev Clin Oncol. 2015;12(8):481–489.
- Seki T, Tamai T, Nakagawa T, et al. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer. 2000;89(6):1245–1251.
- Tan WC, Deng QW, Lin SY, et al. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):264–272.
- Poggi G, Montagna B, DI Cesare P, et al. Microwave ablation of hepatocellular carcinoma using a new percutaneous device: preliminary results. Anticancer Res. 2013;33:1221–1227.
- Liang P, Wang Y, Yu XL, et al. Malignant liver tumors: treatment with percutaneous microwave ablation-complications among cohort of 1136 patients. Radiology. 2009;251(3):933–940.