Abstract
Purpose
To determine the effectiveness of ablation for pulmonary metastases (PM) from hepatocellular carcinoma (HCC).
Methods
Between 2010 and 2017, the study analyzed 39 patients who had a median age of 59 years. Primary HCC was under control and the number of PM was less than 5 (median: 2), with a maximum diameter of ≤60 mm (median: 15 mm). The primary endpoints were overall survival (OS) and local tumor progression-free survival (LTPFS). Secondary endpoints included technique success (TS), complication and tumor response. TS referred to PM treated using the treatment protocol. Multivariate analysis using the Cox proportional hazard model was conducted on the potential risk factors (univariate: p < 0.5) to determine the independent factors (multivariate: p < 0.05).
Results
The TS rate was 100%. Major complications included pneumothorax (n = 3) requiring chest tube placement and pleural effusion requiring drainage (n = 2). Complete ablation was achieved in 32/38 patients (valid percent: 84.2%) at 1 month after ablation. The 1-, 3- and 5-year OS rates were 79.8, 58 and 30.9%, respectively. The 1-, 3- and 5-year LTPFS rates were 60.7, 34.2 and 22.8%, respectively. The extent (unilateral vs. bilateral) of PM (hazard ratio (HR): 0.197, 95% confidence interval (CI): 0.043–0.890, p = 0.035) and the number (≤2 vs. >2) of PM (HR: 0.555, 95% CI: 0.311–0.991, p = 0.047) were found to be the independent risk factors for predicting OS.
Conclusion
Percutaneous thermal ablation is a safe and effective treatment for PM from HCC.
Introduction
In hepatocellular carcinoma (HCC), the lung is the most common site of extrahepatic metastasis [Citation1]. Systemic therapy and supportive care are recommended for HCC patients with extrahepatic metastasis [Citation2]. However, the survival outcomes are still dismal. Sorafenib is associated with median overall survival (OS) of 5–7.13 months for HCC patients with pulmonary metastases (PM) [Citation3,Citation4]. Chemotherapy could provide a median OS of 2.8 months, and supportive care provides a 1-year OS rate of 20% in patients with PM from HCC [Citation5]. Moreover, several published studies demonstrated that surgical resection can provide a 5-year OS rate ranging from 10–66.9% [Citation6–9] in select candidates. However, the majority of patients are not eligible for surgical treatment due to the existence of multiple PM or if the target lesion diameter exceeds 3 cm [Citation10].
Recently, percutaneous thermal ablation was proposed as a treatment option for patients with lung malignancies [Citation11–13]. Inducing necrosis in lung malignancy has been demonstrated in an animal model [Citation14]. In addition, clinical studies investigating the safety of thermal ablation in humans have found that the treatment could achieve high tumor response [Citation15–17], with a 1-year OS rate and a 1-year progression-free survival (PFS) rate of 89–100% and 49–53%, respectively [Citation18–24].
However, only a few small studies have examined the clinical outcomes of thermal ablation therapy for PM from HCC. Hence, we aimed to evaluate the safety and efficacy of thermal ablation for PM originating from HCC.
Patients and methods
Patients
This retrospective study was performed at one single institution with approval from an institutional ethics committee, and written informed consent was obtained before treatment.
From June 2010 to July 2017, a total of 39 HCC patients with PM were treated with percutaneous thermal ablation. (a) Patients aged ≥18 years and ≤80 years, (b) patients who did not receive other treatment for PM besides ablation, (c) patients whose intrahepatic disease was under control (no evidence of local residual disease or recurrence existed) at the time of ablation for the PM diagnosis, (d) patients who had less than 5 PM, (e) patients who had a metastatic lesion diameter of less than 60 mm and (f) patients who were treated with curative intent and had a follow-up period of ≥3 months were included. Treatment decisions were determined after discussions with a multidisciplinary team.
(a) Patients who had uncorrectable coagulopathy or septicemia, (b) patients who refused ablation therapy or received other therapies besides ablation for PM and (c) patients who had other extrahepatic metastasis were excluded. The patient enrollment flow chart is shown in Supplementary Appendix 1.
Ablation procedures
Equipment
All procedures were performed under the guidance of a 16-slice computed tomography (CT) scanner (Aquilion™, Canon Medical Co., Tokyo, Japan). A radiofrequency ablation (RFA) system (RITA Medical Systems, Mountain View, CA) coupled with an RF generator (Model 1500X, RITA Co., USA) and an RF electrode (StarBurst™XL, RITA Co., USA) was used in this study. The RF generator could provide a maximum output power of 200 W. For microwave ablation (MWA) therapy, an MWA system (FORSEA MTC-3CA, Qinghai Microwave Electronic Institute, Nanjing, China) was used in this study. MWA was performed with a frequency of 2450 MHz and provided an output power of 0–120 W. For cryoablation, a cryoablation system (Cryo-HIT™, Galil Medical, Yokneam, Israel) was used.
The choice of ablation techniques depended on the tumor size and location [Citation25,Citation26]. MWA was the preferred method for target lesions larger than 3 cm and/or close to large vessels, or for patients who had an implantable cardiac device, while cryoablation was performed for lesions close to the heart, pericardium or large airways, as it preserves the collagen matrix or peripheral lesions. If a patient could derive advantages from RFA, MWA and cryoablation, the treatment modality was determined by the patient’s preference and the radiologist’s experience.
Ablation procedure
Patients were provided local anesthesia with 2% lidocaine and were placed in an appropriate position. All ablation procedures were performed under CT guidance. A 22-gauge Chiba needle was advanced into the target lesion to lead the antenna/electrode/cryoprobes to the target. For the cryoablation procedure, the cryoprobes were inserted into the target lesion, and 2 or 3 consecutive cycles of freeze (10 min) and thaw (5 min) were performed. For the RFA or MWA procedure, the ablation electrode/antenna was inserted into the target lesion, maintaining a power of 45 W for a total ablation time of 10 min.
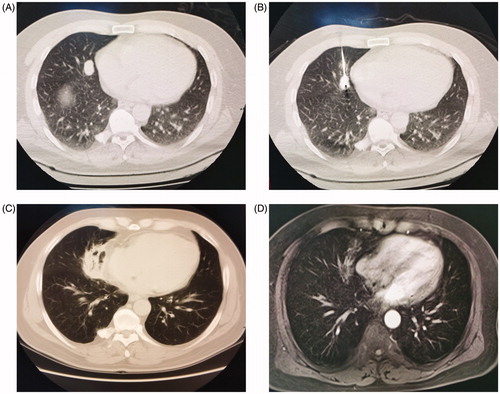
Intraoperative CT scans helped determine the correct position of the antenna/electrode. At the end of the RFA or MWA procedure, the needle track was ablated in order to avoid bleeding along the electrode route. Repeat CT scans were immediately performed to evaluate the TS, ablation margin and complications. Up to 3 PM were ablated on the same side of the lung. The endpoint of each ablation session was marked by the creation of an ablation zone of a circumference of at least 5 mm around the target lesion. The remaining nodules were ablated in the following week in order to minimize the potential risk of ablation-related complications. The ablation procedures are shown in .
Figure 1. (A) Chest CT image before ablation therapy shows a tumor with the maximum diameter of 15 mm. (B) CT image during MWA shows the antenna inserted into the tumor. (C) Follow-up CT scan after 1 month shows air inside the tumor and is evaluated as complete ablation. (D) Contrast enhanced MRI scan after 2 month shows marked involution of the tumor without contrast enhancement.
Assessment of treatment efficacy and follow-up
Tumor response was evaluated per patient by CT at 1 month after ablation, and all assessable patients were followed up at 3-month intervals.
Complications were recorded and classified based on the Society of Interventional Radiology Classification System [Citation27]. A minor complication was described as an event requiring no therapy or nominal therapy, and with no accompanied consequence. A major complication was defined as an event that required major therapy or prolonged hospitalization, or induced potential mortality or permanent adverse sequelae.
Pneumothorax exceeding 30% of a hemithorax, rapidly accumulating with mediastinal shift, or accompanied with respiratory or circulatory distress, was treated with chest tube placement. If the pneumothorax required chest tube placement for more than 72 h, or accorded with the definition of a major complication, it was considered as a major complication. Pleural effusion that exceeded 50% of a hemithorax or symptomatic pleural effusion was also treated with chest tube drainage [Citation28]. Mild pleural effusion was defined as effusion exceeding no more than 50% of a hemothorax.
The primary endpoints were OS and local tumor progression-free survival (LTPFS). OS was defined as the interval from initial ablation to death or the latest date when the patient was still alive. LTPFS was defined as the interval from initial ablation to radiologic evidence of local tumor progression or the latest date of follow-up.
TS was defined as the target tumor treated with the predetermined treatment protocol and covered completely by the ablation zone [Citation27]. Tumor coverage was assessed immediately after ablation. Local tumor response was evaluated using mRECIST at 1 month after ablation [Citation25]. Unchanged morphologic features of the ablation zone or shrinkage without an enhancement at CT were considered as complete ablation. An increase in the overall size of the ablation zone without change in the enhanced zone was considered as incomplete ablation. Enlargement of the ablation zone to more than 10 mm due to the newly developed enhancement at CT was considered as local progression. If local progression or incomplete ablation was detected at 1 month after ablation, up to two ablation sessions were performed.
Statistical analysis
Data were analyzed using SPSS 20.0 for Windows (IBM Corp, Armonk, NY). The baseline and clinical characteristics were expressed as median ± range or frequency (categorical variables). Survival data were depicted by the Kaplan–Meier method and compared using the log-rank test. Factors associated with OS were determined by performing univariate and multivariate analysis using the Cox proportional hazards model. Univariables with p < 0.5 were incorporated into the multivariate analysis. The final model was chosen based on those variables with p < 0.05 in the multivariate analysis. All the statistical tests were two-sided, and p < 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
shows the baseline demographic characteristics of the patients. A total of 39 patients were included in this study. The sample included 33 (33/39, 84.6%) men and 6 (6/39, 15.4) women. The median age of the patients was 59 years (range: 31–78 years). The median number of metastatic lesions per patient was 2 (range: 1–5). The median maximum diameter of the metastatic lesions was 15.0 mm (range: 6.00–52.00 mm). A total of 60 sessions were performed in 39 patients (23 patients had 1 session each, 11 patients had 2 sessions each and 5 patients had 3 sessions each).
Table 1. Demographic and clinical characteristics of included patients.
Tumor response and local control rate
The TS rate was 100%. Thirty-three patients had ablation margins of ≥5 mm: 29 of them were evaluated a having complete ablation, 1 of them was evaluated as having incomplete ablation, and 3 of them were evaluated as having local progression. Five patients had ablation margins of <5 mm: 5 received salvage ablation at 1 month after ablation (3 were evaluated as having complete ablation, 2 were evaluated as having local progression). The remaining 1 did not have the images at 1 month after ablation, but the CT images at 4 months after ablation were evaluated as local progression.
shows the tumor response at 1 month after ablation. A total of 32 patients (32/38, 84.2%) achieved complete ablation, 1 (1/38, 2.6%) was classified as having incomplete ablation, and 5 patients (5/38, 13.2%) had local progression.
Table 2. Tumor response at 1 month after ablation therapies.
Survival outcomes
The median follow-up period was 13.47 months (range: 4.5–67.09 months). shows the OS curves of the entire sample. The median OS rate was 45.14%. The 6-month and 1-, 2-, 3- and 5-year OS rates were 97.4, 79.8, 71.8, 58 and 30.9%, respectively. Twelve events occurred: six of them due to intrahepatic HCC, one of them due to hepatic failure, and five of them were out-of-hospital death with unknown cause.
Figure 2. The Kaplan–Meier estimates of survival for all included patients. (A) The OS rates of all patients with pulmonary metastases (PM) from hepatocellular carcinoma (HCC) treated with thermal ablation. (B) The local progression-free survival rates of all patients with PM from HCC treated with thermal ablation.
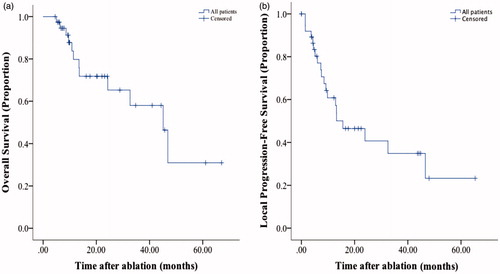
shows the LTPFS curves. The 6-month and 1-, 2-, 3- and 5-year LTPFS rates were 80.4, 60.7, 45.5, 34.2 and 22.8, respectively. Twenty patients had local recurrence after ablation, of which 7 received ablation therapy. Twenty-seven patients had distant recurrence after ablation, and 12 of them received ablation.
and show the OS rates and LTPFS rates in different subgroups divided by the number, laterality of metastases and ablation modalities. No significant difference was detected among all groups.
Figure 3. The Kaplan–Meier estimates of survival for patients in different subgroups. (A) and (C): The overall survival (OS) rates and local progression-free survival (LTPFS) rates of patients with ≤2 or >2 PMs. (B) and (D): the OS rates and LTPFS rates of patients with unilateral or bilateral PM.
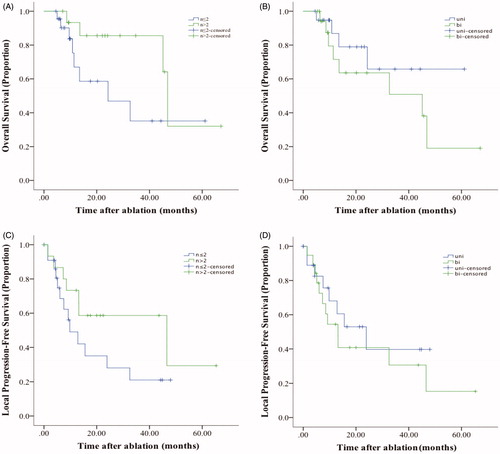
Table 3. Subgroup analysis for overall survival rate, local tumor progression-free survival rate and complete response rate.
Univariate and multivariate cox proportional hazards regression analyses
shows the results of the univariate and multivariate analyses. In the multivariate Cox regression analyses, adjusting for clinically significant univariate factors, the number of (≤2 vs. >2) PM (hazard ratio (HR): 0.555, 95% confidence interval (CI): 0.311–0.991, p = 0.047) and the extent (unilateral vs. bilateral) of PM (HR: 0.197, 95% CI: 0.043–0.890, p = 0.035) were determined as the independent prognostic factors for OS.
Table 4. Univariate and multivariate analysis of all included patients for overall survival in Cox proportional hazard model.
Complications
Major complications were observed in 5 (5/39, 12.8%) patients, including pneumothorax requiring chest tube placement (3 patients; 7.7%) and pleural effusion requiring chest tube drainage (2 patients; 5.1%). Minor complications included mild pneumothorax (18 patients), pneumonia (7 patients), mild hemorrhage (19 patients) and mild pleural effusion (22 patients). No ablation-related deaths were detected.
Discussion
Our study showed a high TS rate with the use of ablation for treatment of PM from HCC. The modality demonstrated an acceptable safety profile, with no ablation-related deaths or life-threatening complications.
Percutaneous ablation therapy could provide promising survival outcomes. Hiraki et al. [Citation29] reported 1- and 3-year OS rates of 87 and 57%, respectively, in 32 patients with PM from HCC after RFA treatment. Li et al. [Citation30] retrospectively evaluated 29 HCC patients with PM who were treated with RFA, and reported 1- and 3-year OS rates of 73.4 and 30%, respectively, with a median OS of 21 months. The survival outcomes in the present study are promising and similar to those reported earlier. A possible reason for the promising survival outcome may be the repeatability of the ablation therapies. Previous studies have found that ablation therapy can be repeatedly performed in patients with recurrent lung tumors [Citation24,Citation31]. Yan et al. [Citation16] demonstrated a 3-year OS rate of 92% in patients with recurrent lung tumors who underwent repeat RFA vs. 45% in those who did not undergo a repeat RFA. However, re-ablation was not the independent risk factor for OS. It is difficult to determine the accurate effect of repeat ablation on survival. Further randomized trials with larger sample sizes are needed to investigate the effect of the ablation session on survival.
Previous studies suggested using surgical resection to treat PM. The 5-year OS rates after resection for unilateral PM were reported to range from 28.2–66.9% [Citation7–9,Citation32,Citation33]. Our findings are comparable to the results obtained using surgical resection. The 30-day mortality rates in patients undergoing lobectomy, segmentectomy and pneumonectomy were reported to be 0.26, 0.31 and 2.45%, respectively [Citation34]. In comparison, the 30-day mortality rate after ablation therapy ranges from 0–0.35% in previous studies [Citation18,Citation21,Citation35,Citation36]. It seems that the mortality rates among ablation, lobectomy and segmentectomy are similar. Even though the rate after pneumonectomy was 1the highest, pneumonectomy is not a conventional therapy for PM. Thus, further randomized trials should be conducted to investigate whether these findings could indicate that a patient at high risk of death within 30 d of surgery would derive more advantages from ablation therapy.
Tumor number is a significant element of complete ablation. A previous study demonstrated that solitary tumors have lower local recurrence rates compared with multiple tumors [Citation37]. Li et al. [Citation30] investigated the prognostic factors associated with survival rate after ablation therapy for PM, and found that PM ≤3 in number were associated with better survival than PM exceeding 3 in number. In the current study, patients with more than 2 metastases faced 0.555 times the risk of death compared to those with ≤2 metastases. Moreover, the extent of PM was another independent risk factor in the current study. However, not only TS, but also the survival rate encouraged bilateral PM after ablation treatment. In the present study, thermal ablation of bilateral PM yielded a TS rate of 100% and the 5-year OS rate of patients with bilateral PM cases after ablation was 19.1%. Our results are comparable to those of a previously reported study about ablation efficacy for PM [Citation38]. It should be noted that the OS rate of bilateral PM patients treated with surgery was only 8.3% [Citation39]. Moreover, pulmonary function was not significantly affected by RFA, whereas it has been shown to reduce severely after bilateral pulmonary metastasectomy [Citation36,Citation40]. Patients with more than 2 PM or patients with bilateral metastases showed improved OS after ablation therapy, albeit with a small magnitude of benefit; thus, these multivariate analysis results could be used to inform the conversation about risks and benefits with patients.
It should be noted that the TS rate was 100%, while the complete ablation rate was only 84.2%. The distinction between TS and complete ablation (or technique effectiveness) should be clarified. Effectiveness should be demonstrated with an appropriate time point, such as immediately after the last course of the ablation protocol, or at 1 week or 1 month after treatment, at which complete ablation of the macroscopic tumor (as evidenced at imaging follow-up) was achieved. On the contrary, TS simply addresses whether the tumor was treated according to protocol and was covered completely, and it can be assessed either during or immediately after the procedure. TS highlights whether the protocol could be effectively performed [Citation41].
Whether different ablation techniques could induce different clinically relevant outcomes, especially RFA and MWA, is still under debate. Comparing the CR rate among different ablation techniques showed no significant differences in the current study. Previous studies indicated that MWA was superior to RFA due to better survival for lung metastases patients [Citation42]. Moreover, a recent randomized, controlled, phase 2 trial comparing the efficacy and safety of RFA and MWA demonstrated that MWA was not superior to RFA in terms of procedure time, local tumor progression, frequency of complications, and OS [Citation43]. Thus, further studies are needed to clarify the difference between these ablation techniques.
According to previous studies, the rate of pneumothorax after thermal ablation ranges from 8.5–50% [Citation21,Citation35,Citation36,Citation44], and the rate of major complications lies between 1.6 and 21% [Citation28,Citation45]. The rates of pneumothorax and pneumothorax requiring chest tube placement in our study were consistent with the findings of earlier studies. Previous research [Citation44] has reported that patients with PM located in the upper lobe are more likely to require chest tube placement for pneumothorax. Pleural effusion is one of the most commonly reported complications for lung cancer, occurring in 1.3–60% of ablation procedures [Citation46]. The findings of our study are similar to previous reports. The most likely cause of pleural effusion is pleurisy induced by the conduction of ablation energy to the pleura [Citation47]. Further, multiple lesions ablated in one ablation procedure, large-sized target lesions, lesions abutting pleura (<10 mm), or procedures of long duration may be associated with pleural effusion requiring treatment [Citation28].
This study has certain limitations. First, a selection bias was unavoidable, given the retrospective nature of this study. Second, the sample size was small, thus limiting the statistical power of the current study. Third, because of the complexity of the technique features (e.g., antenna design and frequency of operation), comparison among MWA, RFA and cryoablation is difficult. Fourth, the patients included in the study were in an advanced stage of HCC, and many patients who reported recurrences after ablation may have received other therapies, such as systemic therapy. However, a detailed analysis of these patients is not possible. Fifth, given the small sample size, the variation within the group and the presence of interaction would affect the stability and accuracy of the Cox proportional hazards model. Sixth, information about other treatments for PM after ablation, such as systemic treatment, and other treatments for local or distant recurrence was insufficient. These aspects would interfere with the analysis of the ablation efficacies.
In conclusion, percutaneous thermal ablation appears to be safe and effective for patients with PM from HCC. Survival outcomes are improved in patients with PM. However, multicenter, randomized and controlled trials with larger sample sizes are needed to confirm these findings.
Supplementary Material
Download PDF (81.9 KB)Acknowledgments
The institutional review board of Ethics Committee has approved this study. Informed consent was obtained from all individual participants included in the study. Human experimentation guidelines of China were followed in the conduct of this clinical research. The work described has not been published or accepted elsewhere, in whole or in part. All the authors listed have seen and approved the manuscript that is enclosed, contributed significantly to the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Uka K, Aikata H, Takaki S, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414–420.
- Network N. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Hepatobiliary Cancers Version 2.2018. 2018 Jun 7. Available from: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- Chen J, Lu S, Zhang Y, et al. Sorafenib monotherapy versus sorafenib combined with regional therapies for hepatocellular carcinoma patients with pulmonary oligometastases: a propensity score-matched analysis. J Cancer. 2018;9:1745–1753.
- Yau T, Chan P, Ng KK, et al. Phase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: presence of lung metastasis predicts poor response. Cancer. 2009;115:428–436.
- Hu Z, Huang P, Zhou Z, et al. Aggressive intrahepatic therapies for synchronous hepatocellular carcinoma with pulmonary metastasis. Clin Transl Oncol. 2018;20:729–739.
- Han KN, Kim YT, Yoon JH, et al. Role of surgical resection for pulmonary metastasis of hepatocellular carcinoma. Lung Cancer. 2010;70:295–300.
- Lam CM, Lo CM, Yuen WK, et al. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg. 1998;85:1198–1200.
- Ohba T, Yano T, Yoshida T, et al. Results of a surgical resection of pulmonary metastasis from hepatocellular carcinoma: prognostic impact of the preoperative serum alpha-fetoprotein level. Surg Today. 2012;42:526–531.
- Takahashi Y, Ikeda N, Nakajima J, et al.; Metastatic Lung Tumor Study Group of Japan. Prognostic analysis of surgical resection for pulmonary metastasis from hepatocellular carcinoma. World J Surg. 2016;40:2178–2185.
- Yano T, Shoji F, Maehara Y. Current status of pulmonary metastasectomy from primary epithelial tumors. Surg Today. 2009;39:91–97.
- de Baere T, Palussiere J, Auperin A, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology. 2006;240:587–596.
- Fernando HC, De Hoyos A, Landreneau RJ, et al. Radiofrequency ablation for the treatment of non-small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg. 2005;129:639–644.
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–275.
- Miao Y, Ni Y, Bosmans H, et al. Radiofrequency ablation for eradication of pulmonary tumor in rabbits. J Surg Res. 2001;99:265–271.
- Zheng A, Ye X, Yang X, et al. Local efficacy and survival after microwave ablation of lung tumors: a retrospective study in 183 patients. J Vasc Interv Radiol. 2016;27:1806–1814.
- Yan TD, King J, Sjarif A, et al. Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol. 2006;13:1529–1537.
- Yamakado K, Hase S, Matsuoka T, et al. Radiofrequency ablation for the treatment of unresectable lung metastases in patients with colorectal cancer: a multicenter study in Japan. J Vasc Interv Radiol. 2007;18:393–398.
- de Baere T, Auperin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol. 2015;26:987–991.
- de Baere T, Tselikas L, Woodrum D, et al. Evaluating cryoablation of metastatic lung tumors in patients – safety and efficacy: the ECLIPSE Trial – interim analysis at 1 year. J Thorac Oncol. 2015;10:1468–1474.
- Rossi S, Dore R, Cascina A, et al. Percutaneous computed tomography-guided radiofrequency thermal ablation of small unresectable lung tumours. Eur Respir J. 2006;27:556–563.
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology. 2011;261:643–651.
- Koelblinger C, Strauss S, Gillams A. Outcome after radiofrequency ablation of sarcoma lung metastases. Cardiovasc Intervent Radiol. 2014;37:147–153.
- Gillams A, Khan Z, Osborn P, et al. Survival after radiofrequency ablation in 122 patients with inoperable colorectal lung metastases. Cardiovasc Intervent Radiol. 2013;36:724–730.
- Chua TC, Al-Alem I, Zhao J, et al. Radiofrequency ablation of concomitant and recurrent pulmonary metastases after surgery for colorectal liver metastases. Ann Surg Oncol. 2012;19:75–81.
- Liu BD, Ye X, Fan WJ, et al. Expert consensus on image-guided radiofrequency ablation of pulmonary tumors: 2018 edition. Thorac Cancer. 2018;9:1194–1208.
- Tafti BA, Genshaft S, Suh R, et al. Lung ablation: indications and techniques. Semin Intervent Radiol. 2019;36:163–175.
- Ahmed M, Solbiati L, Brace CL, et al.; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. J Vasc Interv Radiol. 2014;25:1691–1705 e4.
- Hiraki T, Tajiri N, Mimura H, et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: incidence and risk factors. Radiology. 2006;241:275–283.
- Hiraki T, Yamakado K, Ikeda O, et al. Percutaneous radiofrequency ablation for pulmonary metastases from hepatocellular carcinoma: results of a multicenter study in Japan. J Vasc Interv Radiol. 2011;22:741–748.
- Li X, Wang J, Li W, et al. Percutaneous CT-guided radiofrequency ablation for unresectable hepatocellular carcinoma pulmonary metastases. Int J Hyperthermia. 2012;28:721–728.
- Nakamura T, Matsumine A, Yamakado K, et al. Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcomas [corrected]. Cancer. 2009;115:3774–3781.
- Lee CY, Bae MK, Park IK, et al. Surgical resection for pulmonary metastasis from hepatocellular carcinoma: analysis of prognosis in relation to primary control. J Surg Oncol. 2010 Mar. 1;101:239–243.
- Mizuguchi S, Nishiyama N, Izumi N, et al. Clinical significance of multiple pulmonary metastasectomy for hepatocellular carcinoma. World J Surg. 2016;40:380–387.
- Kuwano H, Amano J, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2010: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2012;60:680–708.
- Chua TC, Sarkar A, Saxena A, et al. Long-term outcome of image-guided percutaneous radiofrequency ablation of lung metastases: an open-labeled prospective trial of 148 patients. Ann Oncol. 2010;21:2017–2022.
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9:621–628.
- Akhan O, Guler E, Akinci D, et al. Radiofrequency ablation for lung tumors: outcomes, effects on survival, and prognostic factors. Diagn Interv Radiol. 2016;22:65–71.
- Palussiere J, Gomez F, Cannella M, et al. Single-session radiofrequency ablation of bilateral lung metastases. Cardiovasc Intervent Radiol. 2012;35:852–859.
- Han KN, Kang CH, Park IK, et al. Thoracoscopic approach to bilateral pulmonary metastasis: is it justified? Interact Cardiovasc Thorac Surg. 2014;18:615–620.
- Welter S, Cheufou D, Zahin M, et al. Short- and mid-term changes in lung function after bilateral pulmonary metastasectomy. Thorac Cardiovasc Surg. 2016;64:139–145.
- Goldberg SN, Grassi CJ, Cardella JF, et al.; International Working Group on Image-Guided Tumor Ablation. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–739.
- Nour-Eldin NA, Exner S, Al-Subhi M, et al. Ablation therapy of non-colorectal cancer lung metastases: retrospective analysis of tumour response post-laser-induced interstitial thermotherapy (LITT), radiofrequency ablation (RFA) and microwave ablation (MWA). Int J Hyperthermia. 2017;33:820–829.
- Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317–325.
- Steinke K, King J, Glenn D, et al. Radiologic appearance and complications of percutaneous computed tomography-guided radiofrequency-ablated pulmonary metastases from colorectal carcinoma. J Comput Assist Tomogr. 2003;27:750–757.
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center’s experiences. AJR Am J Roentgenol. 2011;197:W576–W580.
- Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol. 2008;15:1765–1774.
- Tajiri N, Hiraki T, Mimura H, et al. Measurement of pleural temperature during radiofrequency ablation of lung tumors to investigate its relationship to occurrence of pneumothorax or pleural effusion. Cardiovasc Intervent Radiol. 2008;31:581–586.
