Abstract
Background
Intraoperative neurological monitoring is important in locating and assessing nerves during surgery. This study aimed to investigate the feasibility of neural monitoring during ultrasound-guided radiofrequency ablation (RFA) of thyroid nodules.
Methods
From February 2019 to August 2019, 16 patients (age, 42.8 ± 15.9 years; range, 17–74 years) with benign thyroid nodules who underwent ultrasound-guided RFA with neural monitoring in Zhongshan Hospital, Xiamen University, were included. A neuromonitoring system stimulated the vagus nerve to obtain electromyographic (EMG) signals and predict the function of recurrent laryngeal nerves (RLNs) during RFA. The hydrodissection technique was used to protect the RLN area. Thyroid nodules were treated with the moving-shot technique. The EMG signal value results were recorded and analyzed. All patients underwent laryngoscopic investigation 1 day after the procedure.
Results
Twenty vagus nerves were stimulated preprocedure and postprocedure, and the EMG signals were successfully recorded (100%). The mean initial (before ablation) and final (final ablation) vagus nerve amplitudes were 612.7 ± 130.4 μV (range, 455–882 μV) and 592.7 ± 127.3 μV (range, 410–817 μV), respectively. Based on the EMG signals, all 20 RLNs were judged to be in good condition, consistent with the postprocedure laryngoscopic results. The maximum lesion size and volume at 6 months after RFA were significantly lesser than those at baseline (p < 0.05). The volume reduction rate was 68.5% ± 21.5% (range, 13.0–97.3%). Cosmetic and symptom scores were significantly lower than those at baseline. No complications from neural monitoring occurred.
Conclusions
Neural monitoring during ultrasound-guided RFA of thyroid nodules is feasible to predict RLN function.
Introduction
Thermal ablation is a new technique for the treatment of benign and malignant thyroid nodules and includes laser ablation [Citation1–3], radiofrequency ablation (RFA) [Citation4,Citation5], high-intensity focused ultrasound [Citation6], and microwave ablation [Citation7]. Recently, this technology has been used in the treatment of thyroid nodules [Citation8–12]. Ultrasound interventionalists and surgeons are interested in ablation techniques because the technique is minimally invasive, does not lead to scars, and has therapeutic effects. However, recurrent laryngeal nerve (RLN) injury is one of the most common complications of thyroid thermal ablation and invasive surgery. However, comprehensive evaluations of neurological complications due to thyroid thermal ablation are lacking. Moreover, in most studies, no routine laryngoscopic evaluations were performed before or after the procedure, except in patients with persistent hoarseness and breathing difficulties. Nevertheless, 1.0–9.1% of the patients were reported to have neurological injuries [Citation13–15].
Ultrasound has limitations in visualizing the RLN, which has a diameter of 1–2 mm; hence, the RLN can only be estimated from the position of the nerve relative to adjacent anatomical landmarks [Citation16]. Furthermore, the RLN has anatomical variations, especially on the right side of the body, where approximately 0.6–1.3% of RLNs are nonrecurrent (NRLNs) [Citation17]. Such variations are very likely to cause damage to the RLN. Moreover, the needle tip used in RFA can generate heat up to 100 °C, and irreversible damage to the RLN can occur at 60 °C [Citation18]. Thus, thermal damage is the main mechanism of thyroid RFA injury. Therefore, intraoperative neurological monitoring technology has been widely used in thyroid and parathyroid surgeries and has become an important means of locating nerve position and assessing nerve function during surgery [Citation19,Citation20]. However, there are no reports on neural monitoring in the literature or guidelines for image-guided ablation of the thyroid. Thus, this study aimed to investigate the feasibility of neural monitoring during ultrasound-guided RFA of thyroid nodules and how well EMG signals can predict RLN function.
Methods
This retrospective cohort study was approved by the Medical Ethics Committee of Zhongshan Hospital, which is affiliated with Xiamen University (approval no. xmzsyyky 2019013). Informed consent was obtained from all participants.
Patients
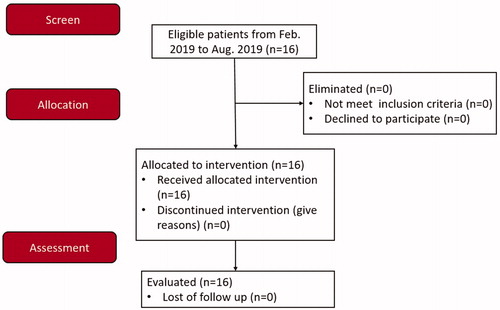
From February 2019 to August 2019, 16 patients (3 men and 13 women) with thyroid nodules were admitted to our hospital. The inclusion criteria for the study were neck discomfort or pain, foreign body sensation, or compressive symptoms; benign thyroid nodules diagnosed by color Doppler ultrasonography; benign, fine-needle aspiration cytopathology and no BRAF mutation; results of laboratory parameters, including routine blood work, coagulation function, blood biochemistry, and thyroid function, within the normal ranges; normal movement of bilateral vocal cords confirmed by preoperative laryngoscopy; and patients refusing to surgery and opting for RFA therapy. Exclusion criteria included malignant thyroid tumors confirmed by fine-needle aspiration or the presence of BRAF mutation, pregnancy, and severe systemic diseases that contraindicated ablation treatment. The characteristic of the patients and the nodules are shown in . The CONSORT flow diagram is presented in .
Table 1. Characteristics of the patients and nodules at baseline.
Neural monitoring and RFA equipment
NIM® Nerve Monitoring System version 3.0 (Medtronic, Minneapolis, MN, USA) was used to record electromyograms (EMGs). Nerves were stimulated with a 22-gauge nerve stimulation needle (Stimuplex® D; B. Braun, Melsungen, Germany). Ambu® Neuroline 715 single-patient surface electrodes were used as recording and grounding electrodes (Ambu, Ballerup, Denmark). Real-time thyroid ultrasonography was performed using high-resolution (6- to 13-MHz linear-array transducer; HFL38) ultrasound (Edge; FUJIFILM SonoSite, Bothell, WA, USA). RFA procedures were performed with a radiofrequency generator (RITA® RFA Systems, 1500X; AngioDynamics, Miami, FL, USA) and 18-gauge internally cooled water circulation electrodes (Angel Medical Technology, Nanjing, China).
Procedure
Equipment Connection and Setup
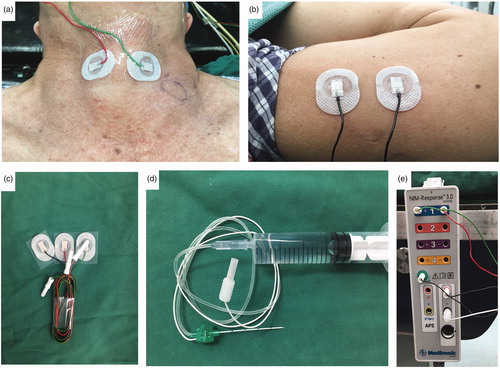
After alcohol disinfection, a pair of cutaneous surface recording electrodes () were attached to the neck at the left and right laminas of the thyroid cartilage and covered with transparent adhesive dressing (). Surface electrodes were attached to the upper arm of the patient as grounding electrodes (). The nerve stimulation needle was connected to a saline syringe (), and all electrodes were connected to an interface-connector box (). The event threshold was set at 100 μV, and the impulse duration and frequency of the stimulation probe were set to 100 ms and 4 Hz, respectively.
Figure 2. Establishment of neural monitoring system (a) A pair of cutaneous, surface-electrodes were attached to the neck skin of the left and right lamina of the thyroid cartilage (b) Another pair cutaneous surface electrodes were attached to the upper arm of the patient (c) Ambu® Neuroline 715 single patient surface electrodes (d) Nerve stimulation needle was connected to a saline syringe as a stimulating electrode (e) All electrodes were connected an interface-connector box.
Initial Vagal Stimulation
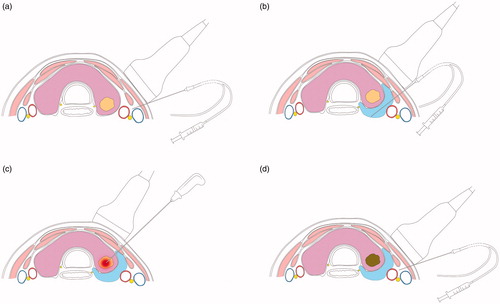
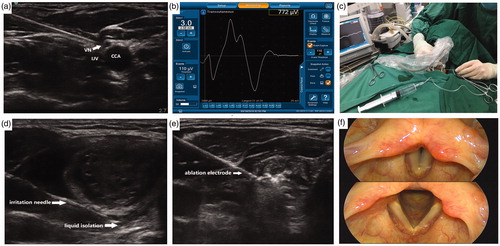
Each patient was placed in the supine position, and the cervical region was sufficiently exposed. All procedures were completed under conventional surgical site disinfection and single-use shop sterile surgical towels. All patients received local anesthesia with 2% lidocaine at the puncture site. Two patients underwent intravenous sedatives without tracheal intubation and muscle relaxants because they were unable to cooperate with the procedure due to anxiety. An ultrasound-guided puncture with a nerve stimulation needle was performed between the lateral common carotid artery and the medial internal jugular vein (). The initial vagus nerve amplitude (V1) was obtained via stimulation using a 3.0-mA stimulus current [Citation21,Citation22] (). Stimulations should be performed at the level of the lower thyroid pole to enable detection of nonrecurrent laryngeal nerves, especially on the right side. If no VN signal could be detected at the inferior thyroid, the superior thyroid should be evaluated to check for the presence of NRLNs. shows the simulation of the initial vagal stimulation.
Figure 3. Procedure of neural monitoring during radiofrequency ablation of thyroid nodules (a) An ultrasoundguided puncture with the nerve-stimulation needle was performed between the lateral common carotid artery (CCA) and the medial internal jugular vein (IJV) and the vagus nerve (VN) was identified (b) EMG amplitudes were measured by NIM® Nerve Monitoring System 3⋅0 (Medtronic) (c) The needle was used to inject saline and continuously detect the recurrent laryngeal nerve with 3.0 mA current (d) A ‘liquid isolation zone’ was formed with a width of approximately 5 mm on the dorsal side of thyroid gland (e) The radiofrequency electrodes were placed into the nodule, and ablation was performed (f) After ablation, a final vagus electromyography (V2) was induced using 3.0 mA stimulus current and fibro laryngoscopies were performed on the first day following the procedure.
Detection of RLN and Hydrodissection Technology
Surgeons often perform hydrodissection [Citation23,Citation24], in which a certain amount of liquid is injected in the area where the RLN traverses, to avoid injury and thermal damage to the RLN during RFA [Citation25,Citation26]. The nerve stimulation needle used as a probe for nerve detection can also be used for injecting normal saline. The needle was used to inject normal saline along the junction of the carotid sheath and thyroid gland and subsequently, into the dorsal side of the thyroid gland. Then, the needle was used to detect the distribution area of the RLN with a continuous 3.0-mA current. If the probe did not detect RLN signals, we assumed the absence of RLN distribution in the area and continued to inject normal saline, which gradually formed a ‘liquid isolation zone’ with a width of approximately 5 mm on the dorsal side of the thyroid gland. (). If the probe detected RLN signals, we assumed the presence of RLNs in the area, and thus, thermal ablation was performed as far from the area as possible. shows the simulation of the RLN detection and hydrodissection technology.
Ultrasound-guided RFA
A real-time, ultrasound-guided transisthmic or lateral approach was selected for the moving-shot technique [Citation23,Citation24]. The radiofrequency electrodes were placed into the deepest and farthest part of the nodule, and ablation was performed with 30–50 W of radiofrequency power. After vaporization and appearance of hyperechoic areas in the ablation area, the needle tip was gradually pulled back to the superficial and proximal areas until the entire nodule was covered by the hyperechoic areas formed by thermal ablation (). In case of mixed nodules, a percutaneous puncture needle was inserted into the cystic portion and the fluid inside was extracted first, followed by ablation of the solid portion. shows the simulation of the ultrasound-guided RFA.
End of RFA Neural Testing
After ablation, the nerve stimulation needle was placed into the lateral part of the carotid sheath under ultrasound guidance, and a final vagus EMG (V2) was induced using a 3.0-mA stimulus current. shows the simulation of the end of the RFA neural testing.
Study end points and follow-up
The primary end point of the study was the feasibility of neural monitoring during ultrasound-guided RFA of thyroid nodules. Neural monitoring was considered feasible if the monitoring needle was placed in the desired position and if nerve stimulation was achieved.
The secondary end point was technical success, which was defined as agreement between the EMG signal and postprocedure laryngoscopic result.
An initial amplitude ≥500 μV was defined as a robust initial EMG goal (V1). Normative baseline EMG indicates the situation when the absolute amplitude of the signal is not less than 50% and the growth of its latency is less than 10% of the initial baseline (V2). Impending adverse EMG was defined as an amplitude decrease >50% and a latency increase >10% of the initial baseline (V2). Loss of signal (LOS) is defined by a normal vocal cord (VC) movement during preprocedure laryngoscopy, a low (<100 μV) or absent EMG response, and troubleshooting in the neural monitoring system.
In the event of impending adverse EMG or LOS, all procedures were terminated. Ice-cooled liquid was injected into the dorsal thyroid membrane and tracheo-esophageal groove to cool the RLN [Citation27]. Stimulations should be performed every 5 min to estimate nerve recovery. The final EMG be considered as the recovery EMG (V2) if the absolute amplitude recovers >50% of the initial baseline (V1). If the amplitude did not reach 50% of the initial baseline within 20 min, RLN injury might have occurred during the ablation. For patients with bilateral nodules, if the signal is not recovered, the contralateral thyroid nodules should be treated in stages. If the final EMG was an LOS or impending adverse EMG, RLN injury should be identified. A normative baseline EMG indicated good nerve function.
All patients underwent laryngoscopy on the first day after the procedure, and those with abnormal vocal cord activity were considered to have RLN injury. The clinical outcomes including energy deposition time, clinical symptoms, volume percentage reduction, cosmetic outcomes, and complications were recorded.
Improvement in clinical symptoms at 6 months was evaluated with a 10-cm visual analog scale (grades 0–10) by the patients, and the cosmetic score (1, no palpable mass; 2, no cosmetic problem but palpable mass; 3, a cosmetic problem on swallowing only; 4, a readily detected cosmetic problem) was measured by a physician [Citation8].
The volume reduction ratio (VRR) was calculated at 6 months post-treatment as follows: VRR (%)= [(initial volume–final volume)×100]/initial volume.
Complications arising from neural monitoring (i.e. pain, local hematoma, vasovagal reaction, bradycardia, hypertension, and vomiting) were recorded. Complications related to RFA were recorded as well.
Statistical analysis
Anonymous data were entered into an Excel 2010 database (Microsoft, Redmond, WA, USA) and then analyzed with SPSS version 19.0 for Windows (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as standard descriptive statistics, including mean, standard deviation, and range. Categorical variables were presented as numbers and frequencies.
The amplitude and latency of V1 were compared with those of V2 using a paired Student t-test. The Wilcoxon signed rank test was used to compare the tumor size, VRR, symptom score, and cosmetic score at baseline versus those at 6-month follow-up. Differences with a p Value < 0.05 were considered statistically significant.
RLN injury identified by neural monitoring was compared with RLN injury identified by laryngoscopic evaluation performed 1 day after the procedure using the chi-square test (Fisher exact test). A p Value < 0.05 was considered significant.
Results
Neural monitoring during ultrasound-guided RFA of thyroid nodules was feasible in all 16 patients (100%). Twenty vagus nerves were stimulated before and after the procedure, and 100% (40/40) EMG signals were obtained.
A total of 16 initial EMG amplitudes (80%) were >500 μV (). The mean ± standard deviation response amplitudes before and after RFA (V1, 612.7 ± 130.4 μV; V2, 592.7 ± 127.3 μV; p < 0.05) were significantly different. Mean response latencies (V1, 6.4 ± 1.0 ms; V2, 6.5 ± 1.0 ms; p > 0.05) were not significantly different between the two groups.
Table 2. Neural monitoring data during ultrasound-guided thyroid nodule ablation.
Technical success was achieved for all 20 nerves (100%). After the ablation of thyroid nodules, the V2 EMGs were not significantly decreased (20/20). All 20 RLNs were judged to be in good condition by neural monitoring during ultrasound-guided RFA of thyroid nodules. All patients had normal pronunciation following the procedure, and none experienced the cough reflex when drinking water or had a decreased vocal tone on the first day following the procedure. Fibrolaryngoscopies were also performed (), and the bilateral vocal cords exhibited good motion, and no vocal cord paralyzes were found. All 20 RLNs related to the RFA treatment were confirmed to be intact (). This result was in agreement with those of the EMG signals during the RFA procedure (p > 0.05).
Table 3. Correlation of electrophysiologic events with RLN function.
RFA procedures lasted between 5 and 18 min (mean duration, 13 min), depending on the size and number of treated nodules.
The baseline maximum nodule lesion size was 2.49 ± 0.73 cm (range, 1.2–3.6 cm), and the lesion volume was 5.46 ± 4.89 ml (range, 0.3–19.5 ml). The maximum nodule lesion size was 1.47 ± 0.44 cm (range, 0.8–2.2 cm; p < 0.05), and the lesion volume was 1.10 ± 0.69 ml (range, 0.1–2.3 ml; p < 0.05) at 6 months after RFA. The volume reduction rate was 68.5 ± 21.5% (range, 13.0–97.3%) at the 6-month follow-up.
The symptom score decreased from 3.6 ± 0.7 at baseline to 1.2 ± 0.4 at 6 months (p < 0.05), and the cosmetic score decreased from 3.4 ± 0.5 at baseline to 1.6 ± 0.6 at 6 months (p < 0.05).
No neural monitoring–related complications, such as injury to the common carotid artery/internal jugular vein, vasovagal reaction, bradycardia, hypertension, or vomiting, were reported. No major complications related to RFA such as nodule rupture, hematoma, tracheal and esophageal rupture, skin burn, transient thyrotoxicosis, or lidocaine toxicity were noted. Pain during RFA treatment occurred in 3 patients (19%), but it was relieved when the generator output was reduced or turned off.
Discussion
In this study, neural monitoring was performed successfully in all procedures, and the EMG amplitudes did not significantly decrease. Moreover, laryngoscopies performed on the first day following RFA showed normal results. The results of our study confirm that neural monitoring is feasible for RFA of thyroid nodules. The main innovation of this study is the use of a neural monitoring system suitable for thermal ablation of thyroid nodules. This procedure can help assesses RLN function and assist in making timely treatment decisions, especially for patients with bilateral thyroid nodules.
Most surgeons and anesthesiologists use EMG endotracheal tube–based surface electrodes as receiving electrodes. However, an experienced anesthesiologist is required to place the surface electrode on the vocal cords through an endotracheal tube. Chiang et al. [Citation28] considered that placing the needle electrode directly in the thyroid cartilage provide a higher and more stable myoelectric signal than using tracheal intubation surface electrodes. Wu et al. [Citation29] used a cutaneous surface electrode to obtain a stable laryngeal EMG signal, which avoids damage from the needle electrode itself when puncturing the thyroid cartilage, but the average amplitude obtained in their study was low. As RFA of the thyroid is usually performed under local anesthesia, rather than through endotracheal intubation, EMG is not needed. As a needle electrode is an invasive tool that may lead to pain intolerance and anxiety while the patient is awake, we selected a cutaneous surface electrode as the receiving electrode. These achieved good EMG signals, with an average value of 612.7 ± 130.4 μV, and 80% of these EMG signals could reach amplitudes >500 μV, meeting the standard of the initial baseline responses [Citation30]. One possible reason for this outcome is that most of the patients in this study were under local anesthesia or intravenous sedatives—no muscle relaxants were used and no skin incisions or anatomical dissections of the flap were performed; thus, the EMG signal was not interrupted, and it was maintained continuously on the path. Therefore, cutaneous surface electrode has more potential applications in thyroid thermal ablation.
Direct stimulation of the vagus nerve is an important step in the evaluation of RLN function. Farizon et al. [Citation31] evaluated RLN injury during thyroid surgery in 195 patients by directly stimulating the vagus nerve, through which asymmetrical, laryngeal muscle adduction function could be evaluated more quickly. This method was able to obtain vagus nerve signals during direct stimulation of the area between the common carotid artery and internal jugular vein [Citation20,Citation32]. In our study, although there was no vocal cord paralysis or hoarseness after the procedure, there was a statistically significant difference in EMG amplitudes before and after the procedure, which confirmed that neural monitoring was feasible in evaluating RLN function.
With the use of energy devices, a safe distance (>3 mm) is generally needed to avoid thermal damage to the nerves [Citation33,Citation34]. Considering the potential errors involved in judging ultrasonic distances and given that RFA is performed in a confined space, we believe that liquid isolation >5 mm on the dorsal side of the thyroid gland (without neural distribution) could ensure the safety of intraglandular ablation. The RLN is close to the dorsal side of the thyroid gland; thus, we would first use the nerve stimulation needle to detect any neural distributions and then to establish a liquid isolation zone. This method could then be used to detect the neural signals of the RLN and confirm the presence or absence of neural distributions before the hydrodissection procedure, indirectly protecting the RLN.
Another advantage of intraoperative neuromonitoring is the ability to provide a basis for the functional outcome of the vocal cords and influence a clinician’s treatment strategy, which provides the opportunity to implement bilateral surgery in stages and thus reduce the risk of bilateral RLN paralysis [Citation35–37]. However, judging whether the RLN has been injured during surgery by assessing the voice is unreliable. Lorenz et al. [Citation37] found that approximately 13% of patients with vocal cord paralysis did not have any vocal problems. Patients with preoperative laryngeal nerve palsy had significantly reduced EMG amplitudes when the vagus nerve or the RLN was irritated during surgery. If one side of the vocal cords is paralyzed, the cords may be fixed in the median position or exhibit limitations in adduction and abduction, and the voice may be either normal or mildly affected. Obvious hoarseness may occur when the vocal cord is fixed in the paramedian or intermediate position. In contrast, when the bilateral vocal cords are paralyzed and fixed in the middle position, inspiratory dyspnea, the three-concave sign, and other symptoms may be observed. Most nerve injuries caused by image-guided ablation of the thyroid involve thermal damage that turns out to be nearly irreversible. Therefore, the purpose of our study was to predict RLN injury through the monitoring of VN signals. In addition, when image-guided ablation is performed in patients with bilateral thyroid nodules, the procedure can help in terminating the surgery if one side of the RLN is damaged. Thus, it can provide an early warning for avoiding bilateral RLN paralysis. As endotracheal intubation is not performed to support breathing during surgery, once an RFA thermal injury causes bilateral vocal cord paralysis and progresses into suffocation, it may be life-threatening.
This study has limitations. First, this study did not have a control group, as this was a feasibility study intended to prove that neural monitoring could be useful in predicting RLN function during RFA of thyroid nodules. Second, although vagus EMG was satisfactorily recorded, it was difficult for the stimulating probe to locate the RLN due to the influence of the hydrodissection procedure; thus, only regional protection could be achieved. Third, this technique can only be used to perform intermittent nerve monitoring. Continuous nerve monitoring may be better for the early detection of nerve injury and can provide direct feedback to the operator, who may then stop the ablation if warranted. Hence, special instruments for continuous monitoring need to be designed. Finally, in addition to the sufficient range of liquid isolation in the RLN distribution area, the temperature of the fluid in the isolation area should also be measured to avoid nerve injury caused by increased temperature due to the prolonged ablation procedure. Therefore, further experimental and prospective randomized controlled clinical trials are required to confirm the feasibility of this technology.
Owing to the wide application of advanced techniques such as lasers and microwave ablation, which also supplement the treatment of benign thyroid nodules, treatment is now more diversified. In addition, more attention has been paid to the safety and efficacy of the technology [Citation38]. This study demonstrated the feasibility of neural monitoring during ultrasound-guided RFA of thyroid nodules to predict RLN function, enabling modification of the treatment strategy. We believe that with the accumulation of experience and the improvement of nerve-monitoring devices, neural monitoring technology will become an important assistive technology in image-guided ablation of thyroid nodules.
Acknowledgments
The authors acknowledge the assistance of Dr. ChunJin Lin for medical illustration.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mauri G, Nicosia L, Della Vigna P, et al. Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography. 2019;38(1):25–36.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperthermia. 2019;36(2):13–20.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: A systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Deandrea M, Trimboli P, Garino F, et al. Long term efficacy of a single session RFA of benign thyroid nodules: a longitudinal 5-year observational study. J Clin Endocrinol Metab. 2019;12:2018–2808.
- Rabuffi P, Spada A, Bosco D, et al. Treatment of thyroid nodules with radiofrequency: a 1-year follow-up experience. J Ultrasound. 2019;22(2):193–199.
- Lang BH, Woo YC, Chiu KW. High intensity focused ultrasound (HIFU) ablation of benign thyroid nodule is safe and efficacious in patients who continue taking an anti-coagulation or anti-platelet agent in the treatment period. Int J Hyperthermia. 2019;36(1):186–190.
- Cui T, Jin C, Jiao D, et al. Safety and efficacy of microwave ablation for benign thyroid nodules and papillary thyroid microcarcinomas: a systematic review and meta-analysis. Eur J Radiol. 2019;118:58–64.
- Kim J-H, Baek JH, Lim HK, et al.; Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. 2017 Thyroid radiofrequency ablation guideline: korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Dobnig H, Zechmann W, Hermann M, et al. Radiofrequency ablation of thyroid nodules: 'Good Clinical Practice Recommendations” for Austria: an interdisciplinary statement from the following professional associations: Austrian Thyroid Association (ÖSDG), Austrian Society for Nuclear Medicine and Molecular Imaging (OGNMB), Austrian Society for Endocrinology and Metabolism (ÖGES), Surgical Endocrinology Working Group (ACE) of the Austrian Surgical Society (OEGCH). Wien Med Wochenschr. 2019;170(1–2):6–14.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2018;20(1):11–22.
- Feng B, Liang P, Cheng Z, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol. 2012;166(6):1031–1037.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27(8):3128–3137.
- Cheng Z, Che Y, Yu S, et al. US-guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study. Sci Rep. 2017;7(1):9554
- Ha EJ, Baek JH, Lee JH. Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol. 2015;16(4):749–766.
- Donatini G, Carnaille B, Dionigi G. Increased detection of non-recurrent inferior laryngeal nerve (NRLN) during thyroid surgery using systematic intraoperative neuromonitoring (IONM)). World J Surg. 2013;37(1):91–93.
- Lin Y-C, Dionigi G, Randolph GW, et al. Electrophysiologic monitoring correlates of recurrent laryngeal nerve heat thermal injury in a porcine model. Laryngoscope. 2015;125(8):E283–90.
- Barczyński M, Konturek A, Pragacz K, et al. Intraoperative nerve monitoring can reduce prevalence of recurrent laryngeal nerve injury in thyroid reoperations: results of a retrospective cohort study. World J Surg. 2014;38(3):599–606.
- Chiang FY, Lee KW, Chen HC, et al. Standardization of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid operation. World J Surg. 2010;34(2):223–229.
- Bacuzzi A, Dralle H, Randolph GW, et al. Safety of continuous intraoperative neuromonitoring (C-IONM) in thyroid surgery. World J Surg. 2016;40(3):768–769.
- Phelan E, Schneider R, Lorenz K, et al. Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: a prospective, multicenter study. Laryngoscope. 2014;124(6):1498–1505.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Mauri G, Pisani Mainini A, Monaco C, et al. Urgent need to apply a common language in image-guided thermal ablations. J Ultrasound. 2018;21(1):77–78.
- Guang Y, He W, Luo Y, et al. Patient satisfaction of radiofrequency ablation for symptomatic benign solid thyroid nodules: our experience for 2-year follow up. BMC Cancer. 2019;19(1):147.
- Cui D, Ding M, Tang X, et al. Efficacy and safety of a combination of hydrodissection and radiofrequency ablation therapy for benign thyroid nodules larger than 2 cm: A retrospective study. J Cancer Res Ther. 2019;15:386–393.
- Chung SR, Baek JH, Choi YJ, et al. Management strategy for nerve damage during radiofrequency ablation of thyroid nodules. Int J Hyperthermia. 2019;36(1):204–210.
- Chiang F-Y, Lu IC, Chang PY, et al. Comparison of EMG signals recorded by surface electrodes on endotracheal tube and thyroid cartilage during monitored thyroidectomy. Kaohsiung J Med Sci. 2017;33(10):503–509.
- Wu C-W, Chiang F-Y, Randolph GW, et al. Transcutaneous recording during intraoperative neuromonitoring in thyroid surgery. Thyroid. 2018;28(11):1500–1507.
- Dionigi G, Wu CW, Kim HY, et al. Severity of recurrent laryngeal nerve injuries in thyroid surgery. World J Surg. 2016;40(6):1373–1381.
- Farizon B, Gavid M, Karkas A, et al. Intraoperative monitoring of the recurrent laryngeal nerve by vagal nerve stimulation in thyroid surgery. Eur Arch Otorhinolaryngol. 2017;274(1):421–426.
- Wu CW, Dionigi G, Chen HC, et al. Vagal nerve stimulation without dissecting the carotid sheath during intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid surgery. Head Neck. 2013;35(10):1443–1447.
- Kwak HY, Dionigi G, Kim D, et al. Thermal injury of the recurrent laryngeal nerve by THUNDERBEAT during thyroid surgery: findings from continuous intraoperative neuromonitoring in a porcine model. J Surg Res. 2016;200(1):177–182.
- Dionigi G, Chiang FY, Kim HY, et al. Safety of LigaSure in recurrent laryngeal nerve dissection-porcine model using continuous monitoring. Laryngoscope. 2017;127(7):1724–1729.
- Sarkis LM, Zaidi N, Norlen O, et al. Bilateral recurrent laryngeal nerve injury in a specialized thyroid surgery unit: would routine intraoperative neuromonitoring alter outcomes? ANZ J Surg. 2017;87(5):364–367.
- Shindo ML, Caruana SM, Kandil E, et al. Management of invasive well-differentiated thyroid cancer: an American Head and Neck Society consensus statement. AHNS consensus statement. Head Neck. 2014;36(10):1379–1390.
- Lorenz K, Abuazab M, Sekulla C, et al. Results of intraoperative neuromonitoring in thyroid surgery and preoperative vocal cord paralysis. World J Surg. 2014;38(3):582–591.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33:911–919.