Abstract
Objective
Hepatocellular carcinoma (HCC) is a notable threat to the longevity of elderly people. However, there is no trial to study the prognosis of these elderly patients after microwave ablation (MWA) treatment. This study investigated whether elderly patients with HCC benefit from MWA similar to younger patients.
Materials and methods
Patients who underwent ultrasound-guided percutaneous MWA were included and divided into four age groups and the prognosis was compared. The senior group (Group S, ≥75 years) was then compared with the younger group (Group Y, <75 years) after a 1:1 propensity score matching was applied. The prognostic outcomes were evaluated and Cox analysis was performed to determine the factors associated with survival.
Results
The four age groups showed a statistically different distribution in terms of sex, size of liver nodules, and the Charlson comorbidity index. Although Group S had a higher Charlson comorbidity index, no significant differences were found between Group S and Group Y in the rates of complete ablation and major complications as well as overall survival and progression-free survival after matching. Cox analysis demonstrated that the size of tumors and Child Pugh grade rather than age or Charlson comorbidity index were significant prognostic factors for overall survival.
Conclusion
The elderly patients with HCC, even though associated with more comorbidities, may achieve acceptable prognostic outcomes following MWA, which are not worse than their younger counterparts.
Introduction
Hepatocellular carcinoma (HCC) is a common malignant tumor in humans. Due to an increase in the aging population, it has become more significant to care for the elderly and elevate their quality of life. For the population aged more than 70 years, liver cancer is the fourth leading cause of cancer-related death worldwide, with the proportion being 6.5% worldwide and 11.7% in China [Citation1] which reduces the life span of the aging population.
Treatment of HCC includes surgical resection, transplantation, ablation, transarterial chemoembolization (TACE) and systemic therapy [Citation2]. Microwave ablation (MWA), which applies antennas radiating microwaves to achieve coagulation of tissue by the heating effect of microwaves, is an effective and safe method for the local treatment of HCC [Citation3,Citation4]. MWA has got more attention because of its faster heat generation and deep penetration into the tissues, as well as its maneuverability and efficacy in cancer treatment. Also, it has been reported that MWA has a lower rate of major complications and increases the preservation of surrounding organs [Citation5,Citation6]. This minimally invasive modality makes it possible to treat patients who are not candidates for conventional modalities such as resection surgery or transplantation.
Elderly patients with liver cancer usually have poor liver function and more comorbidities, and often expect to receive minimal therapy to obtain faster recovery and less peripheral organ damage. It is considered that patients with poor liver function benefit from MWA as their liver function and general condition are improved after the procedure [Citation7]. Therefore, MWA is thought to be an appropriate treatment method for elderly HCC patients.
However, there is no treatment strategy specifically recommended for elderly patients in the liver cancer guidelines [Citation2,Citation8,Citation9]. In addition, whether MWA benefits elderly patients similar to younger patients has not been fully documented. Thus, this study was designed to explore the impact of age, especially aging, on the prognosis of HCC after MWA and determine whether elderly patients have a similar outcome to their younger counterparts.
Materials and methods
Study design
This study was based on a previously collected dataset of patients who underwent MWA of HCC in our department from January 2010 to November 2017. The data were checked and revised according to medical records before analysis. The study protocol was approved by our institutional review board and consent forms were not required due to the retrospective and observational design of this study. The results were reported following the checklist of items included in cohort studies and the brief guidelines for reporting propensity score analysis in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [Citation10,Citation11].
Patients
Patients included in this study were diagnosed with HCC according to the Standardization of Diagnosis and Treatment for Hepatocellular Carcinoma from Chinese Bureau of Medical Administration. Most liver nodules were diagnosed noninvasively by imaging techniques and all were finally proved to be hepatocellular carcinoma (HCC) by pre-ablation pathology. Patients were assessed by a multidisciplinary team in our institute and MWA was performed for appropriate indications.
Ultrasound (US)-guided MWA
Percutaneous MWA guided by US imaging was performed by interventional radiologists with at least 10 years of experience in MWA. The microwave systems (KY-2,00,02,450 MHz and KY-21,00,915 MHz; Kangyou Medical, Nanjing, China) with cool-tip needle antennas of 1.9 mm (15 gauge) in diameter were used. General anesthesia was administered in all patients. The placement and approach of the antennas, as well as the frequency, power output setting, and ablation time were determined according to the size and location of the tumors during the pre-procedure meetings. The detailed MWA protocol is available in our previous reports [Citation7,Citation12]. Approximately 1–3 days after the ablation procedure, contrast-enhanced US or magnetic resonance (MR) imaging was performed to evaluate treatment effectiveness and verify whether complete ablation was achieved.
Follow-up
The follow-up protocol involved measurement of alpha‐fetoprotein (AFP), contrast-enhanced US, as well as computed tomography (CT) or MR imaging at 1 month after ablation, then at 3-month intervals during the first post-procedural year and at 6-month intervals thereafter. Overall survival (OS) was calculated from the date of ablation to the time of death or the last follow-up visit. Tumor‐specific survival (TSS) was defined as the time between the procedure and tumor-specific death. Liver cirrhosis-related death (e.g., gastrointestinal hemorrhage) was not included. Patients who died from other causes such as myocardial infarction were treated as censored for TSS. Progression-free survival (PFS) referred to the time from the procedure to disease progression, including local progression, intrahepatic recurrence and extrahepatic metastasis. Patient follow-up was discontinued at the end of January 2019.
Variables and outcomes
Age, sex, size and number of nodules, comorbidity, serologic biochemical values, AFP and types of liver disease were recorded. The maximum diameter of the main nodule was considered to be the tumor size, while the Charlson comorbidity index was calculated to measure the burden of comorbidity [Citation13,Citation14]. AFP level was divided into <400 µg/L and ≥400 µg/L as some HCC diagnosis criteria set as the cutoff values [Citation8]. Ascites, extrahepatic metastasis, and portal vein tumor thrombus (PVTT) were assessed by imaging and were recorded as disease severity. Tumor staging classification was evaluated using the Barcelona Clinic Liver Cancer (BCLC) system [Citation15]. Liver function was assessed by Child Pugh grade, albumin–bilirubin (ALBI) grade [Citation16], and the Model for End‐stage Liver Disease (MELD) score [Citation17]. Ablation procedure-related parameters were extracted from the procedure records, and included frequency, number of antennas, output power and total ablation time of MWA. The short-term outcomes included the rate of complete ablation and post-ablation major complications, as well as the total and post-ablation hospital stay and the cost of hospitalization. Complete ablation referred to an ablation zone which completely covered the treated tumor under assessment on 1- to 3-day post-ablation US or MR imaging. Major complications were defined as events that led to substantial morbidity and disability [Citation18], including perforation of the gastrointestinal tract, sudden appearance of pleural or peritoneal effusion requiring thoracentesis or abdominocentesis, hemorrhage requiring embolization, bile duct injury and biliary fistula, liver abscess and empyema, tumor seeding, and skin burn requiring resection.
Statistical analysis
Patients were divided into four age groups: group 1, <55 years, group 2, 55–64 years, group 3, 65–74 years and group 4 ≥ 75 years. The epidemiological variables and short- and long-term outcomes of groups 1–3 were then compared with those of group 4. To eliminate the impact of unbalanced variables including tumor burden, liver function and other general conditions, a 1:1 propensity score matching with a caliper of 0.05 was applied in group 4 and the other groups. The matching variables included all unbalanced data except age-related variables including age and the Charlson comorbidity index, which consisted of sex, the number and size of the tumors, ALBI, hepatitis B virus (HBV) infection and HCV infection.
Continuous variables were expressed by the median and interquartile range (IQR) and categorical variables were expressed by number and percentage. To compare the differences, continuous variables were analyzed by the Mann‐Whitney U test and Kruskal‐Wallis test when non-normally distributed, and categorical variables were analyzed by the Chi‐Square test or Fisher exact test as appropriate. OS, TSS, and PFS were analyzed by the Kaplan‐Meier (KM) method and compared by the log‐rank test. Univariate and multivariate Cox analyses were performed to determine the risk or protective factors of OS, which were selected after negotiation. All statistical analyses were 2‐tailed and performed with the statistical software packages R (http://www.R-project.org; The R Foundation) and EmpowerStats (http://www.empowerstats.com; X&Y Solutions, Inc., Boston, MA). A P value less than 0.05 was considered statistically significant.
Results
Patients included
From January 2010 to November 2017, 1112 consecutive patients with HCC were registered in the dataset and considered for analysis in our research. Of these patients, 229 were excluded due to incomplete data. Comparison analysis showed no significant differences in characteristics between the excluded and included patients (). In total, 883 patients were included in this study. The overall and median follow-up times were 105 and 42 months, respectively.
Table 1. Comparison of included and excluded characteristics.
Comparison of baseline characteristics in groups 1–4
The number of included patients in the four age groups was as follows: 313 patients in group 1, 330 in group 2, 170 in group 3 and 70 in group 4. The groups showed significant differences in baseline characteristics including sex, size of nodules, the Charlson comorbidity index, ALBI, proportion with HBV and HCV infection, and a pairwise comparison between groups 1–3 and the group 4 is shown in . Of these variables, the Charlson comorbidity index and proportion of those with HBV infection of group 4 were statistically different from groups 1–3, and significant differences between groups 1, 2 and 4 were observed in terms of sex, size of nodules, proportion of HCV infection and ALBI (marked with *in ).
Table 2. Baseline characteristics of groups 1–4 and pairwise comparison between groups 1–3 and group 4.
Short-term and long-term outcomes in groups 1–4
Short-term and long-term outcomes in the four groups are listed in . With regard to short-term outcomes, the rates of complete ablation were 97.8%, 98.8%, 97.1% and 92.9% in groups 1–4 (p = 0.029), respectively, where significant differences were found between groups 1 and 4 as well as 2 and 4 (p = 0.049 and 0.010). The number of patients with major complications was 10 (3.2%), 12 (3.6%), 2 (1.2%), and 4 (5.7%) in groups 1–4, respectively, which was not statistically different (p = 0.271). However, the total hospital stay was significantly different (p < 0.001) and the longest post-ablation hospital stay was observed in group 4 with a median stay of 7 days (p < 0.001). And pairwise comparison showed that the cost of hospitalization was significantly different between groups 1–3 and 4 (p < 0.001, =0.001, =0.047).
Table 3. Prognostic outcomes for groups 1–4.
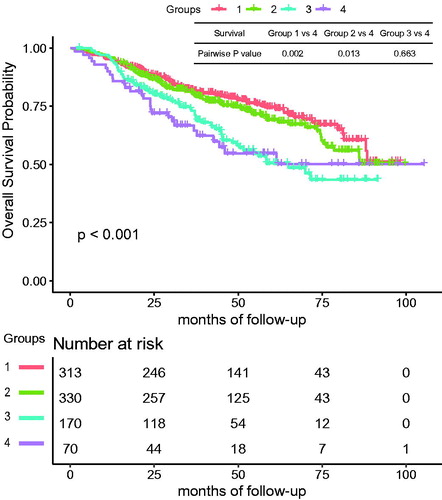
With regard to long-term outcomes, the rates of death were 25.2%, 27.9%, 39.4%, and 40.0% (p = 0.002), and pairwise statistical differences were found between group 1 and 4 (p = 0.013). Correspondingly, the rates of tumor-specific death in groups 1–4 were 21.4%, 23.0%, 33.5% and 32.9% (p = 0.009), respectively, and pairwise statistical differences were observed between group 1 and 4 (p = 0.041). The median OS was 45.9 (IQR: 26.8–66.7), 42.1 (IQR: 26.3–58.4), 37.2 (IQR: 21.9–54.8), and 31.9 (IQR: 21.3–51.5) months in groups 1–4 (p < 0.001), respectively. The KM curve of cumulative OS is shown in and the log rank test found significant differences (p < 0.001) among the age groups. The pairwise log rank test using group 4 as a control showed a significant difference in groups 1 and 2 (p = 0.002, 0.013), but no difference in group 3 (p = 0.663). Nevertheless, there was no significant difference among the four age groups in the rates of progression and median PFS (p = 0.952, 0.080), which were 60.4%, 60.9%, 62.4%, 58.6% and 20.8, 18.5, 16.1, 15.8 months, respectively. The log rank test also showed no difference in PFS (p = 0.526).
Figure 1. The cumulative overall survival of group 1 (<55 years), group 2 (55–64 years), group 3 (65–74 years), and group 4 (≥75 years). The log rank test showed a significant difference among the 4 groups (p < 0.001). Pairwise statistical difference existed between group 1, 2 and 4 (p = 0.002, 0.013).
Prognostic outcomes and factors after matching
To investigate the independent impact of aging on prognosis, group 4 was selected as the senior group (Group S, n = 70) and compared with the other groups. To balance the tumor burden and other unbalanced confounding factors, including sex, the number and size of the tumors, ALBI, HBV and HCV infection, the propensity score was used to match comparable patients to obtain the younger group (Group Y, n = 70) for comparison. Group Y included patients with a median age of 57 years (IQR: 51–63), and other baseline characteristics of the two groups before and after matching are shown in . A comparison of short-term and long-term outcomes before and after matching is shown in .
Table 4. Baseline characteristics of Group Y (<75 years) and Group S (≥75 years).
Table 5. Prognostic outcomes before and after matching.
With regard to short-term outcomes after matching, there was no significant difference in the rates of complete ablation (95.7% in Group Y vs 92.9% in Group S, p = 0.718), and major complications (5.7% in Group Y vs 5.7% in Group S, p = 1.000). Nevertheless, the total hospital stay was statistically different, with a median stay of 12 days (IQR: 9–15) in Group Y and 14 days (IQR: 11–17) in Group S (p = 0.011). In addition, the post-ablation hospital stay was 5 days (IQR: 3–8) in Group Y, and was 7 days (IQR: 5–9) in Group S (p < 0.001). The median cost of hospitalization in Group S was 45 thousand RMB yuan (IQR: 40–52) compared with 43 thousand yuan (IQR: 37–51) in Group Y (p = 0.124) after matching, although there was a significant difference before matching (p < 0.001).
Before matching, the death rate in Group Y and Group S was 29.3% and 40.0% (p = 0.061) and the median OS was 42.4 months (IQR: 25.7–61.0) and 31.9 months (IQR: 21.3–51.5) (p = 0.014), respectively. After matching, the death rate in Group Y and Group S was 40.0% (p = 1.000) and the median OS was 39.4 months (IQR: 21.8–57.1) and 31.9 months (IQR: 21.3–51.5) (p = 0.349), respectively. The log rank test showed no significant difference in the cumulative OS between the two groups after matching (p = 0.784) and the KM curve is presented in . For TSS, the log rank test also showed no significant difference between the two groups (p = 0.843). In addition, after matching, no significant difference in the rate of progression was observed (p = 0.496), and the log rank test showed no difference in PFS between the two groups (p = 0.186). Cox regression analysis of OS revealed that age was not a significant variable associated with survival (HR 1.01, p = 0.296), neither was the Charlson comorbidity index (HR 1.09, p = 0.228). Nevertheless, nodule size (HR = 1.24, p < 0.001), presence of other cancers (HR = 6.37, p = 0.003), Child Pugh grade (HR = 4.10, p = 0.008) and the frequency of microwaves (HR = 4.55, p = 0.019) were independent prognostic factors ().
Figure 2. The cumulative overall survival of Group Y (<75 years) and Group S (≥75 years) after propensity matching. The log rank test showed no significant difference between the two groups (p = 0.784).
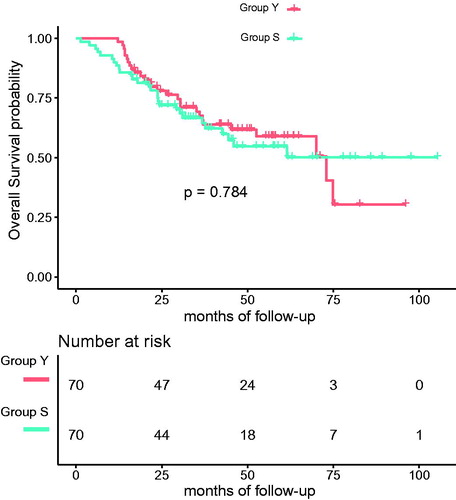
Table 6. Univariate and multivariate Cox regression analyses of overall survival of the patients from Group Y and S.
Discussion
It has been shown that liver weight declines [Citation19] and portal blood flow velocity decreases in elderly people aged >71 years [Citation20], which leads to a diminished repair capability of the liver compared with younger people. Thus, a poorer prognosis is expected in elderly patients following treatment for liver cancer. As the life span of humans continues to increase [Citation21,Citation22], a number of studies have suggested that the lowest age of elderly groups should be 75 years [Citation23,Citation24]. Accordingly, we defined 75 years as the threshold for the senior group in the present study, which has also been applied in several studies on liver cancer in elderly patients [Citation25–27].
In our study, we compared baseline characteristics and prognosis after MWA of HCC in different age groups and found some differences between the senior group and younger groups. When age increased by one decade, the Charlson comorbidity index increased by one point and a pairwise comparison showed that the three younger groups were statistically different from Group S. Significant differences were observed in the distribution of sex, nodule size, hepatitis virus infection and ALBI, mainly between the <55 years group and the 55–64 years group, and Group S. The proportion of females was relatively higher in the older age groups possibly due to the longer life expectancy of females. And the nodule size increased with age, and one of the reasons might be the poorer immune system of elderly patients. Moreover, the probability of liver cancer was higher in those with HCV infection but lower in those with HBV infection as age increased, which was similar to the findings in other studies [Citation28]. This may be attributed to the older age of patients with HCV infection than those with HBV infection [Citation29]. Consequently, elderly patients are a particular population with unique characteristics and direct comparisons may be influenced by a number of interfering factors. As a result, we performed propensity score matching to compare the impact of aging on prognosis.
Before matching, there were no significant differences in the rate of major complications and PFS among the four age groups, which demonstrated the safety of MWA and progression of the malignancy did not differ with age. When tumor burden was matched, the elderly liver cancer patients after MWA achieved survival outcomes which were not worse than those in younger patients as shown in . These findings suggest that compared to younger patients with similar liver function and tumor burden, elderly patients can equally benefit from MWA. Although Group S had a higher Charlson comorbidity index, similar long-term outcomes were observed after MWA, which concurred with the findings of a previous study [Citation30]. Furthermore, elderly patients had a longer hospital stay post-ablation and higher hospitalization cost. This was due to their poor physical status as they required a longer recovery time after ablation than younger patients, which consequently resulted in a longer observation and surveillance time and higher cost.
Cox regression analysis suggested that the size of the tumor, the presence of other cancers, Child Pugh grade, and microwave frequency were independent risk factors for OS. It is acknowledged that the survival of HCC is influenced by the tumor size and the larger generally causes poorer prognosis. The Child Pugh grade, which is a frequently used tool to evaluate liver function, includes subjective variables such as ascites and encephalopathy. It was used as a sensitive index for OS in our study. Although ALBI was statistically different among the four age groups (p < 0.001), it was not a significant prognostic factor for survival. With regard to microwave frequency, the higher frequency (2450 MHz) was considered to have greater power deposition, greater thermal conduction and a lower rate of progression than the lower frequency (915 MHz) [Citation31,Citation32], which accounts for the poorer OS following treatment with 915 MHz in our study.
The prevalence of HCC in elderly people is expected to continue to increase in the near future [Citation29,Citation33]. Minimally invasive therapy is often recommended in elderly patients considering their reduced tolerance to surgery and the presence of comorbidities. There are few studies and reviews on the treatment of HCC in elderly patients by percutaneous or transarterial means, especially percutaneous MWA. In our research, we confirmed that elderly patients (aged ≥75 years) even with a poor comorbidity index, benefited from MWA of HCC similar to younger patients with an overall follow-up time of up to 8 years.
A limitation of this study is its retrospective nature. Although this real-world study based on a database represented the actual situation, the risk factors for survival identified by post-hoc analysis were not dependable. In addition, the number of elderly patients aged more than 75 years was limited and 70 pairs were ultimately included in the analysis after matching. Another limitation is that this study was carried out in one center. As a result,on the one hand, the technical success of MWA partly depended on experience; on the other hand, the liver disease profile of the included patients is characteristic for an Asian population, and most liver diseases in this study were caused by HBV infection, while in a typical cohort in Europe or North America might be caused by HCV infection or heavy alcohol drinking [Citation34]. So further prospective controlled studies with more elderly patients in different centers are needed.
In conclusion, elderly patients (aged ≥75 years) with HCC who undergo MWA may achieve acceptable prognostic outcomes which are not worse than those of their younger counterparts. Despite different liver disease traits of elderly patients and higher incidence of comorbidities, aging per se does not reduce the safety and effectiveness of MWA. We believe that thorough and systematic evaluation is more reasonable than simply classifying aging as a contraindication for MWA.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- International Agency for Research on Cancer, WHO. Estimated number of deaths in 2018, all cancers, both sexes, ages 70+. 2018 [cited 2019 Jun 24]. Available from: http://gco.iarc.fr/today/
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314.
- Seki T, Wakabayashi M, Nakagawa T, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817–825.
- Dong BW, Liang P, Yu XL, et al. Sonographically guided microwave coagulation treatment of liver cancer: an experimental and clinical study. AJR Am J Roentgenol. 1998;171:449–454.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. 2009;251:933–940.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208.
- Liang P, Dong B, Yu X, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299–307.
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430.
- Omata M, Cheng A-L, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370.
- Yao XI, et al. Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst. 2017;109:djw323.
- von Elm E, Altman DG, Egger M, et al.; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457.
- Dong B, Liang P, Yu X, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. AJR Am J Roentgenol. 2003;180:1547–1555.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Quan H, Li B, Couris CM, et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682.
- Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524.
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558.
- Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871.
- Ahmed M, Solbiati L, Brace CL, et al.; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology. 2014;273:241–260.
- Popper H. Aging and the liver. Prog Liver Dis. 1986;8:659–683.
- Zoli M, Iervese T, Abbati S, et al. Portal blood velocity and flow in aging man. Gerontology. 1989;35:61–65.
- Olshansky SJ, Carnes BA, Désesquelles A. Demography. Prospects for human longevity. Science. 2001;291:1491–1492.
- Vaupel JW, Carey JR, Christensen K, et al. Biodemographic trajectories of longevity. Science. 1998;280:855–860.
- Ihle A, Jopp DS, Oris M, et al. Investigating discontinuity of age relations in cognitive functioning, general health status, activity participation, and life satisfaction between young-old and old-old age. Int J Environ Res Public Health. 2016;13:1092.
- Baltes MM. The psychology of the oldest-old: the fourth age. Curr Opin Psychiatry. 1998;11:411–415.
- Oishi K, Itamoto T, Kobayashi T, et al. Hepatectomy for hepatocellular carcinoma in elderly patients aged 75 years or more. J Gastrointest Surg. 2009;13:695–701.
- Kaibori M, et al. Treatment optimization for hepatocellular carcinoma in elderly patients in a Japanese nationwide cohort. Ann Surg. 2019;270:121–130.
- Famularo S, Di Sandro S, Giani A, et al. The impact of age and ageing on hepatocarcinoma surgery: short- and long-term outcomes in a multicentre propensity-matched cohort. Liver Int. 2019;39:894–904.
- Cohen MJ. Trans-arterial chemo-embolization is safe and effective for very elderly patients with hepatocellular carcinoma. World J Gastroenterol. 2013;19:2521.
- Nishikawa H, Kimura T, Kita R, et al. Treatment for hepatocellular carcinoma in elderly patients: a literature review. J Cancer. 2013;4:635–643.
- Shen X, Ma S, Tang X, et al. Clinical outcome in elderly Chinese patients with primary hepatocellular carcinoma treated with percutaneous microwave coagulation therapy (PMCT). Medicine. 2018;97:e11618.
- Curto S, Taj-Eldin M, Fairchild D, et al. Microwave ablation at 915 MHz vs 2.45 GHz: a theoretical and experimental investigation. Med Phys. 2015;42:6152–6161.
- Vogl TJ, Hagar A, Nour-Eldin N-EA, et al. High-frequency versus low-frequency microwave ablation in malignant liver tumours: evaluation of local tumour control and survival. Int J Hyperthermia. 2016;32:868–875.
- Hung AK, Guy J. Hepatocellular carcinoma in the elderly: meta-analysis and systematic literature review. World J Gastroenterol. 2015;21:12197–12210.
- Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477–491.e1.
