Abstract
Purpose
To evaluate feasibility, safety and efficacy of image-guided thermal ablations associated with retrograde pyeloperfusion in patients with centrally located renal tumors.
Materials and methods
48 patients (15 women, 33 men, mean age 69.1 ± 11.8) were treated with image-guided thermal ablation associated with pyeloperfusion for 58 centrally located renal tumors (mean diameter 32.3 ± 7.32 mm). 7 patients had a single kidney. Microwave and radiofrequency ablation were used. All treatments were performed with ultrasound, CT, or fusion imaging guidance under general anesthesia and simultaneous retrograde cold pyeloperfusion technique.
Results
Procedure was feasible in all cases. Technical success and primary technical efficacy were reached in 51/58 (88%) and 45/54 tumors (83%). With a second ablation performed in 5 tumors, secondary technical efficacy was achieved in 50/50 (100%) tumors. Minor and major complications occurred in 8/58 (13%) and 5/58 (8%) tumors. No significative change in renal function occurred after treatment.
During follow-up, 5 recurrences occurred, that were retreated with a second ablation. At last follow up (mean 32.2 ± 22.0 months), 41/48 (85%) treated patients were free from disease. The median TTP and PFS were 27.0 (range, 2.3–80.0) and 26.5 months (range, 2.3–80.0), respectively.
Conclusion
Image-guided thermal ablation associated with protective pyeloperfusion is a feasible, safe, and effective treatment option for patients with central renal tumors with a minimal impact on renal function and relevant potential to avoid nephrectomy.
Introduction
Renal cancer represents 2–3% of all cancers, with an estimated 403.262 new cases and 175.098 deaths worldwide in 2018 [Citation1]. Its detection is increasing owing to a rise in the diagnosis of small asymptomatic renal masses with cross-sectional imaging [Citation2]. Therefore, small renal masses less than 4 cm represent nowadays 48–66% of all renal cancers [Citation3].
While nephron-sparing treatments such as partial nephrectomy are considered the gold standard treatment for renal cell carcinomas (RCC) [Citation4,Citation5], centrally located tumor represent a surgical challenge and often, require a radical nephrectomy. Therefore, a minimally invasive treatment able to achieve tumor cure with preservation of renal function would be extremely useful for these patients. Over the last decade, percutaneous thermal ablation therapies including radiofrequency ablation (RFA), cryoablation and, more recently, microwave ablation (MWA) have emerged as a feasible and effective alternative to surgical approaches, and are particularly applied in patients who are unfit for surgery or with previous ipsi- or contralateral renal surgeries [Citation6–9]. However, considering the higher risk of injury to the collecting system [Citation10] and incomplete tumor ablation [Citation11,Citation12] that these image-guided thermal ablations pose for centrally located renal tumors, protective maneuvers such as pyeloperfusion or hydrodissection have been implemented and applied [Citation13–15] giving new chances of cure to many patients with solitary kidneys, compromised renal function, multiple RCCs or comorbid medical conditions.
At our institution, in order to lower the risk of complications to the collecting system, retrograde pyeloperfusion is systematically applied in case of centrally located renal tumors candidate to an image-guided thermal ablation.
Thus, the purpose of our study was to retrospectively evaluate feasibility, safety and efficacy of image-guided thermal ablations associated with retrograde pyeloperfusion in patients with centrally located renal tumors.
Materials and methods
Institutional review board Approval was obtained and patients’ informed consent was waived.
A retrospective analysis of electronic records and imaging database was undertaken to identify patients who underwent retrograde pyeloperfusion during image-guided thermal ablation of kidney tumors between October 2011 and December 2018. A total number of 48 patients (mean patients age 69.1 ± 11.8, range 37.1–89; 15 F, 33 M), underwent treatment of 58 tumors (mean number of tumor per patient: 1.21; median: 1; range 1–3; mean maximum tumor diameter 32.3 ± 7.32 mm; median 36.5 mm; range, 16–48 mm). Microwave ablation (MWA) was used in 27 tumors, radiofrequency ablation (RFA) in 29 tumors. Two other tumors were treated initially with RF and then with a combined approach (MWA + RFA) in the same session due to their high peripheral vascularization and incomplete ablation at the immediate control post RFA.
All tumors underwent percutaneous biopsy as a separate procedure before the ablation treatment. Of the treated tumors, 51/58 (88%) fell in the T1a group and 7/58 (12%) in the T1b according to the TNM classification criterion. Patients and tumors pathologic characteristics are summarized in . 13/58 (22%) lesions had previously been treated, 1/13 (8%) with a non-radical surgical attempt and the remaining 12/13 (92%) with a percutaneous thermal ablation.
Table 1. Characteristics of 48 patients who underwent thermal ablation for a centrally located renal lesion (n = 58).
Indication to image-guided thermal ablation was taken into a multidisciplinary meeting involving urologists, radiologists, oncologists and radiation therapists. The percutaneous approach was chosen taking into account age, comorbidities, tumor location, size and proximity to critical non-target structures. Due to the central location, it was acknowledged that more than one ablation could have been necessary to achieve technical success. Thus, it was always explained to the patients that the course of treatment might likely consist of two ablations [Citation16]. The ablation technology adopted, RF or MW, depended on various factors such as preference and familiarity of the first operator, morphological characteristics and location of the tumor, intraprocedural morphological results and technology availability. Cryoablation was not considered as it has not yet been adopted at our institution.
All procedures were performed using retrograde pyeloperfusion and the placement of the ureteral stent was performed by an urologist with at least 3 years of experience. Considering the risk for thermal damage to the collecting system in centrally located renal lesions ablation, a proximity of <4 mm to the collecting system including calyces and infundibulum was the indication for pyeloperfusion. Regarding the choice of perfusate, distilled water was adopted for being nonionic and therefore safer than sodium chloride–containing solutions due to their high electrical conductivity, possibly favoring direct thermal injury of the urothelium during ablation.
All procedures were performed by a team of two interventional radiologists, at least one with more than 10 years of experience.
Procedure
All procedures were performed in a dedicated suite equipped with a C-arm (Ziehm Vision RFD Hybrid Edition, Ziehm Vision, Nuremberg, Germany), a computed tomography (CT) (GE Lightspeed, GE Healthcare, Chicago, IL, USA) and an ultrasound (US) machine (GE E9, GE Healthcare, Chicago, USA) equipped with a dedicated fusion imaging software.
All procedures were performed under general anesthesia. As first step, the urologist under cystoscopy and fluoroscopic guidance, placed a 6 F single J ureteral catheter (WIRUTHAN, Teleflex Medical, Westmeath, Ireland) on a hydrophilic guidewire, with the tip located in the renal pelvis. Afterwards, a 14–16-F Foley catheter was placed into the bladder, to which the retrograde ureteral catheter was taped to prevent its displacement. Then, a 500 ml bag of distilled water at about 5 °C was hung about 100 cm above the patient, connected to the ureteral catheter and perfused by continuous gravity drainage into the renal collecting system, frequently monitored throughout all procedure to confirm ongoing perfusion. The ureteral single J was connected to a three-way stopcock, allowing for manual injection in case of a higher injection pressure was needed.
Subsequently, careful patient positioning planning was performed and the most advantageous decubitus was secured with the help of dedicated devices such as vacuum mattress to immobilize the patient body during the procedure. A contrast enhanced CT (CECT) scan was then performed to evaluate the tumor and allow for fusion imaging with US images [Citation15]. On the basis of the size and geometry of the tumor and its relationships with adjacent organs, the best access strategy and the possible need for hydrodissection were decided. Adjunct procedures, such as hydrodissection were applied according to interventional radiologist judgment [Citation15]. The ablative device was than inserted under real time US/CT fusion imaging guidance, and correct device position was confirmed with a CT scan.
Regarding RF, a LeVeen 17 G umbrella-shaped electrode (LeVeen CoAcces RFA, Boston Scientific, Marlborough, MA, USA needle electrode) was adopted using increasing power up to roll-off twice. Regarding MW, a 13 G internally cooled antenna (Emprint, Medtronic, Minneapolis, MN, USA) was used. Power and time were established according to manufacturer indications for liver thermal ablations and to the size and shape of the tumor to be treated on a case-by-case basis.
After ablation, a CECT was always performed to control for immediate result and eventual presence of complications. In case of suspicious persistence of pathological tissue beyond the ablation area, device repositioning and a further ablation were performed in the same manner as previously described. On the basis of operator judgment, further ablation can be postponed to a subsequent session. The ureteral catheter was left in place up to the day after the procedure.
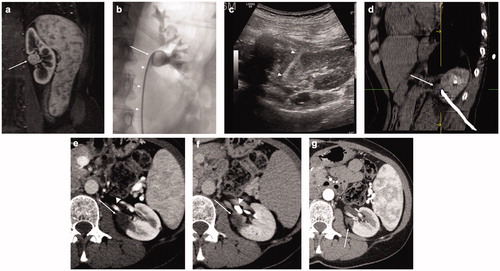
A CECT within 24 h was performed, with also retrograde injection of contrast material through the single J catheter to evaluate treatment outcomes and even minor urinary leakages. Finally, if no complications were observed, the stent was removed after CT and the patient was discharged the day after. A case of a patient with a centrally located lesion treated with image-guided thermal ablation associated with retrograde pyeloperfusion is shown in .
Figure 1. Treatment of a left kidney central tumor with renal sinus extension with image-guided microwave ablation and retrograde pyeloperfusion. (a) T1-weighted, fat-saturated, contrast-enhanced MR image demonstrating a centrally located tumor (white arrow) with extension in close proximity of the renal sinus. (b) Retrograde pyeloperfusion was performed through a single j stent (white arrowheads) placed endoscopically the day of the ablation with the tip located in the renal pelvis (white arrow). (c) Microwave antenna (white arrowheads) was inserted under real-time US visualization. (d) CT confirmed the precise positioning of the microwave antenna at the level of the tumor. (e, f) Contrast-enhanced CT 6 months after treatment demonstrates the correct ablation of the tumor (white arrows) with no residual enhancing tumor either in the arterial phase (e, white arrowhead = arterial branch) or the excretory phase (f, white arrowhead = renal calyx). (g) Contrast-enhanced CT in the arterial phase 12 months after the ablation demonstrating complete ablation with tumor shrinkage (white arrow) and no complications.
Data collection
Clinical, laboratory, imaging pathology and procedural data were collected from the comprehensive electronic medical records.
Clinical data included the patient’s age, gender, BMI, mono-renal status, pre- and post-ablation serum creatinine levels, estimate of the glomerular filtration rate (eGFR), pre- and post-ablation hemoglobin levels, any previous treatment or retreatments.
Pathological data included the tumor size, the histological features and tumor complexity as assessed with the RENAL nephrometry score [Citation17].
Our series included only centrally located tumors defined as those that contacted and grow internally into the renal sinus fat, at a distance of ≤4 mm, according to the classification of Gervais et al. [Citation18].
Procedural data included the type of technique adopted, the duration of the energy delivery, the maximum power delivered, any applied hydrodissection and complications. The intra- and post-procedural complications were recorded and classified according to SIR standards [Citation19]. Urinary leakage was considered as minor when only trace of contrast material outside the collecting system was seen on postoperative CECT images, but within the zone of ablation and without urinoma accumulation, and did not required any treatment.
Follow-ups were performed by CECT or contrast enhanced MRI at established intervals (6 weeks, 3, 6, 12 months) following the first treatment and subsequently on an annual basis.
Technical success, primary and secondary efficacy, local tumor progression, time to local tumor progression (TTP) and progression free survival (PFS) were assessed and defined according to standard terminology as reported by Ahmed et al. [Citation16].
Technical success was defined as a complete treatment according to protocol with full cover of the lesion by the ablation zone on imaging obtained immediately after ablation.
The primary efficacy rate was defined as the percentage of target tumors successfully eradicated following the course of treatment and the secondary efficacy rate as including tumors that have undergone successful repeat ablation following identification of local tumor progression.
The local tumor progression was defined as the appearance of a tumor foci at the edge of the ablation zone after a follow-up beyond that performed for the assessment of technical efficacy.
TTP was defined as time interval between the date of ablation procedure and appearance of local tumor progression.
PFS was defined as time interval between the date of ablation procedure and appearance of local tumor progression or patients’ death.
Data analysis and statistics
Statistical analyses were performed using XLSTAT (Addinsoft, New York, NY, USA), results were reported as means, standard deviation (SD), medians and ranges for the quantitative variables and percentages for the categorical variables. The groups with continuous variables were compared using two-tailed paired Student’s t test; p < 0.05 was considered significant. TTP and PFS were estimated using the Kaplan–Meier method.
Results
Clinical and pathological data are shown in . In addition to pyeloperfusion, hydrodissection was applied in 8/58 (14%) of treated lesions as a further protective maneuver to sensitive adjacent structures.
Mean ablation time in the RF group was 55.00 ± 31.88 min (range, 18–160 min), and 8.61 ± 6.38 min (range, 2–29 min) in the MW group (p < 0.05). Mean energy deployment in the RF group was 83.89 ± 42.70 W (range, 25–180 W) and 87.96 ± 14.76 W (range, 55–100 W) in the MW group (p = 0.64).
According to the RENAL score, there were 5 (9%) low complexity, 28 (48%) moderate complexity and 25 (43%) high complexity tumors. RENAL nephrometry scores are summarized in .
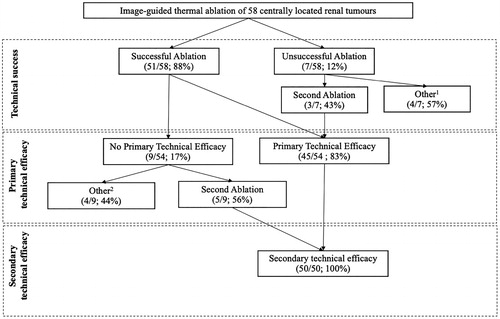
Technical success was achieved in 51/58 (88%) tumors. Of the 7 tumors which showed unsuccessful ablation at the immediate CECT, a second treatment was successfully performed in 3 tumors, while of the remaining four tumors one was treated with total nephrectomy, one underwent active surveillance, two were not retreated due to the death of patients for unrelated reasons. Finally, primary technical efficacy was achieved in 45/54 tumors (83%).
A second ablation was performed in 5 tumors incompletely eradicated, while four tumors did not undergo a second ablation, and were treated with total nephrectomy (1 case), or were not retreated due to the death of patients for unrelated reasons (3 cases). Thus, including patients who underwent a second treatment, complete ablation was achieved in 50/58 initially enrolled tumors (86%), with a secondary efficacy rate achieved in 100% of the 50 tumors who were manageable with a second ablation. shows a flow chart of the ablation results.
Figure 2. Flowchart of the ablation results (per-lesion analysis). 11 total nephrectomy, 1 active surveillance, 2 untreated due to death of patients for unrelated reasons; 21 total nephrectomy, 3 untreated due to death of patients for unrelated reasons.
At a mean imaging follow-up of 32.2 ± 22.0 months (median 26.07; range, 2.3–80.0 months) local tumor progression was identified in 5/50 (10%) of successfully treated tumors. All patients underwent successful retreatment at our institution with thermal ablation. The median TTP was 27.0 (range, 2.3–80.0).
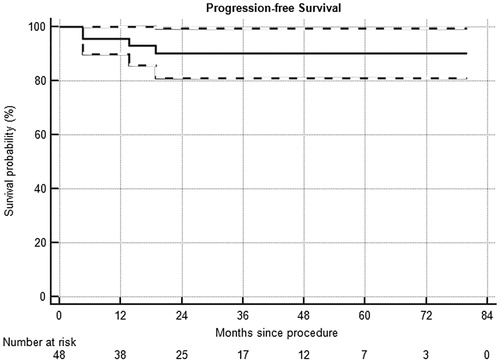
Overall, of the initially included 48 patients, 41/48 (85%) were successfully treated in one or two sessions and free from disease at last follow up, 2/48 (4%) required total nephrectomy, 1/48 (2%) has residual disease and is under active surveillance, and 4/48 (8%) died due to causes unrelated to their tumors. As shown in , the median PFS was 26.5 months (range, 2.3–80.0). The 2-years PFS rate was 90%.
Figure 3. Progression-free survival in a series of 48 patients with kidney lesions treated with thermal ablation. Dashed lines = 95% confidence intervals.
Pre- and post-treatment procedural data at last follow-up (mean imaging follow-up of 32.2 ± 22.0 months, range 2.3–80.0 months), are shown in .
Table 2. Pretreatment and post-treatment characteristics of patients (n = 48) who underwent thermal ablation for a centrally located renal lesion (n = 58).
Regarding the group of patients (7/48, 15% patients; 8/58, 14% tumors) with single kidney, no significant change occurred in mean creatine and eGFR levels after the procedure ablation (p = 0.94; p = 0.83, respectively). Pre- and post-treatment procedural data for the mono-renal patients are shown in .
Table 3. Pretreatment and post-treatment characteristics of mono-renal patients (n = 7) who underwent thermal ablation for a centrally located renal lesion (n = 8).
A total number of 13 complications over 58 procedures (22%) was reported in 12/48 (25%) patients. Minor complications (A, B) accounted for 8/13 (62%) and major complications (C–F) for 5/13 (38%). No severe treatment-related AEs (E, F) occurred. No patient developed a ureteral stricture or needed dialysis over a mean of 32.2 months follow-up (range, 2.3–80.0 months). Complications are listed in .
Table 4. Complications among patients (n = 48) who underwent thermal ablation for a centrally located renal lesion (n = 58), classified according to SIR standards [Citation17].
Discussion
At present, image-guided thermal ablations are widely used as safe and effective alternatives to partial nephrectomy for the treatment of clinical stage T1 renal masses in patients who are not ideal surgical candidates or those with previous surgeries [Citation6,Citation13,Citation20]. However, lower success rates in treating central kidney tumors have been reported, maybe related to less aggressive treatment owing to potential complications of the vascular and collecting system. Retrograde pyeloperfusion during thermal ablation has been advocated to mitigate this effects for central renal tumors, especially for patients with limited surgical options [Citation21,Citation22]. Notably, for patients with central tumors, the main surgical option is often nephrectomy, which can significantly affect renal function, or even determine the need for dialysis after treatment in patients with a single kidney. Thus, in this particular scenario, improving efficacy and lowering complications of minimally invasive treatments such as image-guided thermal ablations would be highly beneficial.
Regarding application of pyeloperfusion to image-guided thermal ablations of kidney tumors, some aspects should be taken into account. A mathematical study [Citation23] regarding RFA performed with pyeloperfusion affirms that it could cause less charring and therefore reduced ablation area. However, Margulis et al. and Hwang et al. [Citation24,Citation25] showed in animal models that pyeloperfusion did not significantly affect ablation size in a comparison of in vivo RFA of normal kidney. Regarding MWA, Isfort et al. [Citation26] team showed in porcine models no impact on the ablation volume with pyeloperfusion even though internal cooling had no effect on reducing thermal damage on the renal pelvis in either peripheral or central lesions. This might be due to the adoption of an anterograde instead of retrograde pyeloperfusion technique which could not assure a higher flow rate of cooling solution and a more efficient cooling effect. Hence, pyeloperfusion should not be considered as a 100% protective maneuver and extreme caution should be taken especially when dealing with central tumors. Furthermore, several aspects such as an ideal flow rate are yet to be defined.
In terms of technical success and efficacy rates our results are in line with other papers evaluating thermal ablation (RF and MW) [Citation27,Citation28] in RCC with concomitant pyeloperfusion even though our series included only central tumors which treatment is usually more challenging [Citation18]. This is further confirmed by the large percentage of high complexity tumors (43%) in our series according to the RENAL nephrometry score.
In our series, with systematical application of pyeloperfusion, it has been possible to achieve complete treatment with image-guided thermal ablation in the majority of patients, including 7 patients with single kidney, who avoided surgical nephrectomy and subsequent dialysis. At the last available follow-up, only two patients in our series finally required total nephrectomy due to renal vein tumor involvement. Furthermore, no patients experienced a significant decrease in renal function after treatment, and no one underwent dialysis. In our series, four patients died due to unrelated causes early after treatment. This fact underlines the fragile conditions of a large percentage of patients that were referred for image-guided thermal ablations.
In terms of adverse events, the major complication rate of 7% (4/58) is in line [Citation22,Citation29] or even lower [Citation30] compared with previous reports. Notably, of the major complications, one case of acute kidney injury and urinary leakage developed on a stage 4 CKD patient. It was promptly resolved with ureteral stent placement and nominal drug therapy, requiring a 5-day hospitalization. No severe adverse events occurred in our series.
In terms of survival outcomes, we were not able to assess longer oncological outcomes due to the relatively short follow-up interval (median 26.5 months) but we had a median TTP of 27.0 months and PFS of 26.5 months that are in line with recent papers evaluating even longer survival outcomes in thermal ablated RCC [Citation31]. Thus, with the systematic application of retrograde pyeloperfusion, it is possible to achieve in centrally located renal tumors clinical results that can be considered similar to what is reported for peripherally located renal tumors.
In fact, the main interest in retrograde pyeloperfusion relies in its potential to protect the collecting system from thermal injury during ablation. In our study, pyeloperfusion was technically successful in all cases, underlying the potential for a wider application of this technique.
In addition to pyeloperfusion, hydrodissection was applied in 14% of our treated lesions as a further protective maneuver to sensitive structures such as the colon, duodenum or pancreas and, in particular, the ureter, when it lay in direct contact to the tumor following stent placement [Citation32].
Regarding our procedures, unlike other authors [Citation33], only one antenna or electrode was used for each ablative session. Furthermore, the availability of a CT scanner in the interventional suite allowed for immediate precise result assessment and eventual retreatment in the same session, minimizing the need of a subsequent retreatment [Citation15,Citation34]. Also, application of image fusion has been reported as a useful tool to guide percutaneous biopsies and ablations, and might help improving tumor visualization and precise targeting, thus optimizing the treatment result [Citation35–38]. Even though immediate postprocedural CT demonstrated residual disease, in 7 cases it was decided not to try to complete the treatment in the initial session. This was due to the operator judgment, and the decision was made on clinical or technical difficulties. Sometimes ablation of large tumors with RFA could be quite long, and frail patients can poorly tolerate long general anesthesia period, or residual vital tissue could be located at the circumference of the tumor, and be difficult to retreat. When dealing with centrally located tumors, a course of treatment of two ablations appear to be reasonable following the principal aim of avoiding critical damage to the organ or surgical nephrectomy. However, re-treatment rates following a suboptimal treatment efficacy, appear to be influenced by pyeloperfusion as stated by Breen et al. [Citation39] when associated with cryoablation. In this regard, low heat-sink effects from renal pelvis cooling in RFA is yet not well understood and the percentage of residual tumor needing secondary treatment in ablated central tumors might be linked to a more prominent vascular-induced heat-sink effect as reported by Dai et al. [Citation30]. These results need to be confirmed and are worthy of further studies in future trials.
In our study, percutaneous cryoablation was not considered as it is not yet part of our armamentarium for the treatment of renal lesions. Recent analyses have indeed indicated that among ablative technologies, cryoablation may produce more consistent results in terms of survival outcomes [Citation40,Citation41] and it could have a superior oncologic efficacy versus RF in the setting of T1b disease [Citation42]. Moreover, as the collagen matrix architecture is preserved, it might be ideal in central tumors, reducing the risk of damage to the calyces and fistula formation [Citation43]. At the same time and although rarer than with other thermal ablation techniques, the risk of injury to the pelviureteric junction and ureter may occur with cryoablation as well, especially when the ureter is directly in contact with ablation zone or if the ablation zone contracts [Citation44]. At this regard, different efforts to avoid ureteral damage to the ureter have been made with cryoablation as well, with pyeloperfusion being effective in both anterograde and retrograde fashion [Citation45].
Our study has some limitations. First, this is a retrospective study, and a predefined protocol for patients’ selection and treatment was not established. Thus, patients’ selection was made based on multidisciplinary discussion, and the operators performing the procedure established choice of technique for treatment. However, the constant application of pyeloperfusion to all patients with centrally located tumors, and the standardization of the procedure in our division, can in some way counterbalance those limitations. Furthermore, the procedures were performed at a reference institution dedicated to cancer care, with all the most recent facilities such as hybrid angio-CT room, top level US machine, and fusion imaging capability, with highly experienced operators in image-guided renal ablations. This make our results difficult to be generalized. However, this underline the importance of performing such procedures with the adequate technological equipment, and that ablations should be performed by experienced interventional radiologists. In our study no control group was available to compare patients treated with pyeloperfusion versus patients treated without pyeloperfusion. However, due to the good results achieved with this technique, the absent complications related to stent insertion, a comparative study at the moment seems not to be justified. Finally, the median follow-up time of our study was lower than 3 years and thus did not allow us for the assessment of long-term oncologic survival outcomes such as overall survival (OS) or cancer-specific survival (CSS).
In conclusion, image guided thermal ablation associated with protective pyeloperfusion can be considered a feasible and safe treatment option for centrally located renal tumors, allowing for kidney function preservation and radical oncological results in a large majority of patients. Moreover, this technique had a minimal impact on renal function and holds the potential of avoiding nephrectomy in mono-renal patients. This technique should always be considered and discussed as a potential treatment option for patients with centrally located renal tumors, particularly in cases were surgical management would be based on total nephrectomy.
Continued and longer follow-up is needed to establish long-term oncologic efficacy and comparison to competing ablation modalities and surgery appear necessary.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- Lightfoot N, Conlon M, Kreiger N, et al. Impact of noninvasive imaging on increased incidental detection of renal cell carcinoma. Eur Urol. 2000;37:521–527.
- Gill IS, Aron M, Gervais DA, et al. Clinical practice. Small renal mass. N Engl J Med. 2010;362:624–634.
- Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198:520–529.
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924.
- Atwell TD, Schmit GD, Boorjian SA, et al. Percutaneous ablation of renal masses measuring 3.0 cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. AJR Am J Roentgenol. 2013;200:461–466.
- Ginzburg S, Tomaszewski JJ, Kutikov A. Focal ablation therapy for renal cancer in the era of active surveillance and minimally invasive partial nephrectomy. Nat Rev Urol. 2017;14:669–682.
- Sartori S, Mauri G, Tombesi P, et al. Ultrasound-guided percutaneous laser ablation is safe and effective in the treatment of small renal tumors in patients at increased bleeding risk. Int J Hyperthermia. 2018;35:19–25.
- Filippiadis D, Mauri G, Marra P, et al. Percutaneous ablation techniques for renal cell carcinoma: current status and future trends. Int J Hyperthermia. 2019;36:21–30.
- Gervais DA, Arellano RS, McGovern FJ, et al. Radiofrequency ablation of renal cell carcinoma: part 2, lessons learned with ablation of 100 tumors. Am J Roentgenol. 2005;185:72–80.
- Ruiz VA, Lönnemark M, Brekkan E, et al. Predictive factors for complete renal tumor ablation using RFA. Acta Radiol. 2016;57:886–893.
- Johnson DB, Saboorian MH, Duchene DA, et al. Nephrectomy after radiofrequency ablation-induced ureteropelvic junction obstruction: potential complication and long-term assessment of ablation adequacy. Urology. 2003;62:351–352.
- Atwell TD, Farrell MA, Callstrom MR, et al. Percutaneous cryoablation of large renal masses: technical feasibility and short-term outcome. AJR Am J Roentgenol. 2007;188:1195–1200.
- Mauri G, Nicosia L, Varano GM, et al. Unusual tumour ablations: report of difficult and interesting cases. Ecancermedicalscience. 2017;11:733.
- Mauri G, Nicosia L, Varano GM, et al. Tips and tricks for a safe and effective image-guided percutaneous renal tumour ablation. Insights Imaging. 2017;8:357–363.
- Ahmed M, Solbiati L, Brace CL, et al.; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273:241–260.
- Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853.
- Gervais DA, McGovern FJ, Wood BJ, et al. Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000;217:665–672.
- Sacks D, McClenny TE, Cardella JF, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:199–202.
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:706–720.
- Cantwell CP, Wah TM, Gervais DA, et al. Protecting the ureter during radiofrequency ablation of renal cell cancer: a pilot study of retrograde pyeloperfusion with cooled dextrose 5% in water. J Vasc Interv Radiol. 2008;19:1034–1040.
- Schultze D, Morris CS, Bhave AD, et al. Radiofrequency ablation of renal transitional cell carcinoma with protective cold saline infusion. J Vasc Interv Radiol. 2003;14:489–492.
- Glamore M, Masterson T, Pease KJ, et al. Mathematical and ex vivo thermal modeling for renal tumor radiofrequency ablation with pyeloperfusion. J Endourol. 2015;29:707–713.
- Margulis V, Matsumoto ED, Taylor G, et al. Retrograde renal cooling during radio frequency ablation to protect from renal collecting system injury. J Urol. 2005;174:350–352.
- Hwang S, Il Cho JY, Kim SH, et al. Protection of the renal collecting system during radiofrequency ablation with antegrade cold dextrose infusion. Radiology. 2010;256:759–766.
- Isfort P, Penzkofer T, Tanaka T, et al. Efficacy of antegrade pyeloperfusion to protect the renal pelvis in kidney microwave ablation using an in vivo swine model. Invest Radiol. 2013;48:863–868.
- Eswara JR, Gervais DA, Mueller PR, et al. Renal radiofrequency ablation with pyeloperfusion. Int J Urol. 2015;22:131–132.
- Samadi K, Arellano R. Comparison of clinical outcome between pyeloperfused versus non-pyeloperfused microwave ablation of renal cell carcinoma. Pol J Radiol. 2019;84:e447–e452.
- Rouvière O, Badet L, Murat FJ, et al. Radiofrequency ablation of renal tumors with an expandable multitined electrode: Results, complications, and pilot evaluation of cooled pyeloperfusion for collecting system protection. Cardiovasc Intervent Radiol. 2008;31:595–603.
- Dai Y, Covarrubias D, Uppot R, et al. Image-guided percutaneous radiofrequency ablation of central renal cell carcinoma: assessment of clinical efficacy and safety in 31 tumors. J Vasc Interv Radiol. 2017;28:1643–1650.
- Marshall HR, Shakeri S, Hosseiny M, et al. Long-term survival after percutaneous radiofrequency ablation of pathologically proven renal cell carcinoma in 100 patients. J Vasc Interv Radiol. 2020;31:15–24.
- Samadi K, Arellano RS. Ureteral protection during microwave ablation of renal cell carcinoma: combined use of pyeloperfusion and hydrodissection. Diagn Interv Radiol. 2018;24:388–391.
- Klapperich ME, Abel EJ, Ziemlewicz TJ, et al. Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology. 2017;284:272–280.
- Mauri G, Porazzi E, Cova L, et al. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. 2014;5:209–216.
- Monfardini L, Gennaro N, Della Vigna P, et al. Cone-beam CT-assisted ablation of renal tumors: preliminary results. Cardiovasc Intervent Radiol. 2019;42:1718–1725.
- Mauri G. Expanding role of virtual navigation and fusion imaging in percutaneous biopsies and ablation. Abdom Imaging. 2015;40:3238–3239.
- Mauri G, Gennaro N, De Beni S, et al. Real-time US-18FDG-PET/CT image fusion for guidance of thermal ablation of 18FDG-PET-positive liver metastases: the added value of contrast enhancement. Cardiovasc Intervent Radiol. 2019;42:60–68.
- Mauri G, De Beni S, Forzoni L, et al. Virtual navigator automatic registration technology in abdominal application. Conf Proc IEEE Eng Med Biol Soc. 2014; 2014:5570–5574.
- Breen DJ, King AJ, Patel N, et al. Image-guided cryoablation for sporadic renal cell carcinoma: three- and 5-year outcomes in 220 patients with biopsy-proven renal cell carcinoma. Radiology. 2018;289:554–561.
- Zargar H, Atwell TD, Cadeddu JA, et al. Cryoablation for small renal masses: selection criteria, complications, and functional and oncologic results. Eur Urol. 2016;69:116–128.
- Zondervan PJ, Buijs M, de la Rosette JJ, et al. Cryoablation of small kidney tumors. Int J Surg. 2016;36:533–540.
- Patel N, Cranston D, Akhtar MZ, et al. Active surveillance of small renal masses offers short-term oncological efficacy equivalent to radical and partial nephrectomy. BJU Int. 2012;110:1270–1275.
- Baust JG, Gage AA, Bjerklund Johansen TE, et al. Mechanisms of cryoablation: clinical consequences on malignant tumors. Cryobiology. 2014;68:1–11.
- Littrup PJ, Ahmed A, Aoun HD, et al. CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol. 2007;18:383–392.
- West B, Keheila M, Smith JC, et al. Efficacy of antegrade and retrograde warm saline pyeloperfusion during renal cryoablation for ureteral preservation. Turk J Urol. 2018;44:142–147.
