Abstract
Imaging-guided percutaneous microwave ablation (MWA) with high thermal efficiency comprises rapid, successful management of small renal cell carcinomas (RCCs) in selected patients. Ultrasound Committee of Chinese Medical Association, Interventional Oncology Committee of Chinese Research Hospital Association developed evidence-based guidelines for MWA of RCCs after systematically reviewing the 1969–2019 literature. Systematic reviews, meta-analyses, randomized controlled trials, cohort, and case–control studies reporting MWA of RCCs were included and levels of evidence assessed. Altogether, 146 articles were identified, of which 35 reported percutaneous MWA for T1a RCCs and 5 articles for T1b RCCs. Guidelines were established based on indications, techniques, safety, and effectiveness of MWA for RCCs, with the goal of standardizing imaging-guided percutaneous MWA treatment of RCCs.
Microwave ablation is recommended for managing small renal cell carcinoma in selected patients.
Imaging protocols are tailored based on the procedural plan, guidance, and evaluation.
Patient’s selection evaluation, updated technique information, clinical efficacy, and complications are recommended to standardize management.
A joint task force (multidisciplinary team) summarized the key elements of the standardized report.
Key points
Introduction
Kidney cancer is currently the ninth most common cancer in men and the 14th most common in women worldwide [Citation1]. Renal cell carcinoma (RCC) comprises more than 90% of kidney cancer, with clear cell (70%), papillary (10–15%), and chromophobe (5%) carcinoma the main histologic types [Citation2]. Several different locoregional therapies for RCC have been performed, mainly including open nephrectomy, laparoscopic nephrectomy, thermal ablation, and radiotherapy [Citation2–5]. Imaging-guided percutaneous ablation has been successfully applied for management of small RCC in selected patients, owing to its advantages of minimal invasion, favorable efficacy, and reproducibility [Citation6–11]. The most widely used thermal ablative techniques for RCC are cryoablation, radiofrequency ablation (RFA), and microwave ablation (MWA) [9–11]. MWA offers many benefits of other ablation techniques with higher intratumoral temperatures, larger tumor ablation volumes, faster ablation times, the ability to use multiple applicators simultaneously, and less dependent on electrical conductivities of tissue. MWA was first adopted in RCC treatment by Dupuy et al. [Citation12] in 2005. They reported the patient was performed percutaneous MWA under computed tomography (CT) guidance. MWA was firstly reported under ultrasound [Citation5] guidance by Liang et al. [Citation13] in 2008 and it is the first report with survival and recurrence result with series cases. After that, MWA of RCC has achieved rapid development by percutaneous, open or laparoscopic approach in China and worldwide over the past decade [Citation14–16]. Several studies have shown that the local tumor control, complications, and long-term survival were equivalent between MWA and cryoablation, RFA or nephrectomy in treatment of T1a RCC [Citation17–20].
Among several MWA techniques, percutaneous MWA shares the advantage of precise positioning with image guidance and minimal invasion, and percutaneous MWA achieved wide application compared with open or laparoscopic approach. Therefore, Ultrasound Committee of Chinese Medical Association, Interventional Oncology Committee of Chinese Research Hospital Association RCC ablation Guideline Panel has compiled these clinical guidelines to provide clinicians with evidence-based information and recommendations for image-guided percutaneous MWA of patients with RCC. The guideline panel is a multidisciplinary group consisting of clinicians with particular expertise in MWA of RCC. To meet the requirements for a multidisciplinary approach, the panel has been reinforced by several other experts, including urologists, medical oncologists, pathologists, radiologists, and biostatisticians. The guideline was established in accordance with the basic principles of evidence-based medicine and the clinical practices on MWA of RCC, and by referring to the USA NCCN Clinical Practice Guidelines in Oncology-Kidney Cancer [Citation6], the European Association of Urology (EAU) Guidelines on RCC [Citation7], and European Society For Medical Oncology [Citation8] Clinical Practice Guidelines on RCC [Citation8].
Evidence acquisition
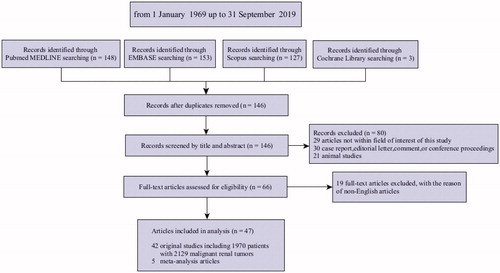
Systematic reviews of the literature were conducted in accordance with PRISMA guidelines [Citation17]. For each literature, elements for inclusion and exclusion, including patient population, intervention, comparison, outcomes, study design, and search terms and restrictions, were developed using an iterative process involving all members of the expert panel to achieve consensus. English-language literature searches were conducted separately using the following databases: Medline, Medline In-Process, Embase, Cochrane Library, Scoups, trial registries, and ISI Web of Science from 1 January 1969 up to 31 September 2019. The search strategy was shown in Supplementary Appendix 1. The search identified a total of 146 articles. Among them, 40 studies reporting percutaneous MWA procedures in 2129 RCC tumors were eligible for inclusion ().
Description of MWA
As one of the energy-based ablation techniques, MWA refers to application of electromagnetic methods with frequencies ≥ 900 MHz to eradicate focal tumors [Citation12]. The rotation of dipole molecules generates most of the heat during MWA [Citation18–21]. Thus heat producing from friction induces cellular death via coagulation necrosis (Supplementary Appendix 2). Microwaves can deliver over a target volume continuously and may produce heating at any water and tissue content. The fast heating of microwaves may overcome the negative effects of rich perfusion of kidney and large ablation area with the use of microwave energy can be achieved [15, 16]. Two kinds of frequencies: 915 and 2450 MHz can be used for MWA. For T1a RCC treatment, only frequency of 2450 MHz is recommended to be adopted, and for larger RCC, a frequency of 915 MHz can be used in selected patients with safe tumor location for its advantage of larger ablation zones [Citation22,Citation23].
There are more than 10 branded microwave systems available currently worldwide. All MWA systems are composed of three basic elements: microwave generator, low-loss flexible coaxial cable and antenna. The design of the antenna has focused mainly on needlelike, cooled-shaft, coaxial-based interstitial antennae [Citation24–26]. Only one microwave machine (Kangyou Medical, Nanjing, China) is equipped with a thermal monitoring system with a separate temperature probe that can continuously measure temperature in real time during ablation [Citation27,Citation28].
Definitions of MWA
Therapeutic effect index including technical success, technical efficacy, complications, residual tumor, local tumor progression (LTP), LTP-free survival, metastasis-free survival (MFS), disease-free survival (DFS), cancer-specific survival (CSS), and overall survival (OS) is introduced in Supplementary Appendix 3 [Citation29–32].
Ancillary procedures are those techniques that are used to separate critical non-target structures from the target ablation zone to avoid non-target thermal injury. That mainly includes hydro-dissection technique and indwelling double J tube in the ureter as an endo-sent technique.
Treatment guidance image
Image is of paramount importance for percutaneous ablation not only for clinical diagnosis and staging of RCC but also procedure planning and even evaluation of ablation effect. The feasibility of the MWA, the site of access, the number and the pathway of the antennae, the necessity of ancillary procedures need to be defined from pre-procedural image [Citation32]. For US guided ablation, three kinds of images are necessary including US, contrast-enhanced US, and either contrast-enhanced CT or magnetic resonance imaging (MRI). For the limited sensitivity of US modality for the detection of small RCCs [Citation33], the use of micro-bubble contrast and MRI/CT may increase the diagnostic accuracy of US and explicit the relationship between the lesion with the adjacent organs [Citation34–36]. For CT guided ablation, contrast-enhanced CT is necessary for ablation planning and probe guidance, if the renal function of the patient permitted. Otherwise plain CT scan combined with noncontrast-enhanced MRI sequences such as diffusion-weighted imaging (DWI) or arterial spin labeling (ASL) is necessary [Citation32].
Diagnosis
Pathological diagnosis is necessary for RCC patients. If the patients need to undergo biopsy to achieve pathological diagnosis, it is preferred to perform intraoperative tumor biopsy using a coaxial system before ablation to offer the opportunity to ablate the biopsy tract to decrease seeding and bleeding. There is consensus to biopsy with an 18-gauge core needle as a sufficient tissue sample is provided with acceptable morbidity [Citation37] (Supplementary Appendix 4).
Indications and contraindications
The main indications and contraindications for percutaneous MWA of RCC are summarized in (Supplementary Appendix 5). For patients with T1a RCC based on the American Joint Committee on Cancer (AJCC) [Citation38], MWA should be considered as curative therapy.
Table 1. Indications and check list for MWA of RCC.
Patient preparation and data required
Patients considered for MWA should be accurately evaluated through clinical history, physical examination, laboratory values and performance status. No specific sign is seen in patients with early-stage RCC. Signs may occur in less than 10% of RCC patients [Citation39] including abdominal palpable mass, lower extremity edema, and hematuria. The main laboratory test items include renal function, serum liver enzymes and electrolytes, routine blood test, routine urinalysis, and test for blood coagulation. Urine cytology should be performed for patients with renal tumors adjacent to or involving renal pelvis. Radionuclide renography should be performed in patients with solitary renal tumor, bilateral renal tumor, abnormal renal function indicators. A full pre-ablation imaging work-up (a combination of contrast-enhanced imaging including US, CT, or MRI) should be performed to stage, locate the lesions, and exclude renal venous thrombosis and metastases accurately (). Chest X-ray and electrocardiogram are the common approaches for pre-operative examination in RCC patients. Chest CT, cerebral MRI/CT, radionuclide bone scan, and PET-CT may be considered with a need to confirm whether there is any distant metastasis.
Techniques
The guidance image is performed to choose the safest intercostal or subcostal needle access before MWA. If the patient is not contraindicated, general anesthesia is recommended. After the anesthesia, the antenna is placed into the chosen area of the tumor. The shortest path between the skin and the target is selected while avoiding puncture of other organs or vessels. In the multiple-needles procedure, two antennae directly connected to the MW generator are inserted into the tumor in parallel 1–2.5 cm apart. At each insertion, the tip of the needle is placed in the deepest part of the tumor. Multiple thermal zones are created along the major axis of the needle antenna by simply withdrawing the antenna from the preceding thermal lesion, and reactivating the MW generator. If necessary, due to tumor size, multiple overlapping ablations are usually needed to cover the entire tumor with a safety margin. In general, the microwave energy application is suggested to set at 50–60 W for 5–10 min in a session. For tumors less than 2.0 cm, one antenna is preferred, and for tumors measuring 2.0 cm or greater, two antennae are preferred to be inserted simultaneously for multiple-channel MW equipment, otherwise, two or more insertions will be needed by one antenna for single-channel MW equipment.
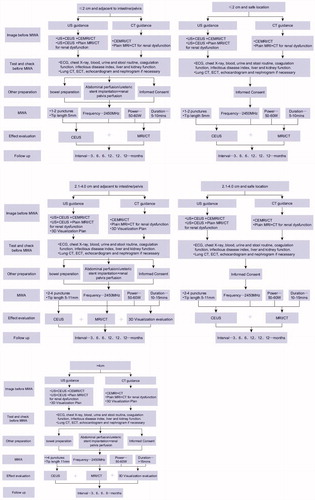
The size of the ablation zone can be roughly judged by US and CT during the guided procedure and be precisely judged by contrast enhanced US and CT/MRI after MWA (Supplementary Appendix 6 and ).
Figure 2. Flow diagram of technique procedure for microwave ablation of renal cell carcinoma with different size and location. MWA: microwave ablation; US: ultrasound; CEUS: contrast-enhanced ultrasound; CECT: contrast-enhanced computed tomography; CEMRI: contrast-enhanced magnetic resonance imaging; ECG: electrocardiograph; ECT: emission-computed tomography.
Care after MWA
After the MWA, the patient undergoes recovery for 4–6 h of bed rest. Then the patients can be observed for 2–3 additional days and both contrast enhanced US and MRI/CT are performed to evaluate the ablation effect. If the images show incomplete ablation, the second session needs to be performed to ablate the residual tumor. There is no consensus on the use of antibiotics after the ablation procedure. According to the review of articles reporting RFA, cryoablation, and MWA of renal masses, potentially infectious complications occurred infrequently in 74/6952 patients (1.06%) [Citation40]. Therefore, prophylactic antibiotics for routine T1a renal tumor ablation are not recommended, but for the diabetic patients, patients with a ureteric stent placed for pyeloperfusion, patients with multiple or large tumors or with tumor adjacent to intestine, prophylactic antibiotics is recommended to use [Citation32]. Patients can be discharged from the hospital when their renal images show complete necrosis of tumor and they have no major complication or feel no severe pain.
Follow up
For ≤4 cm RCC, patients need be observed on an outpatient basis at 3 months post MWA. At the first visit, the level of pain, the ability to pass urine, the serum renal function index, and the presence of any hematuria are assessed, and the skin puncture point is examined. One of the contrast-enhanced images including US, CT, and MRI scan needs to be performed to evaluate the effect of ablation. If complete ablation is achieved, then routine contrast-enhanced image is repeated to monitor for recurrence or metastasis at 6-month intervals during the first year and then annually after MWA. If there is any suspicion of tumor residual or disease progression, a new ablation can be arranged if the patient meet the criterion. For >4 cm RCC, a follow-up principle is recommended as at 3, 6, and 12 months after MWA and then at 6-month intervals during the patient’s lifetime. The ablation zone will shrink gradually and the margin of the ablated tissue may be replaced gradually by fat that evolves to form a crescent-like band or “halo” [Citation41].
Effectiveness
There is an extensive evidence in the literatures from meta-analysis, cohort studies, and case series on the technical outcomes, the safety and the effectiveness of the use of MWA for the treatment of T1a RCCs [Citation15,Citation27,Citation42–53] (). All the literatures reported the MWA of RCC under US or CT guidance. The majority therapeutic studies provided in this practice document meet the Levels of Evidence (LoE) 2–4, as suggested by the center for Evidence-Based Medicine [Citation8,Citation58], illustrated in . One systematic review for percutaneous MWA of T1a RCC with 13 papers since 2012 showed pooled technical success rate and technical efficacy rate were 97.3% and 97.6%, respectively. The meta-analytic pooled LTP was 2.1%. The 1-, 2-, 3-, and 5-year pooled CSS were 99.1%, 98.4%, 97.6%, and 96.9%, respectively, while the OS were 98.3%, 94.9%, 86.8%, and 81.9%. In terms of major complications, a 1.8% of meta-analytic pooled incidence was found (15). Study with the largest number of patients is reported in the single-center retrospective series from Liang et al. in China ([Citation59], LoE 4). The authors included 185 patients with 192 sporadic T1a RCCs that were treated with US-guided percutaneous MWA. In the study, during the median followed up of 42 months, the overall occurrence of LTP was 3.2% per patient. The OS rates at 1, 3, and 5 years were 98.3%, 94.0%, and 86.3%, respectively. The largest sample report on CT-guided percutaneous MWA of RCC is from Wells et al. in USA ([Citation51], LoE 4), which showed the LTP rate was 1% (1/100) and 3-year OS was 91%. By now four reports have compared surgery and MWA in RCC patients with comparative oncologic survival outcomes ([Citation27,Citation42,Citation59,Citation60], LoE 3). In addition, MWA has also been compared with cryoablation and RFA in treating small RCC from case control study. Three techniques achieved comparable treatment response and complication in T1a RCC, but MWA is associated with shorter treatment times and less sedation than RFA or cryoablation ([Citation61,Citation62], LoE 3). Two literatures reported the high power MWA for T1a RCC by using the equipment with the frequency of 915 MHz, but that induced a very high major complication rate of 11.5% and 20%, respectively ([Citation63,Citation64], LoE 4). Therefore, high-volume and high-power ablation is not recommended for T1 a RCC for the over large ablation zone.
Table 2. Major researches of MWA in RCC treatment (tumor number ≥ 50).
Table 3. Levels of evidence and grades of recommendation (adapted from the ESMO Clinical Practice Guidelines for RCC(8)).
The MWA for the treatment of RCC with T1b stage or more is with the aim of cytoreduction and symptom relief. MWA only achieves preliminary clinical application for reducing the tumor volume with an acceptable safety. The reports are very limited and the sample size is small [Citation28,Citation48,Citation50,Citation65,Citation66]. It is difficult to obtain strong reference data in terms of complete ablation, tumor progression, long-term survival and complications with the LoE level 4.
Combined treatment with other modalities
The therapeutic efficacy of MWA can be augmented by other therapies. For patients with tumors adjacent to intestinal tract, renal pelvis and ureter, artificial ascites, intrapelvic saline perfusion, ureteral stent placement, and temperature monitoring with a separate temperature probe should be combined with MWA. Real-time virtual navigation system and three-dimensional visualization techniques provide an appealing alternative option to be used in large or complex RCC ablation, enabling the physician to perform precise therapy to improve effectiveness ([Citation65,Citation67], LoE 4).
For patients in whom MWA cannot achieve complete necrosis, radiotherapy can be a useful supplement [Citation8]. For palliation of local and symptomatic metastatic RCC disease or to prevent the progression of metastatic disease in critical sites, MWA can be combined with immuno- or targeted therapies [Citation6–8].
Complication
The side effect of MWA of RCC mainly includes low-grade fever, pain, and transient hematuria, although occurring frequently, which rarely if ever result in substantial morbidity and do not require any further treatments. Major complication includes death related to procedure, uncontrollable bleeding, bowel perforation, abscess, ureteral stricture, urinary fistula, and tumor seeding, etc. (). After summarizing the total literatures on MWA of T1a RCC with 1582 procedures, the major complications (Clavien Grades III and V) of MWA of RCC are relatively low ( and Table S1). These can be controlled by surgical operation, interventional approach and medical therapy [Citation49–52,Citation68–71]. Based on the systematic literatures analysis of MWA for RCC in safety and effectiveness with the evaluation of evidence level, we performed a key recommendation on preparation, technique, and care during MWA procedure in patients with RCC ().
Table 4. Complications during and after 1582 procedures of MWA of T1a RCC.
Table 5. Key recommendations on preparation, technique, and care during MWA procedure in patients with RCC.
Conclusion
Percutaneous MWA represents a valid treatment of T1a RCCs with excellent long-term technical and functional outcomes and a very low complication rate. Chinese panel has written and approved the guidelines to promote the cost-effective use of high-quality MWA therapeutic procedures for RCC. The guidelines will be updated when data or publications might change a prior recommendation or when the panel feels clarifications are required for the oncology community.
Author contributions
Dr Liang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Liang and Yu Jie. Acquisition of data: Liang, Yu Jie, Dong, Yu Xiao-Ling, Cheng, Han, Yang, Guo, Liu, Wang, Hu, Dong, Pan and Sai. Analysis and interpretation of data: Liang, Yu Jie and Yu Xiao-ling. Drafting of the manuscript: Liang and Yu Jie. Critical revision of the manuscript for important intellectual content: Yu Jie and Liang
Supplemental Material
Download PDF (238.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67(3):519–530.
- Srigley JR, ISUP Renal Tumor Panel, Delahunt B, Eble JN, et al. The International Society of Urological Pathology (ISUP) vancouver classification of renal neoplasia. Am J Surg Pathol. 2013;37(10):1469–1489.
- Bamias A, Escudier B, Sternberg CN, et al. Current clinical practice guidelines for the treatment of renal cell carcinoma: a systematic review and critical evaluation. Oncologist. 2017;22(6):667–679.
- Dengina N, Tsimafeyeu I, Mitin T. Current role of radiotherapy for renal-cell carcinoma: review. Clin Genitourin Cancer. 2017;15(2):183–187.
- Alghamdi A, Alkhateeb S, Alghamdi K, et al. Saudi oncology society and saudi urology association combined clinical management guidelines for renal cell carcinoma. Urol Ann. 2016;8(2):136–140.
- Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(6):804–834.
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924.
- Escudier B, Porta C, Schmidinger M, et al.; ESMO Guidelines Committee. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v58–v68.
- Krokidis ME, Kitrou P, Spiliopoulos S, et al. Image-guided minimally invasive treatment for small renal cell carcinoma. Insights Imaging. 2018;9(3):385–390.
- Prins FM, Kerkmeijer LGW, Pronk AA, et al. Renal cell carcinoma: alternative nephron-sparing treatment options for small renal masses, a systematic review. J Endourol. 2017;31(10):963–975.
- Wah TM. Image-guided ablation of renal cell carcinoma. Clin Radiol. 2017;72(8):636–644.
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25(Suppl 1):S69–S83.
- Liang P, Wang Y, Zhang D, et al. Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J Urol. 2008;180(3):844–848; discussion 848.
- Floridi C, De Bernardi I, Fontana F, et al. Microwave ablation of renal tumors: state of the art and development trends. Radiol Med. 2014;119(7):533–540.
- Choi SH, Kim JW, Kim JH, et al. Efficacy and safety of microwave ablation for malignant renal tumors: an updated systematic review and meta-analysis of the literature since 2012. Korean J Radiol. 2018;19(5):938–949.
- Cornelis FH, Marcelin C, Bernhard JC. Microwave ablation of renal tumors: a narrative review of technical considerations and clinical results. Diagn Interv Imaging. 2017;98(4):287–297.
- Moher D, Liberati A, Tetzlaff J, et al.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341.
- Liang P, Yu J, Lu MD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol. 2013;19(33):5430–5438.
- Yu J, Liang P. Status and advancement of microwave ablation in China. Int J Hyperthermia. 2017;33(3):278–287.
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38(3):135–143.
- Tanaka M, Sato M. Microwave heating of water, ice, and saline solution: molecular dynamics study. J Chem Phys. 2007;126(3):034509.
- Sun Y, Wang Y, Ni X, et al. Comparison of ablation zone between 915- and 2,450-MHz cooled-shaft microwave antenna: results in in vivo porcine livers. Am J Roentgenol. 2009;192(2):511–514.
- Vogl TJ, Roman A, Nour-Eldin NA, et al. A comparison between 915 MHz and 2450 MHz microwave ablation systems for the treatment of small diameter lung metastases. Diagn Interv Radiol. 2018;24(1):31–37.
- Bertram JM, Yang D, Converse MC, et al. A review of coaxial-based interstitial antennas for hepatic microwave ablation. Crit Rev Biomed Eng. 2006;34(3):187–213.
- Longo I, Gentili GB, Cerretelli M, et al. A coaxial antenna with miniaturized choke for minimally invasive interstitial heating. IEEE Trans Biomed Eng. 2003;50(1):82–88.
- Kuang M, Lu MD, Xie XY, et al. Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna–experimental and clinical studies. Radiology. 2007;242(3):914–924.
- Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: intermediate-term results. Radiology. 2014;270(3):880–887.
- Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology. 2012;263(3):900–908.
- Ahmed M, Technology Assessment Committee of the Society of Interventional R. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014;25(11):1706–1708.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Clark TW, Millward SF, Gervais DA, et al.; Technology Assessment Committee of the Society of Interventional Radiology. Reporting standards for percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol. 2009;20(7 Suppl):S409–S416.
- Krokidis ME, Orsi F, Katsanos K, et al. CIRSE guidelines on percutaneous ablation of small renal cell carcinoma. Cardiovasc Interv Radiol. 2017;40(2):177–191.
- Gulati M, Cheng J, Loo JT, et al. Pictorial review: renal ultrasound. Clin Imaging. 2018;51:133–154.
- Meloni MF, Smolock A, Cantisani V, et al. Contrast enhanced ultrasound in the evaluation and percutaneous treatment of hepatic and renal tumors. Eur J Radiol. 2015;84(9):1666–1674.
- Sparchez Z, Radu P, Sparchez M, et al. Contrast enhanced ultrasound of renal masses. A reappraisal of EFSUMB recommendations and possible emerging applications. Med Ultrason. 2015;17(2):219–226.
- Wang ZJ, Westphalen AC, Zagoria RJ. CT and MRI of small renal masses. Br J Radiol. 2018;91(1087):20180131.
- Tsivian M, Rampersaud EN Jr, del Pilar Laguna Pes M, et al. Small renal mass biopsy-how, what and when: report from an international consensus panel. BJU Int. 2014;113(6):854–863.
- Kim SP, Alt AL, Weight CJ, et al. Independent validation of the 2010 American Joint Committee on Cancer TNM classification for renal cell carcinoma: results from a large, single institution cohort. J Urol. 2011;185(6):2035–2039.
- Guo J, Ma J, Sun Y, et al. Chinese guidelines on the management of renal cell carcinoma (2015 edition). Ann Transl Med. 2015;3(19):279.
- Crawford D, vanSonnenberg E, Kang P. Infectious outcomes from renal tumor ablation: prophylactic antibiotics or not? Cardiovasc Interv Radiol. 2018;41(10):1573–1578.
- Svatek RS, Sims R, Anderson JK, et al. Magnetic resonance imaging characteristics of renal tumors after radiofrequency ablation. Urology. 2006;67(3):508–512.
- Yu J, Zhang G, Liang P, et al. Midterm results of percutaneous microwave ablation under ultrasound guidance versus retroperitoneal laparoscopic radial nephrectomy for small renal cell carcinoma. Abdom Imaging. 2015;40(8):3248–3256.
- Novanta A, Herpe G, Vesselle G, et al. Chart for renal tumor microwave ablation from human study. Diagn Interv Imaging. 2018;99(10):609–614.
- Dvorak P, Hoffmann P, Brodak M, et al. Percutaneous radiofrequency and microwave ablation in the treatment of renal tumors – 10 years of experience. Wideochir Inne Tech Maloinwazyjne. 2017;12(4):394–402.
- Filippiadis DK, Gkizas C, Chrysofos M, et al. Percutaneous microwave ablation of renal cell carcinoma using a high power microwave system: focus upon safety and efficacy. Int J Hyperthermia. 2018;34(7):1077–1081.
- Abboud SE, Patel T, Soriano S, et al. Long-term clinical outcomes following radiofrequency and microwave ablation of renal cell carcinoma at a single VA Medical Center. Curr Probl Diagn Radiol. 2018;47(2):98–102.
- Cheng Z, Yu X, Han Z, et al. Ultrasound-guided hydrodissection for assisting percutaneous microwave ablation of renal cell carcinomas adjacent to intestinal tracts: a preliminary clinical study. Int J Hyperthermia. 2018;34(3):315–320.
- Toro-Gutierrez JS, Espejo-Herrero JJ, Lombardo-Galera MS, et al. Renal tumors percutaneous ablation by microwave. Initial experience. Arch Esp Urol. 2017;70(5):525–533.
- Chan P, Velasco S, Vesselle G, et al. Percutaneous microwave ablation of renal cancers under CT guidance: safety and efficacy with a 2-year follow-up. Clin Radiol. 2017;72(9):786–792.
- Ierardi AM, Puliti A, Angileri SA, et al. Microwave ablation of malignant renal tumours: intermediate-term results and usefulness of RENAL and mRENAL scores for predicting outcomes and complications. Med Oncol. 2017;34(5):97.
- Klapperich ME, Abel EJ, Ziemlewicz TJ, et al. Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology. 2017;284(1):272–280.
- Mansilla AV, Bivins EE Jr, Contreras F, et al. CT-guided microwave ablation of 45 renal tumors: analysis of procedure complexity utilizing a percutaneous renal ablation complexity scoring system. J Vasc Interv Radiol. 2017;28(2):222–229.
- Horn JC, Patel RS, Kim E. Percutaneous microwave ablation of renal tumors using a gas-cooled 2.4-GHz probe: technique and initial results. J Vasc Interv Radiol. 2014;25(3):448–453.
- Dong X, Li X, Yu J, et al. Complications of ultrasound-guided percutaneous microwave ablation of renal cell carcinoma. Onco Targets Ther. 2016;9:5903–5909.
- Mu MJ, Yu J, Liang P, et al. Long-term effects of ultrasound-guided microwave ablation in the treatment of small renal cell carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36(5):622–627.
- Moreland AJ, Ziemlewicz TJ, Best SL, et al. High-powered microwave ablation of t1a renal cell carcinoma: safety and initial clinical evaluation. J Endourol. 2014;28(9):1046–1052.
- Li X, Liang P, Yu J, et al. Role of contrast-enhanced ultrasound in evaluating the efficiency of ultrasound guided percutaneous microwave ablation in patients with renal cell carcinoma. Radiol Oncol. 2013;47(4):398–404.
- Jenssen C, Gilja OH, Serra AL, et al. European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Policy Document Development Strategy – Clinical Practice Guidelines, Position Statements and Technological Reviews. Ultrasound Int Open. 2019;5(1):E2–E10.
- Yu J, Zhang X, Liu H, et al. Percutaneous microwave ablation versus laparoscopic partial nephrectomy for cT1a renal cell carcinoma: a propensity-matched cohort study of 1955 patients. Radiology. 2020;294(3):698–706.
- Guan W, Bai J, Liu J, et al. Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol. 2012;106(3):316–321.
- Zhou W, Arellano RS. Thermal ablation of T1c renal cell carcinoma: a comparative assessment of technical performance, procedural outcome, and safety of microwave ablation, radiofrequency ablation, and cryoablation. J Vasc Interv Radiol. 2018;29(7):943–951.
- Martin J, Athreya S. Meta-analysis of cryoablation versus microwave ablation for small renal masses: is there a difference in outcome? Diagn Interv Radiol. 2013;19(6):501–507.
- Castle SM, Salas N, Leveillee RJ. Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology. 2011;77(4):792–797.
- Thompson SM, Schmitz JJ, Thompson RH, et al. Introduction of microwave ablation into a renal ablation practice: valuable lessons learned. Am J Roentgenol. 2018;211(6):1381–1389.
- Li X, Yu J, Liang P, et al. Combination therapy of three-dimensional (3D) visualisation operative treatment planning system and US-guided percutaneous microwave ablation in larger renal cell carcinomas (D ≥ 4 cm): preliminary results. Int J Hyperthermia. 2017;33(3):271–277.
- Bartoletti R, Meliani E, Simonato A, et al. Microwave-induced thermoablation with Amica-probe is a safe and reproducible method to treat solid renal masses: results from a phase I study. Oncol Rep. 2012;28(4):1243–1248.
- Gao Y, Liang P, Yu X, et al. Microwave treatment of renal cell carcinoma adjacent to renal sinus. Eur J Radiol. 2016;85(11):2083–2089.
- Lin Y, Liang P, Yu XL, et al. Percutaneous microwave ablation of renal cell carcinoma is safe in patients with renal dysfunction. Int J Hyperthermia. 2017;33(4):440–445.
- Bai J, Hu Z, Guan W, et al. Initial experience with retroperitoneoscopic microwave ablation of clinical T(1a) renal tumors. J Endourol. 2010;24(12):2017–2022.
- Zhou W, Herwald SE, Uppot RN, et al. Risk assessment of chronic kidney disease following microwave ablation for stage T1 renal cell carcinoma. J Vasc Interv Radiol. 2018;29(12):1685–1691.
- Sui G, Luo Q, Du J, et al. Clinical application of ultrasound-guided percutaneous microwave ablation in the treatment of T1aN0M0 stage renal carcinoma. J Med Ultrason (2001). 2019;46(2):217–222.