Abstract
Purpose
To compare the effectiveness of radiofrequency ablation (RFA) for benign thyroid nodules (BTNs) among groups presenting with different nodule volumes.
Materials and methods
This retrospective study evaluated 186 patients with BTNs who underwent ultrasound guided RFA treatment. The BTNs were categorized into small (≤10 ml); medium (10-30 ml); and large (>30 ml) according to the initial volume of BTNs before ablation. The RFA procedures were performed using the moving shot technique. The volume reduction ratio (VRR) of each nodule, cosmetic score, symptomatic score, and complications were analyzed at 1, 3, and 6 months after RFA treatment and the three groups compared.
Results
At 1-month follow-up, the large nodules group showed significantly greater VRR compared to the other two groups (small, 31.88% ± 37.91; medium, 38.9% ± 19.18; large, 48.7% ± 20.43, p = .03). At 6-month follow-up, there was no significant difference of VRR among the three groups (small, 74.6% ± 20.92; medium, 68.1% ± 17.07; large, 75.0% ± 11.88). The most common presented complication was temporary vocal palsy (6 patients; small, n = 1; medium, n = 1; large, n = 3). Additionally, one skin burn, one hematoma, and one nodular rupture of BTNs occurred after the procedures. The complication rate of the large nodules group was highest among the three groups and showed a considerable difference (8 patients; small, n = 1, 2.1%; medium, n = 2, 4.5%; large, n = 5, 11.4%, p = .061).
Conclusions
RFA was confirmed as effective in patients with large thyroid nodule (>30ml), with therapeutic efficacy similar to patients with smaller thyroid nodules.
1. Introduction
In recent years, a growing number of studies investigating the treatment of benign thyroid nodules through minimally invasive image-guided thermal ablation, including radiofrequency (RFA), laser ablation (LA), microwave ablation, and high-intensity-focus ultrasound (HIFU) have reported encouraging results. In particular, RFA is feasible, safe, and effective in the treatment of benign thyroid nodules (BTNs) when performed by highly experienced operators [Citation1–5]. According to the guidelines of the Korean Society of Thyroid Radiology and the Italian minimally-invasive treatments of the thyroid (MITT) group, thyroid RFA was used as a first-line treatment or as an alternative to surgery for patients with nonfunctioning solid BTNs complaining of symptomatic or cosmetic issues [Citation4–6]. By using RFA, significant volume reduction ratio (VRR) with cosmetic and/or symptomatic improvement can be achieved between 1 and 6 months with a low recurrence rate at 2 years [Citation7,Citation8]. Compared to surgical resection, the relatively low complication rate [Citation9] has allowed RFA to become a common alternative treatment modality for BTNs in patients unwilling or unable to undergo surgery [Citation10].
Nodules exceeding 2 cm in diameter present physicians with patients suffering a variety of symptoms, cosmetic issues, and clinical concerns [Citation11]. Patients seeking treatment due to nodule-related mass are often considered subjective in nature, associated with individual neck circumference or nodule location (i.e. isthmus) [Citation6]. At present, no strict criteria defining nodule size or volume have been established regarding thyroid RFA treatment [Citation6]. However, it is reasonable to infer that nodular size may affect the total ablation time [Citation12], patient comfort during the procedure, and the immediate or delayed complication rate. Moreover, nodular volume may also associate with factors influencing therapeutic success, including initial incomplete ablation [Citation12], VRR, number of RFA sessions, and long-term nodular recurrence rate. Whether or not nodular size can predict the efficacy and safety of RFA remains unclear. In the present study, we aim to investigate the therapeutic success of RFA for treatment of BTNs of various sizes and to evaluate the potential associations between nodular volume, VRR, and complication rate.
2. Method
2.1. Patients
From July 2016 to June 2018, more than 300 patients underwent RFA for treatment of BTNs at the Kaohsiung Chang Gung Memorial Hospital Medical Center in Taiwan. Patients with BTNs presented with nodule-related symptoms, cosmetic issues, or were identified incidentally. Patients visited otolaryngologists, surgeons, or internal medicine physicians, and were subsequently transferred to the radiology department for assessment of nodular composition by sonography or CT/MRI. Nodules were then classified as solid, predominant solid, predominant cystic, or cyst [Citation13]. Ultrasound guided fine-needle aspiration cytology (FNAC) or core needle biopsy was performed to confirm the benign nature of the nodules. At least two benign cytological results or a single benign cytological result with ultrasound appearance was considered low risk of malignancy [Citation4,Citation6].
Patients had no contraindication for surgery, however they refused surgery over concerns of post-treatment scarring, complications, side effects, or thyroid functional changes. The demographic data records of these patients and their follow-up outcomes were retrospectively analyzed. The exclusion criteria were patients with cyst or predominant cystic nodule, patients with pathological result as follicular neoplasm or malignancy, patients who did not return for each follow-up session (1-month, 3-month, and 6-month), and patients who received RFA for more than one nodule concurrently. Finally, 136 patients presenting with solid benign nodule who underwent RFA for a single nodule were enrolled in this study (). The patients were further divided into three groups, according to the size of BTNs assessed by sonography evaluation. This study was a retrospective study and approved by the institutional review board, and informed consent was obtained from all patients prior to the procedure.
2.2. Pre-ablation assessment
At each visit, complaints including nodule-related symptom score, cosmetic score, and thyroid function were recorded. The nodule-related symptom score was obtained by patients filling out a questionnaire concerning five clinical symptoms: compression, cough, difficulty swallowing, voice change, and pain. For each positive symptom, we allocated one point, therefore the symptom scores ranged from 0 to 5. The cosmetic score was obtained using the following scale: 0, no visible or palpable mass; 1, not visible but palpable mass; 2, visible when swallowing only; 3, an easily visible mass [Citation6]. The nodular size and sonographic composition were evaluated.
Three orthogonal diameters of the tumors (the largest diameter and two other perpendicular ones) were measured by sonography. The volume of the tumor was calculated using the following equation: V = πabc/6 (V: volume; a: the largest diameter; b and c: the other two perpendicular diameters) [Citation6]. To evaluate the association between nodule volume and treatment efficacy, patients were categorized into three groups [Citation14]: (1) Small: a pre-ablation nodule volume <10Ml, (2) Medium: a pre-ablation nodule volume of 10-30 ml, (3) Large: a pre-ablation nodule volume >30 ml.
2.3. Ablation technique
RFA was performed in an outpatient setting for all patients. The ablation procedure was performed by a radiologist with over 10 years of experience in US-guided procedures. Prior to ablation, a solution of 2% lidocaine hydrochloride was used as local anesthesia at the puncture site and around the thyroid gland. In accordance with US examination guidelines, the electrode tip size was chosen based on tumor size, and status of the surrounding critical structures. () An internally cooled electrode (18 gauge, with 5 mm, 7 mm or 1-cm active tip) with RF generator (VIVA, STARmed and M2004, RF Medical) were used. The trans-isthmic approach, passing through thyroid parenchyma with careful observation of the vessels along the approach route was used. An electrode was inserted into the thyroid nodule at the deepest and most remote portion of the nodule. With application of the moving shot technique [Citation15], the BTN was sequentially ablated by moving the electrode tip backward and forward, bottom to top. Ablation termination was determined when all visual field of the nodule had changed to transient hyperechoic zones. After ablation, patients were referred to the otolaryngology department for examination with flexible fiberoptic laryngoscopy to check for occurrence of vocal cord paralysis.
Table 1. Patient and nodule characteristics based on nodule volume.
2.4. Follow-up evaluation
The follow-up sonographic evaluations were performed to assess the VRR at one month, three months, and six months post-RFA. The symptomatic score and cosmetic score were also evaluated at time of follow-up. The VRR was assessed by US imaging and was calculated by the following equation: volume reduction ratio (%) = initial volume (ml) – final volume (ml) × 100/initial volume. Major and minor complications were assessed according to the standard terminology of Society of Interventional Radiology (SIR) [Citation16]. Minor complications include SIR classification A–B; A: No therapy, no consequence; B: Nominal therapy, no consequence; includes overnight admission for observation only. Major complications include SIR classification C–F; C: Require therapy, minor hospitalization (<48 h); D: Require major therapy, prolonged hospitalization (>48 h); E: Permanent adverse sequelae; F: Death.
2.5. Analysis and statistics
Statistical analysis was performed by using SPSS, version 22 (SPSS, Inc. Chicago, IL, USA). All data were given as the mean ± standard deviation (SD). Demographic characteristics and sonographic results among the BTNs subgroups were compared. Group comparison for continuous variables data were performed by using the Mann–Whitney U Test and Kruskal–Wallis Test. Standard Chi-Square, and Fisher’s Exact Test were used for group comparison of categorical data. Generalized estimation equation for repeated measure analysis was used to examine the relationship of nodule volume and VRR among groups at each follow-up time. Point p value <.05 was considered significant.
3. Results
3.1. Demographic characteristics
All enrolled patients had a single ablated thyroid nodule and received ablation treatment for the first time. All nodules were evaluated by sonography and defined as solid or predominantly solid component. The demographic data of the 136 index BTNs based on pre-RFA volume groups are presented in . Of 136 BTNs, 48 nodules (38.7%) constitute small nodular group; 44 nodules (29.2%) constitute medium nodular group; and the remaining 44 nodules (32.1%) constitute large nodular group. Among the 136 patients, there were no differences in age or sex distribution, serum T3, T4 or TSH levels between the three groups.
3.2. VRR
The baseline nodular volume and respective changes are presented in and . Prior to ablation, the overall mean baseline volume of the BTNs was 31.9 ± 39.1 ml. After ablation, the 1-month, 3-month, and 6-month mean volumes were 15.8 ± 17.2 ml, 11.5 ± 18.3 ml, and 8.1 ± 10.3 ml, respectively. The results showed that the overall nodular volume reduced significantly after RFA treatment over time (time effect, p < .001). The volume in each nodular volume group also showed significant reduction over time (time effect, p < .001). At the 1-month follow-up, the VVR showed significant reduction in the large nodules group (51.3% ± 19.4), compared to the small nodules group (28.9% ± 41.9, p < .001) and medium nodules group (41.9% ± 19.1, p = .043); the medium nodular VRR was significant higher than the small nodular VRR (p = .012). At the 6-month follow-up, there were no significant differences of VRR among the three groups (small, 71.7% ± 22.0; medium, 69.0% ± 16.4; large, 72.2% ± 17.3, p = .165).
Figure 2. Changes in nodular volume and VRR of the three volume groups of BTN at each follow-up. Changes in nodular volume (A) and VRR (B) of the different echogenicity groups of BTN at each follow-up. BTN: benign thyroid nodule; VRR: volume reduction ratio.
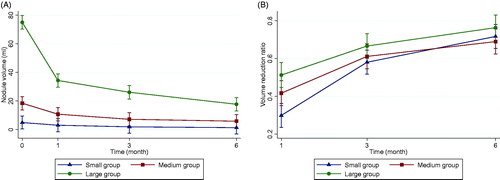
Table 2. Outcomes based on nodule volumea.
3.3. Cosmetic and symptomatic scores
The cosmetic and symptomatic scores are presented in . Prior to RFA treatment, there were 118 (86.8%) patients with cosmetic concerns, defined as a cosmetic score >0. The mean cosmetic score was elevated in the larger nodular groups, and highest in large nodules before RFA (p < .001). The overall cosmetic score improved from 2.2 ± 1.1 to 0.7 ± 0.9 at the 6-month follow-up (p < .001), with each group achieving significant improvement (p < .001). The cosmetic score at the 6-month follow-up remained significantly higher in large nodular group than small nodular group (p < .001) and medium nodular group (p = .007).
Before RFA treatment, there were 86 (63.2%) patients with nodular related symptoms, defined as a symptoms score >0. The mean symptomatic score of large nodules was significantly higher than small nodules (p < .001) but similar to the medium nodules (p = .295). The overall symptomatic score improved from 1.1 ± 1.0 to 0.02 ± 0.13 at the 6-month follow-up (p < .001), with each group achieving significant improvement (p < .001). The symptomatic scores at the 6-month follow-up showed no significant difference among the three groups (p < .5).
3.4. Complications
The complications post-RFA treatment are presented in . A total of 8 (5.9%) immediate or delayed complications occurred after the RFA procedure, including 1 major complication and 7 minor complications [Citation16]. One case of nodular rupture (in the large nodular group) who was hospitalized for drainage (SIR class C) accounted for the major complication. The minor complications included 5 temporary vocal paralyzes (5 patients: small group, n = 1; medium group, n = 1; large group, n = 3), one skin burn (medium group, n = 1), and one hematoma (large group, n = 1). Most patients with vocal paralysis recovered within 3 months. All of the minor complications belong to SIR class B. The complication rates showed a considerable difference among the 3 groups (8 patients: small group, n = 1, 2.1%; medium group, n = 2, 4.5%; large group, n = 5, 11.4%, p = .061).
4. Discussion
The present study of patients with BTNs covering a 6-month follow-up period demonstrated that RFA resulted in significant reductions in nodular volume, as well as improved cosmetic and symptomatic status. Previous literature has reported a VRR range of 52.1-86.1% at 6 months post-ablation [Citation17]. Similarly, this study observed the mean VRR of all nodules was 72.2 ± 17.3% at the 6-month follow-up. While the effects of RFA treatment are notable, previous studies have reported the most effective reduction rate may be observed in groups consisting of patients with small nodules [Citation7,Citation18,Citation19]. However, the VRR reported herein was similar among the three groups of different nodule sizes at the 6-month follow-up. Moreover, the VRR at the 1-month follow-up was higher in the large nodule group (51.3%) than the small nodule (28.9%, p < .001) and medium nodule (41.6%, p = .012) groups, indicating that larger- size nodules indeed present an enhanced VRR compared to smaller nodules. This finding may help clinicians to better educate and manage patient expectations prior to RFA treatment.
The energy delivered per nodular volume is considered an important factor affecting the VRR after RFA [Citation20]. Previous studies have reported the higher energy per nodule volume received, the greater VRR of nodule achieved [Citation18,Citation20]. We therefore compared the energy per nodule volume delivered among the three nodular volume groups. The results showed that although the total energy delivered was higher in the large nodules group, the increase in energy does not correspond with the increase in nodule volume, hence there was a progressive fall in energy per nodule volume from the smaller nodular group to the larger nodular group (). This finding is consist with studies that have shown higher energy does not always translate into greater nodular shrinkage [Citation21].
To investigate the reduced VRR as well as the higher delivered energy per volume in the small nodule group, we evaluated the image immediately after ablation. We found that during RFA, the small nodule ablation had probably extended beyond the nodular borders, resulting in a mild increase of nodular size observed on sonography immediately after ablation. With more of the marginal region ablated, the more volume increased immediately after ablation. The initially enlarged small nodules resulting in lower VRR at the 1-month follow-up was due to the VRR being calculated by nodular volume before ablation and the volume of the ablated region at 1, 3, and 6 months after ablation. . In patients with large nodules, a safety margin was observed to avoid surrounding organ damage and complications during the RFA procedure. Due to no or limited over-treated regions, the ablated large nodules did not exhibit increased size immediately after ablation. The extended area of ablation of the small nodules may also explain why the lower VRR at 1-month did not indicate a lower RFA efficacy in the small nodules, because the three groups achieved similar VRR at the 6-month follow-up. The VRR of the three groups achieved similar results (small group, 74.6% ± 20.92; medium group, 68.1% ± 17.07; large group, 75.0% ± 11.88, p = .165). Therefore, our results demonstrate that despite the fall in energy per volume, the large nodules still exhibited initial and final treatment responses and ablation success rates comparable to the small and medium nodules.
There remain other factors which may influence the VRR of nodules which have been associated with initial therapeutic success. These include an ill-defined nodule margin, undertreated peripheral portion, location of nodule close to the danger triangle area or carotid artery, prominent peripheral vascularization [Citation18], and tissue temperature during the procedure [Citation22]; however, these factors were not evaluated in this study. Further investigation is therefore necessary to clarify the influences of these factors.
Regarding cosmetic outcomes, the overall cosmetic score improved significantly post-RFA, while any wounds and scarring conditions were ameliorated during the follow-up period. Although the cosmetic scores of the different nodular volume subgroups improved significantly, the scores of the larger nodules were higher than those of the smaller nodules. In the larger nodule group, the residual volume after single RFA procedure was greater than that of the smaller nodule group during the follow-up period. This is the primary factor influencing the higher cosmetic scores of the larger nodular group patients. Since the foremost objectives of thyroid nodule ablation are the resolution of cosmetic concerns and nodule-related symptoms, subsequent RFA procedures may be necessary to achieve similar cosmetic satisfaction rates as those noted in the smaller nodular group.
Laser treatment is another minimal invasive thermal ablation option for thyroid nodules [Citation23,Citation24]. In a study by Pacella [Citation25], the laser technique showed greater efficacy than RFA among larger nodules (VRR at 6 months: LA, 69% ± 19%; RFA, 50% ± 21%, p = .001.). Although our study did not compare these two techniques, our results indeed demonstrate that large nodules can achieve VRR to 72.2%±17.3% at 6 months by RFA, a rate which is comparable with the reported laser treatment among nodules larger than 30 ml. As such, according to a report by Mauri et al., the operator’s skills could be crucial in determining the extent of nodule VRR [Citation26].
Surgery remains the standard treatment recommended by several guidelines for patients with symptomatic BTNs, autonomously functioning nodules, malignant or suspicious nodules [Citation27]. The advantages of surgery include preventing possible false-negative malignancies with prior benign cytology results; however, the false-negative malignancies rate of a benign cytology diagnosis are low [Citation27]. Therefore, minimal invasive procedures have increasingly become an alternative treatment for BTNs. Furthermore, a study by Dobrinja, et al. reported that a single session of RFA does not affect subsequent thyroid surgery and/or histological diagnosis [Citation28]. According to previous studies, through RFA, benign thyroid disease patients can achieve a lower complication rate, less postoperative discomfort, better cosmetic outcome, and a lower postoperative hypothyroidism rate compared to surgery [Citation9,Citation29]. Both RFA and surgery may be effective methods for treating nodule-related clinical problems, however RFA is not as effective as surgery for the treatment of hot nodules [Citation9,Citation29,Citation30]. Of note, the patients included in this study refused surgery by choice, not due to contraindications for thyroidectomy. According to our results, RFA is suitable and effective at treating benign thyroid nodules.
This study showed a low complication rate and no life-threatening complications, similar to the findings of previous studies [Citation31]. Vocal cord paralysis was the most common minor complication observed herein; however, relevant literature addressing the association of nodular size with vocal paralysis is lacking. In this study, vocal paralysis was most commonly observed in the large nodule group (5 patients: small, n = 1; medium, n = 1; large, n = 3). All of them presented as transient unilateral vocal paralysis, which recovered within several minutes, hours or months with conservative treatment or observation, and without hospitalization. The potential mechanisms of vocal cord paralysis are related to stretching of the nerve during the procedure, hemorrhage [Citation21], lidocaine injection, or RFA-induced thermal injury rather than permanent nerve damage [Citation31–35]. Previous studies have indeed noted the safety margin of normal thyroid parenchyma around the lesion and an understanding of peri-thyroidal anatomy are critical for the prevention of vocal paralysis [Citation31–35]. However, deep regions of thyroid structure may be obscured on the sonographic image in large size nodules. Further study is necessary to evaluate the correlation between nodular size and vocal cord palsy.
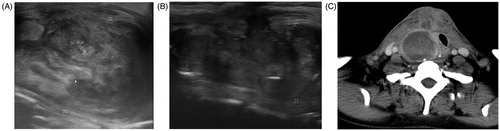
Another important complication observed in this study was delayed nodular rupture in a patient who required hospitalization, defined as a major complication (SIR class C). The patient with nodular rupture received debridement and antibiotic treatment, then gradually recovering within 10 months. The imaging characteristics of the ruptured BTN were superficial location, solid composition, and larger size, hence belonging to the large nodular group. This patient had received previous partial thyroidectomy, and there were no complete anterior thyroid capsules surrounding the thyroid nodule. The incomplete capsule site was afterward the nodular rupture site on sonography (). The rupture may have resulted from tearing of the tumor wall and thyroid capsule at a weak point [Citation20–22,Citation31,Citation33,Citation36]. Other potential causes of nodule rupture may have included the large nodular size, location near the anterior thyroid capsule, solid component nodule, excessive RFA power, and longer ablation time. In this study, the two complications were observed only in the large nodule group, including hematoma and nodular rupture. As the number of complications observed in this study was small, future investigation is necessary to confirm the association between the aforementioned complications and nodular size.
Figure 3. Goiter rupture before and after RFA. Ultrasound and CT obtained after nodular rupture of the patient with previous partial thyroidectomy history and received thyroid RFA. (A) Incomplete anterior thyroid capsules surrounding the thyroid nodule noted before RFA. (B)The incomplete capsule site is afterward the nodular rupture site on sonography. (C) Post-contrast CT scan shows a ruptured nodule into the right anterior neck. RFA: radiofrequency ablation.
There are several limitations to this study. First, as a retrospective single-center study, uncontrolled bias could have been introduced despite the relatively large sample size. Second, the absence of information regarding nodular margins, location, and peripheral vascularity could influence the VRR differences. Further prospective studies are required to address some these issues. Third, it is difficult to clarify the associations between nodular volume and each complication due to the small number of complications in each subgroup. Fourth, the observation time was relatively short at 6 months, although most patients indeed continued follow-up in other institutions. Finally, we did not compare outcomes among different ablation treatments, including radiofrequency, laser, microwave, and high-intensity-focus ultrasound. A future longitudinal follow-up study or a comparative study could be designed to analyze and evaluate longer-term outcomes.
In conclusion, this study demonstrates that single-session RFA is an effective treatment strategy to ameliorate cosmetic and symptomatic issues by reducing the nodular volume; nevertheless, for larger nodules, post-procedural cosmetic issues may persist, thereby requiring additional RFA treatment.
Disclosure satement
No potential conflict of interest was reported by the author(s).
Acknowledgments
The authors wish to thank the MRI Core Facility of Chang Gung Memorial Hospital, and all the subjects who participated in this study. The authors would also like to thank James Waddell for the proofreading and revision of this manuscript.
References
- Pacella CM. Correction to letter: Image‑guided thermal ablation of benign thyroid nodules. J Ultrasound. 2018;21(1):79.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperthermia. 2019;36(2):13–20.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Dietrich CF, Muller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Kim JH, Baek JH, Lim HK, Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology, et al. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632–655.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23(4):1044–1049.
- Spiezia S, Garberoglio R, Milone F, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009;19(3):219–225.
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:934595.
- Mauri G, Sconfienza LM. Percutaneous ablation holds the potential to substitute for surgery as first choice treatment for symptomatic benign thyroid nodules. Int J Hyperthermia. 2017;33(3):301–302.
- Deandrea M, Sung JY, Limone P, et al. Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid. 2015;25(8):890–896.
- Ahn HS, Kim SJ, Park SH, Seo M. Radiofrequency ablation of benign thyroid nodules: evaluation of the treatment efficacy using ultrasonography. Ultrasonography. 2016;35(3):244–252.
- Andrioli M, Carzaniga C, Persani L. Standardized ultrasound report for thyroid nodules: the endocrinologist's viewpoint. Eur Thyroid J. 2013;2(1):37–48.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Ha EJ, Baek JH, Lee JH. Moving-shot versus fixed electrode techniques for radiofrequency ablation: comparison in an ex-vivo bovine liver tissue model. Korean J Radiol. 2014;15(6):836–843.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202.
- Kim YS, Rhim H, Tae K, et al. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006;16(4):361–367.
- Deandrea M, Garino F, Alberto M, et al. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicentre prospective study. Eur J Endocrinol. 2019;180(1):79–87.
- Cui D, Ding M, Tang X, et al. Efficacy and safety of a combination of hydrodissection and radiofrequency ablation therapy for benign thyroid nodules larger than 2 cm: a retrospective study. J Cancer Res Ther. 2019;15(2):386–393.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and Safety of Radiofrequency Ablation for Benign Thyroid Nodules: A Prospective Multicenter Study. Korean J Radiol. 2018;19(1):167–174.
- Huh JY, Baek JH, Choi H, et al. Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session – prospective randomized study. Radiology. 2012;263(3):909–916.
- Zhao CK, Xu HX, Lu F, et al. Factors associated with initial incomplete ablation for benign thyroid nodules after radiofrequency ablation: first results of CEUS evaluation. CH. 2017;65(4):393–405.
- Papini E, Rago T, Gambelunghe G, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014;99(10):3653–3659.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20(11):1253–1261.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33(8):1–919.
- Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia. 2017;33(3):295–299.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Dobrinja C, Bernardi S, Fabris B, et al. Surgical and pathological changes after radiofrequency ablation of thyroid nodules. Int J Endocrinol. 2015;2015:576576.
- Bernardi S, Dobrinja C, Carere A, et al. Patient satisfaction after thyroid RFA versus surgery for benign thyroid nodules: a telephone survey. Int J Hyperthermia. 2018;35(1):150–158.
- Yue WW, Wang SR, Li XL, et al. Quality of life and cost-effectiveness of radiofrequency ablation versus open surgery for benign thyroid nodules: a retrospective cohort study. Sci Rep. 2016;6(1):37838.
- Wang JF, Wu T, Hu KP, et al. Complications following radiofrequency ablation of benign thyroid nodules: a systematic review. Chin Med J. 2017;130(11):1361–1370.
- Baek JH, Lee JH, Sung JY, Korean Society of Thyroid Radiology, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27(8):3128–3137.
- Chung SR, Baek JH, Choi YJ, et al. Management strategy for nerve damage during radiofrequency ablation of thyroid nodules. Int J Hyperthermia. 2019;36(1):204–210.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33(8):920–930.
- Shin JH, Jung SL, Baek JH, et al. Rupture of benign thyroid tumors after radio-frequency ablation. AJNR Am J Neuroradiol. 2011;32(11):2165–2169.