?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Pancreatic cancer is typically diagnosed in a late stage with limited therapeutic options. For those patients, ultrasound-guided high-intensity focused ultrasound (US-HIFU) can improve local control and alleviate pain. However, MRI-guided HIFU (MR-HIFU) has not yet been studied extensively in this context. To facilitate related research and accelerate clinical translation, we report a workflow for the in vivo HIFU ablation of the porcine pancreas under MRI guidance.
Materials and methods
The pancreases of five healthy German landrace pigs (35–58 kg) were sonicated using a clinical MR-HIFU system. Acoustic access to the pancreas was supported by a specialized diet and a hydrogel compression device for bowel displacement. Organ motion was suspended using periods of apnea. The size of the resulting thermal lesions was assessed using the thermal threshold- and dose profiles, non-perfused volume, and gross examination. The effect of the compression device on beam path length was assessed using MRI imaging.
Results
Eight of ten treatments resulted in clearly visible damage in the target tissue upon gross examination. Five treatments resulted in coagulative necrosis. Good agreement between the four metrics for lesion size and a clear correlation between the delivered energy dose and the resulting lesion size were found. The compression device notably shortened the intra-abdominal beam path.
Conclusions
We demonstrated a workflow for HIFU treatment of the porcine pancreas in-vivo under MRI-guidance. This development bears significance for the development of MR-guided HIFU interventions on the pancreas as the pig is the preferred animal model for the translation of pre-clinical research into clinical application.
Introduction
Despite its moderately low incidence, pancreatic cancer (PaC) caused 4.5% of all cancer fatalities worldwide in 2018, making it the seventh most likely cause of cancer-related deaths [Citation1]. PaC exhibits the lowest 5-year relative survival rate of any cancer [Citation2]. This stems from a confluence of unfavorable characteristics, namely the late onset of symptoms, rapid involvement of the adjacent arteries, early development of metastases, and the ineffectiveness of systemic therapy [Citation3–6]. As a result, resection is often impossible at time of diagnosis, leaving patients with limited therapeutic options, such as palliative chemotherapy [Citation7]. The growing tumor mass often leads to complications, including duodenal- or biliary obstruction that requires the placement of stents or surgical intervention [Citation7,Citation8]. Furthermore, the pressure exerted on the surrounding nerves often induces abdominal and back pain, which is currently treated according to the WHO guidelines recommending a combination of analgesics escalating from nonopioids to strong opioids and adjuvant treatments [Citation9,Citation10]. In case the pain cannot be alleviated in this manner, celiac plexus block (CPB) and celiac plexus neurolysis (CPN) is applied [Citation7]. However, the effectiveness of these techniques has been questioned and has not shown a significant benefit in quality of life over a placebo in a double-blinded randomized control trial [Citation11]. Thus, patients diagnosed with advanced PaC are in urgent need of alternative treatment options and effective analgesia free of debilitating side-effects. Thermal ablation of PaC using high-intensity focused ultrasound (HIFU) offers a noninvasive method for tumor debulking and pain reduction and has therefore been explored in several clinical studies since the beginning of the century [Citation12–21].
In HIFU treatments, ultrasound waves produced by a purpose-built transducer are focused inside the target tissue. Energy dissipation in focus leads to a sharp temperature increase in a small ellipsoid-shaped volume of a few millimeters in diameter and length, rapidly inducing a lesion of coagulative necrosis and even thermal fixation [Citation22,Citation23]. Accumulated evidence shows that HIFU-mediated tumor ablation leads to long-term pain relief in a large proportion of patients suffering from PaC [Citation15,Citation16,Citation24–31], improves local control [Citation16,Citation25,Citation27,Citation29], and may even offer survival benefits over chemo- and radio-chemotherapy [Citation17,Citation21,Citation32].
During ablation, the accumulated thermal damage in the target tissue is commonly estimated using the thermal dose concept introduced by Sapareto et al. The thermal dose is measured in cumulative equivalent minutes at 43 °C, CEM43 [Citation33,Citation34]:
(1)
(1)
where
is the temperature in °C,
is the time of the thermal exposure in minutes, and
is the base of the exponential expression which determines the dose rate. Though sensitivity to thermal damage is tissue dependent, several experimental studies showed that 240 CEM43 is a thermal dose threshold at which coagulative necrosis is induced in most tissues [Citation35–40]. Consequently, most tissues heated to temperatures above 57 °C undergo coagulative necrosis within less than a second. The exponential temperature dependence of the thermal dose provides a strong argument for accurate temperature monitoring of the target tissue, together with feedback-controlled heating to ensure swift, safe, and complete ablation [Citation36,Citation41,Citation42].
HIFU treatments can be guided by diagnostic ultrasound (US-HIFU) [Citation43] or magnetic resonance imaging (MR-HIFU) [Citation44]. In treating abdominal organs like the pancreas, US guidance is convenient because acoustically opaque obstacles like gas pockets and bones, which would block the HIFU beam and thereby lead to prefocal heating and tissue damage, can easily be identified. Monitoring of target tissue ablation is based on changes in echogenicity and elasticity that are associated with coagulative tissue necrosis [Citation43,Citation45–47]. However, an ultrasound-based method for measuring tissue temperatures above 50 °C for the purpose of monitoring the treatment and off-site heating has not yet been reported [Citation48–51].
During MR-guided thermal therapy, the proton resonance frequency of water, which shifts linearly with per Kelvin of temperature increase in all water-rich tissues [Citation52], is often exploited for temperature monitoring. Proton Resonance Frequency Shift (PRFS) thermometry therefore provides noninvasive, volumetric, and radiation-free monitoring of the temperature along the beam path in the full temperature range that is of interest for thermal ablation [Citation53]. Consequently, MR-guided HIFU systems that allow near-real time imaging of the induced temperature elevation have been developed [Citation36,Citation42,Citation54]. Moreover, the use of MR-thermometry data in a feedback controller enables ablation of tissue defined by a given thermal dose [Citation36,Citation41,Citation55]. Among other indications, MR-guided HIFU is currently clinically approved for ablation of uterine fibroids, ablation of prostate cancer and the treatment of essential tremor as well as several musculoskeletal conditions [Citation56–58]. Despite the success of MR-HIFU in treating these conditions and the good results that have been achieved with US-HIFU in treating PaC, only one study in six patients has been published describing MR-HIFU ablation of PaC [Citation59].
In preparation for a planned clinical trial using MR-HIFU for ablation of PaC, we performed a feasibility study in a healthy swine model to establish a protocol for MR-guided ablation of the porcine pancreas using a commercially available MR-HIFU device that is already approved for the treatment of uterine fibroids and bone metastasis. The porcine pancreas differs from the human pancreas in that it is larger and that its main duct drains into the duodenum directly instead of joining the common bile duct [Citation60,Citation61]. It consists of the duodenal lobe, the connecting lobe and the splenic lobe, which resemble the head, uncinate process, and the body and tail of the pancreas in humans in their anatomical arrangement, respectively [Citation62]. As in humans, the head of the porcine pancreas (duodenal lobe) is connected to the duodenum, the body (splenic lobe) follows the curvature of the stomach, passes the portal vein on the anterior side and extends to the left kidney (tail end of the splenic lobe) [Citation63,Citation64]. The unique anatomical arrangement of the colon in pigs complicates ventral acoustic access to the pancreas compared to humans. In the latter, only the transverse mesocolon overlaps the head and neck of the pancreas while in the former, most of the colon is arranged in a series of spirals located in the upper left quadrant of the abdomen, blocking access to the duodenal lobe of the pancreas [Citation61,Citation63]. Thorough bowel preparation is therefore required to minimize feces and air pockets in the colon. Despite this obstacle, the similarity in anatomical dimensions and the structural resemblance of the human and porcine pancreas make the pig the predominant model for preclinical studies on HIFU treatment of PaC [Citation65–71].
Here, we describe the developed protocol and discuss the difficulties which arose from the anatomical location of the pancreas during our experiments using MR-HIFU. The predictive power of the applied ultrasound energy and the measurement of the lesion size via the diameter of the thermal dose- and thermal threshold profiles and contrast-enhanced MRI are evaluated as well. Acoustic access to the pancreas was supported by a custom compression spacer and its effect on the distance from the skin to the pancreas was assessed.
Materials and methods
Animal model
In this study, the feasibility of selective ablation of the porcine pancreas using MR-HIFU was investigated in five healthy, female German landrace pigs between 35 and 58 kg in weight. The experimental protocol was approved by the State Agency for Nature, Environment and Consumer Protection of North Rhine Westphalia. The animals underwent bowel preparation starting 96 h prior to induction of anesthesia (). From that time point on, they were fed Dutch vanilla custard (“Vla”) exclusively with free access to water. Starting at
and ending at
a laxative was added to the custard (200 g per animal and day; Agiolax® Madaus, MEDA Pharma). From
until the experiment, the pigs were fasted with free access to water and were fed three capsules of Simeticone (Lefax Intense 250 mg, Bayer, Germany) to promote elimination of gas pockets.
At the beginning of the experiment, the animals received an intramuscular injection of Atropine (0.02 mg/kg; WDT, Germany) and Tiletamin/Zolazepam (10 mg/kg; Zoletil, Virbac, Germany). Anesthesia was induced and maintained intravenously using Propofol (induction: 1.66 mg/kg i.v., maintenance: 4.0–9.5 mg/kg/h as required; Fresenius, Bad Homburg, Germany). Analgesia was achieved by intravenous administration of buprenorphine (0.02 mg/kg; Buprenovet Multidose, Bayer, Germany). The pigs were intubated and ventilated under pressure-control (Hamilton C1, Heinen + Löwenstein, Switzerland) at 30% oxygen, 14 breaths/min, and a positive end-expiratory pressure (PEEP) of 5–8 mmHg. The tidal volume was adjusted as needed to maintain normocapnia (PaCO2 35–45 mmHg). A catheter (18 G; Arrow International, Reading, USA) for the continuous administration of propofol and Ringer’s solution (5 ml/kg/h; Fresenius Kabi, Germany) was placed in the right external jugular vein using the Seldinger technique. The transportation to the MR-HIFU lab was performed with the animal in prone position. During the experiment, several phases of apnea at 10 mbar of airway pressure were induced to suspend motion for imaging and sonication. To prevent hypoxia, all animals were ventilated with 100% oxygen beforehand for preoxygenation and denitrogenisation until end expiratory oxygen concentrations reached >85%. Before the first sonication, a dose of Buscopan (2 mg/kg i.v., Sanofi, Paris, France) was administered to suppress peristaltic movement. After the sonications, animals were euthanized using sodium-pentobarbital (150 mg/kg i.v., Euthadorm®, CP-Pharma, Burgdorf, Germany). Laparotomy was performed and sites of thermal injury along the beam path were documented if present.
The study was preceded by a pilot study in three animals in which the used MRI sequences and anesthesia protocols were optimized (data not shown), the depth of the pancreas inside the animal without a compression spacer was assessed, and sonication of the pancreas without the addition of a compression spacer was attempted, which proved to be unfeasible due to the lack of acoustic access to the pancreas. The animals in the pilot study were prepared in the same way as the animals in the subsequent feasibility study, with the exception that these animals instead were fed Calshake (Fresenius, Bad Homburg, Germany) and were not given a laxative.
Experimental setup
The experiments were performed using a clinical MR-HIFU system (3T Achieva®, Philips Healthcare, Best, The Netherlands, and Sonalleve® V2 HIFU, Profound Medical, Mississauga, Canada), which was operated using the Sonalleve® software release 3.5.1271.1817. The employed Sonalleve® V2 HIFU System has not yet been approved by the FDA for the treatment of PaC. The patient table of the used system contains a 256-element phased array transducer immersed in an oil bath with a radius of curvature of 140 mm and an aperture of 135.9 mm [Citation72].
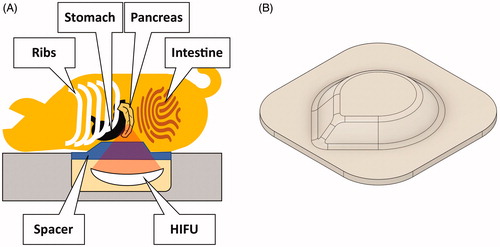
A polyacrylamide spacer with a drop-shaped dome, which displaced the bowels and thereby facilitated acoustic access to the pancreas, was interposed between the HIFU window and the animal (). The influence of a similar spacer on the quality of temperature mapping was studied previously by Ferrer et al. [Citation73]. A thin layer of degassed and demineralized water was applied to the HIFU window to establish acoustic coupling to the spacer. The compression spacer was positioned on the HIFU window with the dome’s point toward the intended cranial direction of the animal. The spacer was held in place using a frame of adhesive tape applied to the edges of the HIFU window. Acoustic coupling was further optimized by shaving and washing of the pig’s abdomen as well as the application of degassed ultrasound gel to both the pig’s abdomen and the spacer. The animal was rolled onto the spacer along the head-tail axis to prevent the trapping of air bubbles, bringing it into prone position with the xiphoid process at the cranial end of the HIFU window.
Figure 1. (A) Experimental setup. The pigs were positioned on the patient table in prone position. The abdomen was compressed using a custom-made hydrogel spacer (B) inserted between the animal and the table’s acoustic membrane.
Spacer fabrication
All chemicals used to create the spacers were purchased from Sigma Aldrich (Sigma-Aldrich Corporation, St. Louis, MO) and their respective ratios are detailed in . The shape of the spacer was chosen based on T1-weighted MRI imaging data from the pilot study and was modeled in Fusion 360 (Autodesk, San Rafael, CA). Using a 3D printer (Prusa i3 MK3, Prusa Research, Czech Republic), a model of the spacer was manufactured from polylactic acid. Using this model, a mold of the spacer was created from silicone (HS620, Versandhandel Blioch, Ahrensburg, Germany). All necessary ingredients for the hydrogel were mixed under stirring. APS was added last to initialize polymerization and the solution was poured into the mold. Ten minutes after the onset of polymerization, the spacer was removed from the mold and stored in an airtight container. For a technical drawing of the spacer and the acoustic properties of the material, see Supplementary Materials S1 and S2.
Table 1. Composition of the spacers used in this study.
MR imaging
All images were acquired using the HIFU table’s window coil and the HIFU pelvis coil (Model 905051-F, Philips Healthcare, Best, The Netherlands). Motion artifacts and organ displacement were avoided by inducing apnea. All scan protocols and sonications were started with a delay of about 5 s after reaching the set apnea pressure to provide enough time for the settlement of organ movement.
Treatment planning
The treatments were planned using 3D T1-weighted turbo field echo (TFE) sequences with and without spectral attenuated inversion recovery (SPAIR) fat suppression. The used parameters were TE = 1.46 ms, TR = 3.2 ms, FA = 10°, slice thickness = 4 mm, number of slices = 150, TFE factor = 69, NSA = 1, SENSE factor (RL, FH) = (2, 1.5), ACQ (RECON) voxel MPS = 1.79 × 1.82 (1.52 × 1.53) mm, water-fat shift = 0.7 pixels, startup echoes = 0, fold-over direction = RL, and no TFE pre-pulses. This resulted in an acquisition duration of 13.4 s.
Treatment monitoring
All sonications were monitored using PRFS-thermometry via an RF-spoiled gradient echo sequence (T1-FFE) with TR = 30 ms, TE = 19.5 ms (animals 1, 2 and 3) or 15 ms (animals 4 and 5), FOV (RL, HF) = 400 × 250 mm, EPI factor = 9, number of slices = 6, reconstructed voxel dimensions = 2.5 × 2.5 × 7 mm3, resulting in an acquisition time of 2.66 s (animals 1, 2, and 3) or 2.25 s (animals 4 and 5) for a full set of slices. Fat suppression was performed using selective excitation. The MR thermometry slices were positioned in four stacks, namely the focus- (3 slices), sagittal- (1 slice), near field- (1 slice), and far field (1 slice) stacks. The focus stack was positioned at the HIFU focus point and was oriented perpendicular to the transducer axis to provide a complete view of the volumetric temperature distribution in the target area. The sagittal stack was positioned to show the long axis of the HIFU focus. The nearfield stack was positioned deep to the subcutaneous fat layer to monitor excess heat generation in the abdominal muscles. The far-field stack was positioned behind the focus to monitor heat generation at the spine.
Post-interventional MRI
The sonications were evaluated using the 3D T1-weighted TFE sequences described above, both before and one minute after intravenous contrast agent injection (0.1 mmol/kg, Dotagraf, Jenapharm GmbH & Co. KG, Jena, Germany). The parameters and field of view of the post-treatment scans were kept the same as the planning scans. For evaluation, the images acquired after contrast agent injection were subtracted from their pre-injection counterpart to reveal non-perfused volumes (NPVs).
Treatment protocol
The splenic and duodenal lobes of the pancreas were identified using the planning images and one target point was selected in each. Care was taken to maintain a distance of 1.5 cm between the two target points and to avoid intersection of the ultrasound beam with air pockets, feces, and bones. Cessation of peristaltic motion was verified by continuous temperature mapping. Each sonication was preceded by test sonications (fixed focus position, 1.2 MHz) using an acoustic output power between 100 and 150 W, depending on the observed heating efficiency, to verify acoustic coupling and alignment of the focus point with the target volume. Offsets between the observed and intended center of heating were corrected by the adjustment of the transducer position and beam steering. Next, the target points were sonicated in two separate apnea phases using a sonication protocol with fixed acoustic output power of either 300 or 400 W and a circular eight-point focus trajectory with a diameter of 4 mm (4 mm regular sonication cell, max. sonication time 60 s, 1.2 MHz, first described by Köhler et al. [Citation42]). According to simulations performed by the manufacturer, this sonication protocol results in a spatial peak, time-averaged intensity of 3.64 W/cm2 per Watt of acoustic output power in water. The sonication power was chosen based on clinical research published on US-HIFU treatment of PaC [Citation26,Citation31,Citation32,Citation74]. The sonications were stopped manually when a safety hazard, such as spontaneous breathing or excessive off-target heating, was observed or when the temperature in the target area was deemed high enough by the operator to achieve rapid ablation. This resulted in sonication times between 7.5 and 29.9 s and thermal lesions of various diameters.
Data analysis
Evaluation of sublethal sonications
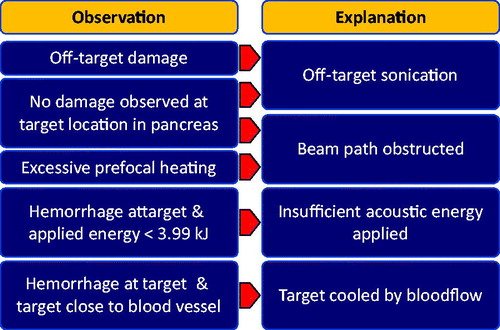
The sonications that did not lead to a visible or palpable thermal lesion in the pancreas upon excision of the organ were documented and possible causes were analyzed. The scheme displayed in shows possible explanations for the absence of a thermal lesion in the pancreas and the observations that were considered in judging the most likely explanation for each individual case. The threshold of 3.99 kJ was used for judging the applied energy insufficient to create a thermal lesion as this was the smallest energy which led to a thermal lesion in the experiments.
Histology
The pancreas was excised in toto. The treated areas of the pancreas were marked with surgical clips and fixated in a 4% v/v solution of formaldehyde. Rapid fixation of the pancreas is essential due to the early onset of autolysis in this organ and the slow penetration of formaldehyde into bulky tissue samples [Citation75,Citation76]. After 48 h, the samples were transferred to phosphate-buffered saline and stored until gross examination (see Analysis of the lesion size). After gross examination, the fixated tissue samples were trimmed and sliced through the center of the target area, perpendicular to the sample’s surface. They were dehydrated using increasing concentrations of alcohol (2-propanol, Th. Geyer Hamburg GmbH, Hamburg, Germany), treated with an intermedium (Histo-Clear, National Diagnostics, Atlanta, Georgia, USA) and embedded in paraffin (Paraplast Plus, Sigma Life Sciences, Darmstadt, Germany). The embedded samples were sliced and stained using hematoxylin and eosin. Examination was performed using a brightfield microscope (BX53, Olympus, Japan).
Analysis of the lesion size
For the evaluation of the lesion size by gross examination, each piece was placed on an even surface next to a ruler and a photograph was taken to generate a near-isometric view of the sample with a reference scale. The photographs were then analyzed using the Measure tool of ImageJ, Version 1.52a (National Institutes of Health, USA; http://imagej.nih.gov/ij). The longest and the shortest axis of the ablated area visible as white necrotic core on the tissue piece’s surface were measured. The average of both measurements will be referred to as the lesion size. The lesion size was correlated with the applied acoustic energy (transducer output sonication time) and the acoustic energy after attenuation by the spacer and the prefocal tissue. The acoustic energy after attenuation will be referred to as the delivered energy
and is calculated from the applied power
and the sonication time
based on the combined attenuation coefficient
of the spacer at 37 °C and the prefocal tissue, using the parameters determined by Hwang et al. [Citation67] for the latter:
(2)
(2)
(3)
(3)
(4)
(4)
where
is the thickness of the spacer along the transducer axis,
is the thickness of the abdominal wall (skin to the deep surface of the abdominal muscles) and
is the total length of the prefocal beam path inside the animal along the transducer axis. The attenuation coefficients reported by Hwang et al. were measured for the acoustic frequency of 1 MHz and were therefore adjusted to 1.2 MHz using a linear model of the attenuation coefficient’s frequency (
) dependence [Citation77]:
(5)
(5)
For the thermal threshold- and dosimetry-based prediction of the lesion size, the spatial extent of the area which exceeded 57 °C at any time during the treatment and the extent of the area which accumulated a thermal dose of 240 CEM43 was assessed. To this end, the lengths along the longest and the shortest axes of the corresponding contour plots in the central slice of the focus stack were measured using a custom python application (thermal threshold) and the Sonalleve GUI measurement tool (thermal dose). The arithmetic mean of the respective measurements will be referred to as the thermal threshold prediction and the dosimetry prediction
of the lesion size. The measurements were performed on the first slice acquired after the end of the respective sonication. The NPV diameter was measured perpendicular to the transducer axis in the transverse imaging planes of the contrast enhanced MR scans. The measurements were performed from the center of one hyperemic rim to the other and will be referred to as the NPV prediction of the lesion size
The quality of
and
as predictors for the lesion size was assessed via the mean squared deviation (MSD) from the lesion size found in gross examination.
Analysis of the compression spacer’s influence on the intraabdominal beam path length
The depth of each pig’s pancreas as shown in the acquired anatomical MRI images was measured using the “Measurements and Tools > Length” tool of the RadiAnt software (Medixant, Poznan, Poland). Both the distance of the organ’s surface closest to the HIFU window (ventral limit) and the organ’s surface farthest from the HIFU window (dorsal limit) to the skin of the abdomen were measured in a vertical line. Additionally, each measurement was repeated between the respective surface of the organ and the HIFU window. The measured distances were then used to assess the effect of the spacer’s influence on the length of the beam path inside the abdominal cavity.
Statistics
The correlation of the lesion size with and
was analyzed via linear regression and the coefficient of determination using the linear_model.LinearRegression module and the LinearRegression.score method of the sklearn python 2.7 package (version 0.20.1). The spacer’s influence on the length of the beam path was analyzed via Welch’s t-test using the stats.ttest_ind module of the scipy python 2.7 package (version 1.1.0).
Results
Overview
The sonication results of the study group are summarized in . Eight out of ten sonications resulted in visible damage at the target site. Five sonications induced a lesion of coagulative necrosis, which was confirmed by histology. Injury of the abdominal skin, abdominal wall, the spleen, or the stomach was not observed in any of the animals. One sonication resulted in clearly discernible bowel damage (animal 3). Hyperemia in the omental fat adjacent to the pancreas was observed in two cases (animals 3 and 4). In sonication 3/2, 400 W of acoustic power were applied as the selected volume lay particularly deep inside the animal, resulting in poor heating efficiency during the test sonications.
Table 2. Sonication results overview.
Analysis of sublethal sonications
Of the five target sites that did not show a thermal lesion, two exhibited no damage at all (1/1 and 2/2). According to the arguments outlined in , this may have been caused by insufficient heating or a migration of the pancreas out of the HIFU focus. Sonication 1/1 showed very minor heating at the target location in both the focus slice and the sagittal slice, but strong prefocal heating and heating offset from the target volume (), which prompted early cancelation with low overall energy being delivered. We therefore believe that the acoustic energy deposited in the pancreas was not sufficient to induce damage. During sonication 2/2, spontaneous breathing occurred due to insufficient depth of anesthesia. As a result, the target volume was displaced out of the HIFU focus and the sonication was canceled before damage was induced ().
Figure 3. Snapshots of thermometry maps acquired during sublethal sonications. (A) Sonication 1/1: offset of thermal dose profile in focus slice and prefocal heating in sagittal slice. (B) Sonication 1/2: strong focal heating despite small amount of delivered energy and irregular shape of heated volume. (C) Sonication 2/2: motion artifacts due to respiration in focus slice; insufficient heating measured in sagittal slice before movement. (D) Sonication 3/2: signal void indicative of air or solid near focus observed in focus slice and strong prefocal heating in sagittal slice. (E) Sonication 5/2: limited heating in the focus, only mild prefocal heating. (F) Sonication 5/2: proximity of the target area to the portal vein likely prevented sufficient heating for the creation of a thermal lesion.
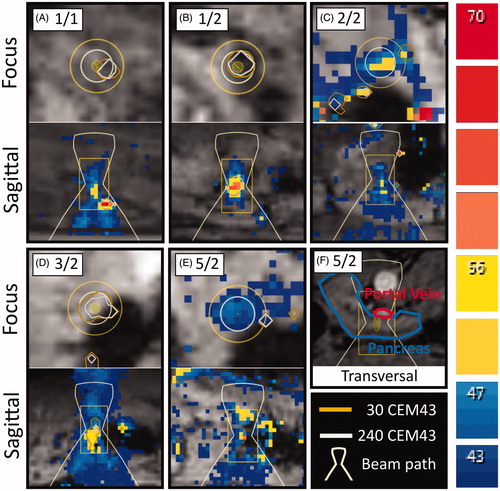
Sonications 1/2, 3/2 and 5/2 resulted in damage at the target site but did not lead to the formation of a thermal lesion. In sonication 1/2, this can be attributed to the relatively small amount of acoustic energy that reached the pancreas. The sagittal temperature map also shows a small deviation of the heated area’s shape from the usual elongated form. It is therefore possible that the ultrasound was absorbed mainly before the pancreas (). Sonication 3/2 was performed with the highest amount of energy among all experiments and prefocal heating was observed only close to the intended focal volume. The target volume was placed in an area of the pancreas directly adjacent to the bowels, which sustained damage that was clearly discernible during gross examination. It is therefore likely that a large part of the acoustic energy was absorbed by stool in the adjacent bowel loop, leading only to hyperemia in the pancreas (). Sonication 5/2 was performed with high acoustic energy compared to other sonications, exhibited limited prefocal heating (), did not induce any visible damage to other organs, and did not lead to a thermal lesion. MR-imaging showed that the center of the target volume was located at a distance of only 5 mm to the surface of the portal vein. It is therefore likely that the perfusion and cooling of the target volume by the nearby blood vessel prevented sufficient buildup of heat ().
Coagulative necrosis was predicted by the thermal dose measurement in three sonications that did not produce a thermal lesion in the pancreas (1/1, 1/2, and 3/2). In sonication 3/2, this can be attributed to the displacement of the pancreas out of the HIFU focus as damage was induced in the bowels. During sonications 1/1 and 1/2, no motion or erratic temperature measurements were observed in the target volume. Creation of a false positive by thermometry artifacts can therefore be ruled out. Damage to other organs was not observed during examination.
Histology
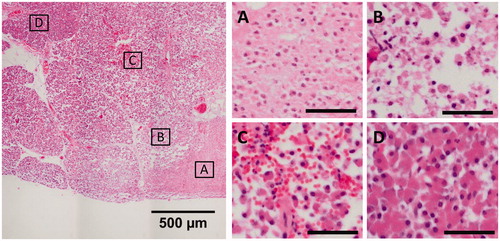
Coagulative necrosis in the focal area was confirmed by histology in all samples which showed a white necrotic core in gross examination (2/1, 3/2, 4/1, 4/2, and 5/1). The focal areas of these samples exhibited coherent cell bodies with fragmented nuclei and no hemorrhage, indicative of coagulative necrosis (). The focal areas were surrounded by a border region of low cellular cohesion () and hemorrhage (), which then transitioned into healthy pancreas parenchyma ().
Figure 4. Representative images of the different degrees of thermal damage induced by HIFU in the porcine pancreas, from sample 4/2. The scale bars in the subfigures represent 50 µm. (A) Center of the focal area, exhibiting coherent cells with fragmented nuclei and no hemorrhage. (B, C) Border region, exhibiting a loss of cohesion between cells and hemorrhage. (D) Healthy pancreas parenchyma.
Analysis of the lesion size
The fixated pancreas samples are shown in . In , the diameters of the thermal lesions created throughout the experiments are drawn versus the applied ultrasound energy and the delivered ultrasound energy
The coefficients of determination
of the ordinary least squares (OLS) regression for the applied and the delivered energy are 0.61 and 0.96, respectively. The resulting linear approximations
and
are
(6)
(6)
(7)
(7)
Figure 5. Fixated pancreas samples. Red arrows indicate thermal lesions of coagulative necrosis. Yellow arrows indicate non-ablative tissue damage.
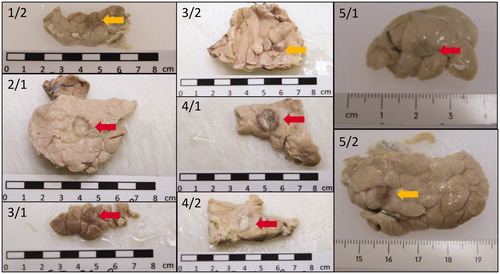
Figure 6. Predictive power of different parameters for the lesion size found in gross examination after fixation. Lesion size versus (A) applied acoustic energy, (B) delivered acoustic energy. Measurement of the lesion size via (C) temperature threshold monitoring (57 °C), (D) thermal dose monitoring (240 CEM43), and (E) post-treatment contrast enhanced MRI. OLS: ordinary least squares; MSD: mean squared deviation; NPV: non-perfused volume.
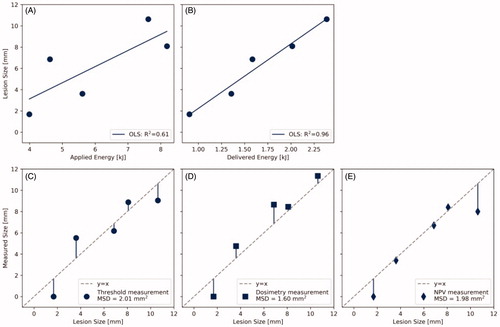
The MSD of the thermal threshold monitoring, thermal dose monitoring and NPV measurement were 2.01, 1.60, and 1.98 mm2, respectively. The corresponding lesion size predictions
and
are plotted against the results of gross examination in .
Analysis of the compression spacer’s influence on the beam path length
Only two animals from the pilot study were included in the analysis to obtain data on the length of the beam path without a spacer as an error in the positioning of the third animal prevented the measurement of the pancreas’ depth in the manner described above. Instead, pig 4 was scanned both with and without a spacer. The weight of the animals appeared to have an influence of approximately 1.05 on the ventral limit of the pancreas without a spacer and 0.29
with a spacer ().
Figure 7. (A) Depth of the pancreas from the skin without a spacer (n = 3) and (B) the depth from the skin with a spacer (n = 5) versus animal weight. (C) Side-by-side comparison of ventral and dorsal limits. Probability values were obtained using Welch’s t-test.
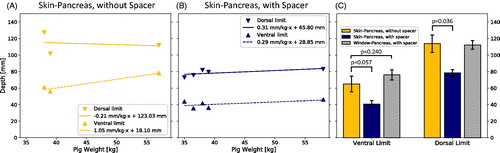
The depths of the ventral and dorsal limits of the pancreas with and without spacer are shown in . The average depth of the ventral limit from the skin with and without the addition of a spacer was 41 ± 4 and 65 ± 10 mm, respectively. The depths of the dorsal limits of the pancreas from the skin with and without spacer were 79 ± 4 and 114 ± 11 mm, respectively. On average, the acoustic beam path from the skin was therefore shortened by 24 ± 6 mm for the most ventral part of the pancreas and by 35 ± 6 mm for the most dorsal part. The average depths of the ventral and dorsal limits of the pancreas from the surface of the table’s acoustic window with a spacer were 76 ± 6 and 112 ± 5 mm, respectively. The overall length of the beam path from the acoustic window therefore remained largely unaffected by the addition of a spacer.
Discussion
We introduced an experimental setup and procedure which enables sonication of the porcine pancreas under MR guidance using a clinical MR-HIFU system. The use of a custom hydrogel spacer appeared a necessary addition to create an acoustic window by compressing tissue located in front of the pancreas and to displace bowel loops. The spacer notably shortened the distance that needed to be penetrated by the ultrasound inside the animals’ abdomen, reducing ultrasound attenuation and the risk of bowel loop interposition without significantly increasing the overall distance between the HIFU window and the pancreas. Furthermore, bowel preparation prior to treatment appeared to be crucial as the presence of feces or air pockets in the bowel led to strong prefocal absorption of ultrasound with off-target heating in the pilot study (data not shown). If bowel loop interposition occurred despite the use of bowel preparation and a compression spacer, this could often be resolved by massaging the animal’s abdomen with hand motions in caudal direction. Ablations were performed during short periods of apnea in expiration, using a PEEP of 10 mbar. This allowed the use of established MRI sequences for planning, PRFS thermometry and post-interventional imaging as well as the use of standard sonication protocols without the need for motion compensation in imaging or treatment. Above protocol allowed successful MR-guided HIFU sonication of the porcine pancreas in 8 out of 10 attempts. One sonication likely failed to induce damage in the pancreas due to the partial obstruction of the beam path and one failed due to insufficient suppression of respiration, leading to movement of the pancreas. In three of the eight successful sonications, the acoustic energy delivered to the pancreas was insufficient to induce coagulative necrosis in the focal area. The reasons that were identified for this were the application of too little acoustic energy, the displacement of the pancreas out of the HIFU focus after acquisition of the planning images, and the rapid cooling of the target tissue by the adjacent portal vein.
The successful delivery of focused ultrasound energy to the porcine pancreas achieved with this technique shows that MRI has potential for the planning and monitoring of HIFU treatments of the pancreas: Acoustically opaque areas, i.e., gas pockets in the stomach or bowels, solid bowel content, and bones, can be identified and avoided due to the capability to display them in high-contrast, high-resolution cross-sections with an overlay of the exact HIFU beam path. In PaC patients, nearby critical organs, i.e., the stomach, major blood vessels and the common bile duct (possibly containing a stent), can also be made visible using MRI [Citation78–80] and should not be in the focal area during sonication, but successful US-HIFU treatments close to these structures have already been demonstrated [Citation26]. Fiducial markers, which may be present due to prior radiotherapy, can also be visualized [Citation81,Citation82] and should be avoided. During the sonication, MR guidance enables near-real-time monitoring of the temperature in the target volume and adjacent tissue. These measurements were used to track the areas that received the lethal thermal dose of 240 CEM43 and the areas which exceeded the temperature threshold of 57 °C. The diameter of these areas matched the diameter of the thermal lesions found upon gross examination on the scale of the voxel size, with the thermal dose performing slightly better than the threshold. In this study, we used sonication protocols with fixed acoustic power and beam trajectories and no feedback mechanisms to control the lesion size. Since a good agreement was found between the diameter of the thermal dose profile and the diameter of the resulting thermal lesion, it should be feasible to create thermal lesions of a specific size in the pancreas with high accuracy using feedback algorithms, such as previously published by Enholm et al. [Citation41]. We have also found that the size of the created thermal lesions correlated well with the applied acoustic energy after accounting for attenuation by the spacer and prefocal tissue, opening the possibility to control the lesion size via this parameter. This can easily be implemented in the current workflow as the required measurements can be extracted directly from the imaging data used for treatment planning.
The main limitations of this study are the absence of a pancreatic tumor in the treated animals, the small number of the induced thermal lesions, and that tissues may change in volume during fixation by a certain percentage [Citation83]. The linear models and predictive powers we derived in this study will therefore not necessarily be representative of the relationships in the clinical application. Furthermore, as the animals were euthanized without an observation period following the treatments, the study does not allow conclusions concerning the frequency and severity of potential adverse events. This is of note as the thermal lesions of two sonications in which coagulative necrosis was indicated by thermal dosimetry were not accounted for during dissection of the animals, leaving open the question of where exactly they formed. Finally, with one exception, the depth of each animal’s pancreas was assessed only either with or without a spacer. While there is little doubt that the spacer does shorten the intra-abdominal beam path to the pancreas, the magnitude of the observed difference may have been distorted by differences in the weight, anatomy, and preparation of the animals.
In conclusion, despite some inherent limits to its transferability, the pig is the best preclinical model for the translational development of HIFU technology targeting PaC due to the resemblance of the porcine and human pancreas as well as the similar anatomical dimensions. We have therefore established a treatment protocol which allows the HIFU sonication of the porcine pancreas using a clinical MR-HIFU system and described it here in detail. The size of the thermal lesions created in the pancreas correlated well with the attenuation-corrected ultrasound energy and can be monitored using PRFS thermometry. Apart from the compression spacer, the software and hardware employed is already in clinical use for the MR-guided HIFU treatment of uterine fibroids and bone metastases. The presented methods and findings should therefore facilitate further research and thereby accelerate clinical translation of this promising application.
Supplemental Material
Download PDF (248.5 KB)Acknowledgements
We would like to thank Joo Ha Hwang (Stanford University, Department of Medicine, Gastroenterology and Hepatology) for sharing his expertise in the treatment of the porcine pancreas using HIFU and Irmgard Henke (University of Cologne, Department of Medicine and University Hospital of Cologne, Experimental Medicine) as well as Jan Ulrich Becker (University of Cologne, Department of Medicine and University Hospital of Cologne, Pathology) for their help and advice regarding histology. We thank the CMMC Microscopy Facility and Florian Küster (University of Cologne, Center for Molecular Medicine Cologne) for their support in microscopy. The compression spacers were manufactured with the friendly support of Jörg-Bernd Bonekamp (Soluxx GmbH, Cologne, Germany). Thanks to Lisa Braunstein and the Therapy Ultrasound team of the Joint Department of Physics/Division of Radiotherapy and Imaging (The Institute of Cancer Research, London, UK) for provision of the acoustic characterization data. Thanks to Simon Hubertus (Profound Medical, Mississauga, Canada) for the acoustic field simulations of the used HIFU sonication protocol.
Disclosure statement
Edwin Heijman is employed by Philips. Sin Yuin Yeo is partly employed by Profound Medical.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA. CAA Cancer J Clin. 2019;69(1):7–34.
- Zhang Q, Zeng L, Chen Y, et al. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Pract. 2016;2016:8962321–8962310.
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049.
- Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2–11.
- Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18(16):4266–4276.
- Moffat GT, Epstein AS, O'Reilly EM. Pancreatic cancer—a disease in need: optimizing and integrating supportive care. Cancer. 2019;125(22):3927–3929.
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378(9791):607–620.
- Carlson CL. Effectiveness of the World Health Organization Cancer Pain Relief Guidelines: an integrative review. J Pain Res. 2016;9:515–534.
- WHO. Cancer pain relief with a guide to opioid availability. 2nd ed. Geneva, Switzerland: World Health Organization; 1996.
- Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. J Am Med Assoc. 2004;291(9):1092–1099.
- Xiong L. The preliminary clinical results of the treatment for advanced pancreatic carcinoma by high intensity focused ultrasound. Chin J Gen Surg. 2001;16:345–347.
- Xu Y, Wang G, Gu Y, et al. The acesodyne effect of high intensity focused ultrasound on the treatment of advanced pancreatic carcinoma. Clin Med J China. 2003;10:322–323.
- Yuan C, Yang L, Cheng Y. Observation of high intensity focused ultrasound treating 40 cases of cancer of pancreas. Chlin J Clin Hep. 2003;19:145–146.
- Xiong LL, Hwang JH, Huang XB, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. J. Pancreas. 2009;10:123–129.
- Gao HF, Wang K, Meng ZQ, et al. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepatogastroenterology. 2013;60(128):1906–1910.
- Vidal-Jove J, Perich E, Del Castillo MA. Ultrasound guided high intensity focused ultrasound for malignant tumors: the Spanish experience of survival advantage in stage III and IV pancreatic cancer. Ultrason Sonochem. 2015;27:703–706.
- Dimcevski G, Kotopoulis S, Bjånes T, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Control Release. 2016;243:172–181.
- Marinova M, Rauch M, Mücke M, et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: evaluation of feasibility, reduction of tumour volume and pain intensity. Eur Radiol. 2016;26(11):4047–4056.
- Ji Y, Zhang Y, Zhu J, et al. Response of patients with locally advanced pancreatic adenocarcinoma to high-intensity focused ultrasound treatment: a single-center, prospective, case series in China. CMAR. 2018;10:4439–4446.
- Marinova M, Huxold HC, Henseler J, et al. Clinical effectiveness and potential survival benefit of US-guided high-intensity focused ultrasound therapy in patients with advanced-stage pancreatic cancer. Ultraschall Med. 2019;40(5):625–637.
- Wu F, Wang ZB, Cao YD, et al. Heat fixation of cancer cells ablated with high-intensity-focused ultrasound in patients with breast cancer. Am J Surg. 2006;192(2):179–184.
- Ter Haar G, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23(2):89–104.
- Dababou S, Marrocchio C, Rosenberg J, et al. A meta-analysis of palliative treatment of pancreatic cancer with high intensity focused ultrasound. J Ther Ultrasound. 2017;5:9.
- Zhao H, Yang G, Wang D, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010;21(4):447–452.
- Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol. 2010;195(3):W245–252.
- Wang K, Chen Z, Meng Z, et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia. 2011;27(2):101–107.
- Sung HY, Jung SE, Cho SH, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011;40(7):1080–1086.
- Sofuni A, Moriyasu F, Sano T, et al. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J Gastroenterol. 2014;20(28):9570–9577.
- Li P-Z, Zhu S-H, He W, et al. High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer. Hbpd Int. 2012;11(6):655–660.
- Orgera G, Krokidis M, Monfardini L, et al. High intensity focused ultrasound ablation of pancreatic neuroendocrine tumours: report of two cases. Cardiovasc Intervent Radiol. 2011;34(2):419–423.
- Lv W, Yan T, Wang G, et al. High-intensity focused ultrasound therapy in combination with gemcitabine for unresectable pancreatic carcinoma. Ther. Clin. Risk Manag. 2016;12:687–691.
- Sapareto SA, Hopwood LE, Dewey WC. Combined effects of X irradiation and hyperthermia on CHO cells for various temperatures and orders of application. Radiat Res. 1978;73(2):221–233.
- Sapareto S. a, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol. 1984;10(6):787–800.
- Damianou C, Hynynen K. The effect of various physical parameters on the size and shape of necrosed tissue volume during ultrasound surgery. J Acoust Soc Am. 1994;95(3):1641–1649.
- Chung AH, Jolesz FA, Hynynen K. Thermal dosimetry of a focused ultrasound beam in vivo by magnetic resonance imaging. Med Phys. 1999;26(9):2017–2026.
- McDannold N, Hynynen K, Wolf D, et al. MRI evaluation of thermal ablation of tumors with focused ultrasound. J Magn Reson Imaging. 1998;8:91–100.
- Van Rhoon GC, Samaras T, Yarmolenko PS, et al. CEM43C thermal dose thresholds: a potential guide for magnetic resonance radiofrequency exposure levels? Eur Radiol. 2013;23(8):2215–2227.
- Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia. 2011;27(4):320–343.
- Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–294.
- Enholm JK, Köhler M, Quesson B, et al. Improved volumetric MR-HIFU ablation by Robust binary feedback control. IEEE Trans Biomed Eng. 2010;57(1):103–113.
- Köhler M, Mougenot C, Quesson B, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys. 2009;36(8):3521–3535.
- Ebbini ES, Ter Haar G. Ultrasound-guided therapeutic focused ultrasound: current status and future directions. Int J Hyperthermia. 2015;31(2):77–89.
- Hynynen K. MRIgHIFU: a tool for image-guided therapeutics. J Magn Reson Imaging. 2011;34(3):482–493.
- Shahmirzadi D, Hou GY, Chen J, et al. Ex vivo characterization of canine liver tissue viscoelasticity after high-intensity focused ultrasound ablation. Ultrasound Med Biol. 2014;40(2):341–350.
- Zhang S, Zhou F, Wan M, et al. Feasibility of using Nakagami distribution in evaluating the formation of ultrasound-induced thermal lesions. J Acoust Soc Am. 2012;131(6):4836–4844.
- Shankar PM. Ultrasonic tissue characterization using a generalized Nakagami model. IEEE Trans Ultrason Ferroelectr Freq Control. 2001;48(6):1716–1720.
- Lewis MA, Staruch RM, Chopra R. Thermometry and ablation monitoring with ultrasound. Int J Hyperthermia. 2015;31(2):163–181.
- Bamber J, Hill C. Ultrasonic attenuation and propagation speed in mammalian tissues as a function of temperature. Ultrasound Med Biol. 1979;5(2):149–157.
- Teixeira CA, Alvarenga AV, Cortela G, et al. Feasibility of non-invasive temperature estimation by the assessment of the average gray-level content of B-mode images. Ultrasonics. 2014;54(6):1692–1702.
- Maraghechi B, Kolios MC, Tavakkoli J. Feasibility of detecting change in backscattered energy of acoustic harmonics in locally heated tissues. Int J Hyperth. 2019;36:964–974.
- Ishihara Y, Calderon A, Watanabe H, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34(6):814–823.
- Hall LD, Talagala SL. Mapping of pH and temperature distribution using chemical-shift-resolved tomography. J Magn Reson. 1985;65(3):501–505.
- Vimeux F, De Zwart JA, Palussiére J, et al. Real-time control of focused ultrasound heating based on rapid MR thermometry. Invest Radiol. 1999;34(3):190–193.
- Daum DR, Hynynen K. Thermal dose optimization via temporal switching in ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control. 1998;45(1):208–215.
- Siedek F, Yeo SY, Heijman E, et al. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU): technical background and overview of current clinical applications (part 1). Rofo. 2019;191(6):522–530.
- Siedek F, Yeo SY, Heijman E, et al. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU): overview of emerging applications (part 2). Rofo. 2019;191(6):531–539.
- Focused Ultrasound Foundation. State of the Technology [Internet]. [cited 2019 Aug 15]. Available from: https://www.fusfoundation.org/the-technology/state-of-the-technology.
- Anzidei M, Marincola BC, Bezzi M, et al. Magnetic resonance-guided high-intensity focused ultrasound treatment of locally advanced pancreatic adenocarcinoma: preliminary experience for pain palliation and local tumor control. Invest Radiol. 2014;49(12):759–765.
- Ligresti D, Kuo YT, Baraldo S, et al. EUS anatomy of the pancreatobiliary system in a swine model: the WISE experience. Endosc Ultrasound. 2019;8(4):249–254.
- Swindle MM, Makin A, Herron AJ, et al. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012;49(2):344–356.
- Ferrer J, Scott WE, Weegman BP, et al. Pig Pancreas Anatomy: Implications for Pancreas Procurement, preservation, and islet isolation. Transplantation. 2008;86(11):1503–1510.
- Mahadevan V. Anatomy of the pancreas and spleen. Surgery. 2016;34(6):261–265.
- Swindle MM, Smith AC. Comparative anatomy and physiology of the pig. Scand J Lab Anim Sci. 1998;25:11–21.
- Khokhlova TD, Hwang JH. HIFU for palliative treatment of pancreatic cancer. J Gastrointest Oncol. 2011;2:83–95.
- Liu CX, Gao XS, Xiong LL, et al. A preclinical in vivo investigation of high-intensity focused ultrasound combined with radiotherapy. Ultrasound Med Biol. 2011;37(1):69–77.
- Hwang JH, Wang YN, Warren C, et al. Preclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumors. Ultrasound Med Biol. 2009;35(6):967–975.
- Xie B, Li YY, Jia L, et al. Experimental ablation of the pancreas with High Intensity Focused Ultrasound (HIFU) in a porcine model. Int J Med Sci. 2011;8(1):9–15.
- Dupré A, Melodelima D, Pflieger H, et al. Thermal ablation of the pancreas with intraoperative high-intensity focused ultrasound: safety and efficacy in a porcine model. Pancreas. 2017;46(2):219–224.
- Chang W, Lee JY, Lee JH, et al. A portable high-intensity focused ultrasound system for the pancreas with 3d electronic steering: a preclinical study in a swine model. Ultrasonography. 2018;37(4):298–306.
- Li T, Khokhlova T, Maloney E, et al. Endoscopic high-intensity focused US: technical aspects and studies in an in vivo porcine model. Gastrointest Endosc. 2015;81(5):1243–1250.
- Kothapalli S, Altman MB, Partanen A, et al. Acoustic field characterization of a clinical magnetic resonance-guided high-intensity focused ultrasound system inside the magnet bore. Med Phys. 2017;44(9):4890–4899.
- Ferrer CJ, Bartels LW, van Stralen M, et al. Fluid filling of the digestive tract for improved proton resonance frequency shift-based MR thermometry in the pancreas. J Magn Reson Imaging. 2018;47(3):692–701.
- Strunk HM, Henseler J, Rauch M, et al. Clinical use of high-intensity focused ultrasound (HIFU) for tumor and pain reduction in advanced pancreatic cancer. Rofo. 2016;188(7):662–670.
- Shimizu M, Hayashi T, Saitoh Y, et al. Postmortem autolysis in the pancreas: multivariate statistical study. The influence of clinicopathological conditions. Pancreas. 1990;5(1):91–94.
- Eltoum I, Fredenburgh J, Myers RB, et al. Introduction to the theory and practice of fixation of tissues. J Histotechnol. 2001;24(3):173–190.
- Szabo TL. Time domain wave equations for Lossy media obeying a frequency power law. J Acoust Soc Am. 1994;96(1):491–500.
- Bottomley PA. In-vivo soft tissue NMR imaging of the rat thorax and abdomen. Experientia. 1981;37(7):768–770.
- Wallner BK, Schumacher KA, Weidenmaier W, et al. Dilated biliary tract: evaluation with MR cholangiography with a T2-weighted contrast-enhanced fast sequence. Radiology. 1991;181(3):805–808.
- Wedeen V, Meuli R, Edelman R, et al. Projective imaging of pulsatile flow with magnetic resonance. Science. 1985;230(4728):946–948.
- Mougenot C, Moonen C. Magnetic resonance-guided high intensity focused ultrasound in the presence of biopsy markers. J Ther Ultrasound. 2017;5:4–13.
- Gurney-Champion OJ, Lens E, Van Der Horst A, et al. Visibility and artifacts of gold fiducial markers used for image guided radiation therapy of pancreatic cancer on MRI. Med Phys. 2015;42(5):2638–2647.
- Stowell RE. Effect on tissue volume of various methods of fixation, dehydration, and embedding. Biotech. Histochem. 1941;16(2):67–83.