Abstract
The blood-brain and blood-tumor barriers represent highly specialized structures responsible for tight regulation of molecular transit into the central nervous system. Under normal circumstances, the relative impermeability of the blood-brain barrier (BBB) protects the brain from circulating toxins and contributes to a brain microenvironment necessary for optimal neuronal function. However, in the context of tumors and other diseases of central nervous system, the BBB and the more recently appreciated blood-tumor barrier (BTB) represent barriers that prevent effective drug delivery. Overcoming both barriers to optimize treatment of central nervous system diseases remains the subject of intense scientific investigation. Although many newer technologies have been developed to overcome these barriers, thermal therapy, which dates back to the 1890 s, has been known to disrupt the BBB since at least the early 1980s. Recently, as a result of several technological advances, laser interstitial thermal therapy (LITT), a method of delivering targeted thermal therapy, has gained widespread use as a surgical technique to ablate brain tumors. In addition, accumulating evidence indicates that laser ablation may also increase local BBB/BTB permeability after treatment. We herein review the structure and function of the BBB and BTB and the impact of thermal injury, including LITT, on barrier function.
Introduction
The blood-brain and blood tumor barriers (BBB and BTB) regulate not only the composition of the central nervous system (CNS) microenvironment but also the entry of potentially therapeutic drugs. These barriers render targeted delivery of drugs to the CNS more challenging than delivery to other organs. The restrictive nature of the BBB has contributed to the therapeutic failures in glioblastoma (GBM), the most common malignant primary brain tumor [Citation1]. GBM carries a median survival of approximately 15 months and a five-year survival of less than 10% [Citation2]. Although many drugs capable of killing these cancer cells in vitro have been developed, only temozolomide has been found to be effective when administered systemically in vivo. Furthermore, angiogenesis in glioblastoma leads to the formation of abnormal tumor microvessels and the creation of the BTB, which has only limited and heterogeneous permeability and introduces additional complexity in drug delivery to tumor tissue [Citation3]. Thus, understanding and overcoming the BBB and BTB to treat CNS diseases such as GBM remain active areas of investigation.
Several strategies to enhance delivery of therapeutic compounds to brain tumors, such as direct delivery to the tissue, use of carrier molecules, and local physical disruption of the BBB have been developed [Citation4–9]. In particular, local disruption of the BBB and BTB has the dual benefit of increasing permeability to otherwise brain-impermeant drugs and mitigating the impact of these drugs on normal parenchyma.
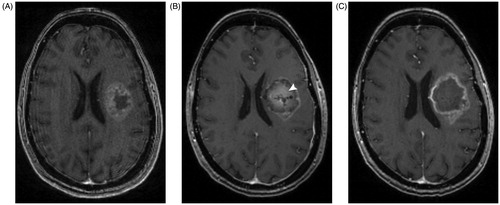
Thermal therapy as a treatment for a variety of conditions has been explored for over a century. The development of microwave and laser probes drove the evolution of thermal therapy from whole-body hyperthermia to the creation of focal thermal lesions. Laser interstitial thermal therapy (LITT) has become a frequently used treatment for CNS lesions in the past decade, with recent evidence suggesting that LITT may create local disruption of the BBB and BTB [Citation10]. LITT is a stereotactic, minimally invasive, cytoreductive technique that can be used to create targeted and conformal thermal injury to treat a wide array of CNS lesions [Citation11–13]. The technique was first described in 1983 but only recently gained traction, primarily due to technological advances, such as MRI thermometry and improved laser probe design [Citation14,Citation15]. On post-procedural MRI scans, increased contrast enhancement can be observed at the edge of the LITT treatment area (). This and other imaging markers of increased BBB permeability support the hypothesis that LITT disrupts the BBB and BTB [Citation10].
Figure 1. Pre- and post-operative MRI from a patient treated with laster interstital thermal therapy. A patient with glioblastoma underwent left-sided LITT. (A) Preoperative T1 MRI with contrast shows a rim-enhancing lesion with central hypointensity. (B) MRI performed 1 day after LITT shows the characteristic targetoid appearance of a lesion treated with LITT, with an area of central enhancement surrounded by a hyperintense rim. White arrowhead points to the area of central contrast-enhancement that was not present prior to treatment. (C) MRI performed 2 months later shows resolution of the central enhancement seen post operatively.
In this review we first describe the biology and physiology of the BBB and BTB. We then highlight the challenges these barriers create for treatment of CNS diseases, particularly brain tumors. Lastly, we briefly describe LITT, the effect that thermal therapy has on the BBB and BTB, and the implications for the treatment of CNS disease.
The blood-brain and blood-tumor barriers
Blood brain barrier physiology
Neural signaling requires a unique and finely controlled biochemical environment. Cellular associations at the BBB maintain homeostasis through passive selective permeability, active molecular and cellular transport, and regulation of local cerebral blood flow. The functional unit of the BBB is comprised of specialized endothelial cells, astrocytes, pericytes, microglia, and neurons and is known as the neurovascular unit [Citation16]. Evolutionary conservation of the function of the BBB has allowed research of the BBB in invertebrates and non-human vertebrates to translate into human studies. Examples of conserved components include endothelial cells, pericytes, and perivascular glial cells [Citation17].
The vascular capillary lumen is separated from the CNS by a single layer of specialized endothelial cells connected to each other by tight junctions and adherens junctions. Tight junctions, comprised of occludin, junctional adhesion molecules (JAMs), and claudins 3 and 5, among others, are a hallmark of the BBB and contribute to the significant reduction in permeability of macromolecules and polar solutes from the plasma in the capillary lumen into brain parenchyma through paracellular diffusion. Occludin and claudins interact with ZO-1, ZO-2, ZO-3, and cingulin, proteins involved with regulation of intracellular architecture. Adherens junctions are comprised of cadherin proteins that provide an intercellular link between cytoplasmic alpha, beta, and gamma catenin, and are essential for providing structural support to tight junction complexes. The tight junctional complex maintains a precisely controlled microenvironment by significantly limiting the diffusion of solutes and small molecules [Citation16]. Junctional properties are dynamic and can be influenced by local circulating and CNS factors [Citation18]. The barrier function of the BBB is significantly altered by not only the expression of tight junctional proteins but also their organization [Citation19].
Adjacent to the endothelial layer is a basement membrane composed of a 30–40 nm thick lamina consisting of type IV collagen, laminin, and fibronectin, which intimately interact with pericytes and the end-foot processes of astrocytes [Citation20]. Pericytes are cells that reside at intervals along capillary walls and play a role in controlling capillary diameter through intracellular actin fibers, which is important for regulating metabolic supply in response to neuronal activity [Citation21]. Recent research suggests that the classic definition of pericytes can be subdivided into subtypes that serve unique roles depending on the organ in which they are located and their location along the capillary bed. For example, single cell sequencing data from mice suggests that lung and brain pericytes are unique cell populations, and pericytes near the arteriole end of the capillary bed are involved in regulating cerebral blood flow while those in the middle of the capillary bed are more important for maintaining the blood-brain barrier [Citation22,Citation23]. They are also critical for the development of the BBB during embryogenesis, and the disruption of pericyte-endothelial cell interactions may lead to BBB dysfunction in the setting of CNS disease [Citation24]. Astrocytes interact with endothelial cells and pericytes via end-feet processes to modulate the expression and polarization of tight junction proteins and secrete factors that support the function of neighboring cells. Astrocytes also play a role in modulating cerebral blood flow to match glucose and oxygen supply with neuronal demand [Citation25].
Passive diffusion of molecules into the CNS is primarily determined by lipid solubility, which allows movement through the lipid bilayer of the endothelial cells that comprise the BBB [Citation26]. Dissolved gases such as oxygen and carbon dioxide move down their concentration gradients and do not require active transport. Paracellular diffusion limits the movement of even small molecules essential to CNS function, such as glucose and amino acids, and therefore, BBB solute carriers (SLCs) expressed in the endothelial cell membrane are necessary for their transport into the CNS. Notable examples include GLUT1 for glucose transport and LAT1 for large neutral amino acid transport. SLCs and tight junctions work together to establish and maintain molecular gradients across the BBB. In contrast, large molecules are transported across the BBB by endocytosis, which allows solutes such as peptides to enter the CNS. This mechanism may be unique to the endothelial cells of the BBB, as transcytosis here routes molecules away from the degradative lysosomal pathway, unlike in in peripheral tissues, which typically involves the lysosomal degradation pathway [Citation27].
Active transport across the BBB occurs via ATP-binding cassette (ABC) transporters. ABC transporters are a superfamily of proteins that are ATP-driven, membrane-bound transporters located on the luminal surface that transport small molecules back into circulation, and include P-glycoprotein (P-gp) and multidrug resistance-associated proteins (MRPs) [Citation28,Citation29]. Members of this superfamily of proteins have been implicated in the development of the multidrug resistance (MDR) phenotype. For example, MRP1 has been found in several CNS tumors including oligodendrogliomas, anaplastic astrocytomas, and GBM, and its degree of expression has been associated with higher grade gliomas [Citation28,Citation30]. Indeed, there is evidence that chemotherapeutics may induce MRP1 expression and contribute to resistance [Citation31]. Thus the efficacy of therapeutic agents may also be influenced by potential efflux in addition to entry mechanisms.
The CNS is thought to be an ‘immune-privileged’ site, but mechanisms for cellular entry into the CNS do exist. For example, during embryogenesis and in pathologic conditions, cells from the peripheral circulation derived from the monocyte lineage enter the brain via the BBB and differentiate into microglia. Furthermore, in the setting of an intact BBB, subsets of T and B cells have been observed to enter the CNS via diapedesis through endothelial cells limited to CSF spaces including the ventricular system and subarachnoid space, which in turn drain to deep cervical lymph nodes [Citation32,Citation33]. Interstitial fluid, distinct from CSF, drains from the CNS parenchyma along basement membranes in the walls of cerebral capillaries Moreover, dural lymphatics have been recently shown to drain CSF into deep cervical lymph nodes are separated from the CNS parenchyma by the glia limitans and the arachnoid blood-CSF barrier [Citation33]. Lastly, in the setting of pathological conditions such as infection, tight junctions may be opened and circulating macrophages and mononuclear leukocytes enter the CNS via transcellular or paracellular mechanisms [Citation34]. Dendritic cells have also been shown to migrate out of the CNS to peripheral tissues to activate T cells and initiate an immune response against CNS antigens [Citation35].
Blood-tumor barrier physiology
BBB dysfunction is a hallmark of most high-grade gliomas and intracranial metastases and is best illustrated by the appearance of these tumors on various conventional MR sequences. For example, extravasation of gadolinium, a hydrophilic contrast agent, on T1-weighted sequences is a marker of the physical disruption of the BBB and is seen in virtually all cases of high-grade glioma and metastatic CNS disease. In addition, beyond the contrast-enhancing portion, there is often a T2-hyperintense region indicating abnormal accumulation of fluid or vasogenic edema. These areas notably allow diffusion of fluid without the accumulation of gadolinium as seen in more central regions of the tumor. Positron emission tomography imaging of tracers such as 11C-methionine, 18F-fluoro-ethyl tyrosine, and 3,4-dihydroxy-6-18F-fluoro-l-phenylalanine have shown active transport of these molecules across the BBB and accumulate inside the tumor tissue. These studies suggest that there are varying degrees of BTB/BBB disruption in the setting of brain tumors [Citation36,Citation37].
A well-known characteristic of GBM is angiogenesis and neovascularization via a vascular endothelial growth factor (VEGF)-mediated mechanism that produces the immature, dilated, and leaky blood vessels of the blood-tumor barrier (BTB). In a mouse model of glioma, glioma cells displace the astrocyte end-feet from the endothelial surface, which in turn disrupts tight junctions and allows extravasation of various molecules from circulating blood into brain parenchyma [Citation38]. Consistently, downregulation of critical BBB tight junction proteins such as claudin-1 and -5 has been found in human glioblastoma [Citation39]. Aquaporins are membrane transport proteins that mediate water transport and play a key role in maintaining BBB integrity. Aquaporin-4 overexpression in astrocytes is believed to be a compensatory result of the loss of end-feet and increase in the volume of the perivascular space [Citation40]. Moreover, as glioma cells infiltrate, they hijack the autoregulatory function of native astrocytes and independently mediate vascular tone [Citation38]. The unique microenvironment created by the BTB, that of low oxygen tension and high interstitial pressure, is thought to contribute to selection of tumor cells with a more malignant phenotype [Citation41].
BTB permeability is a dynamic phenomenon that evolves throughout the process of tumorigenesis, proliferation, and infiltration. The neurovascular unit exhibits different properties in different regions of the tumor. The BTB is more permeable in the tumor core compared to the tumor periphery, and a nearly impermeable BBB can be seen in the surrounding normal parenchyma [Citation42]. Concordantly, imaging studies have demonstrated heterogeneous permeability to small and large molecules, and heterogeneous perfusion as seen on dynamic susceptibility contrast (DSC)-MRI [Citation43]. Anti-cancer agents have been described to have varying degrees of distribution across the normal BBB and BTB [Citation36]. Heterogeneous permeability of the BTB may be secondary to alterations in cell types that normally make up the neurovascular unit [Citation44]. In a model of metastatic breast cancer, increased expression of a subpopulation of pericytes was associated with increased local BTB permeability, suggesting that atypical cellular heterogeneity contributes to heterogeneous BBB/BTB permeability in these lesions [Citation45].
BTB permeability also varies between tumor types. Medulloblastoma, a childhood brain tumor, has vastly different prognosis depending on subtype. Phoenix et al. demonstrated that WNT-medulloblastoma, a curable form of medulloblastoma, exhibits paracrine signals mediated by mutant beta-catenin, which produces fenestrated endothelial cells that allows the intracellular accumulation of chemotherapeutics [Citation46]. In contrast, SHH-medulloblastoma, a more treatment-resistant subtype, exhibits a less permeable BTB, which is thought to play a role in its resistance to chemotherapy [Citation46]. These results suggest that BTB permeability is a heterogeneous phenomenon that impacts drug delivery, and therefore efficacy of treatment, to target tissues.
Circumventing the BBB and BTB
Under normal circumstances, the biochemical and mechanical properties of the BBB and BTB prevent the penetration of many therapeutic agents into the CNS [Citation42]. Although brain tumors can alter and even increase permeability of the BBB, these BTB effects are heterogeneous, and in any case, treating the region of near normal BBB permeability in the tumor periphery remains challenging [Citation47]. These regions can be visualized on T1-weighted MRI as those that do not exhibit gadolinium enhancement but may be hyperintense on T2-weighted sequences [Citation48]. Attempts to overcome the challenges of drug delivery to CNS tumors range from development of new drugs or drug combinations to mechanical disruption of the BBB and BTB.
Many pharmacologic agents are susceptible to multidrug resistance ABC transporters that actively efflux drugs out of the brain. Unfortunately, attempts to develop an effective drug efflux transporter blocker have failed in clinical trials [Citation49]. Osmotic blood-brain barrier disruption has also been attempted to circumvent the BBB and the BTB. In early experiments, Rapoport et al. injected intra-arterial mannitol to draw free water away from the endothelial cells and thus create an opening of the tight junctions for several hours during which chemotherapeutics could be administered [Citation4, Citation50]. More recent studies have investigated the use of mannitol to enhance delivery of stem cells and their growth factors across the BBB as well as combining mannitol to temozolomide to increase BBB permeability [Citation51,Citation52]. Unfortunately, the clinical benefit of utilizing chemotherapeutics in this manner has not been clearly established, and this method has been associated with toxicity, such as permanent hearing loss, which is thought to be due to the nonselective entry of inflammatory markers and resulting neurotoxicity as well as the unclear time course of BBB permeability in relation to mannitol administration [Citation53].
Besides chemical means to temporarily disrupt the BBB, ultrasound has also been used to mediate BBB permeability to enhance drug delivery and remains an active area of research. Focused ultrasound (FUS) with circulating microbubbles is able to noninvasively and transiently open the BBB in a controllable manner [Citation54,Citation55]. Low amplitude FUS causes repetitive expansion and contraction of microbubbles to induce shear stress on cells and eventually create pores through cell membranes [Citation56]. High amplitude FUS can create shockwaves and microjets, which may temporarily disrupt tight junctions, increase permeability, and enhance drug delivery [Citation57]. Preliminary results from a phase I/II clinical trial (NCT02253212) utilizing an implanted ultrasound device on patients with recurrent GBM show that this technique produces transient BBB disruption for the administration of carboplatin [Citation58]. At present, although a number of clinical trials are underway, few clinical applications for FUS have been developed in comparison to LITT ().
Table 1. Comparison of focused ultrasound (FUS) and laser interstitial thermal therapy (LITT).
More recently, bone marrow and X-linked non-receptor tyrosine kinase (BMX) inhibitor ibrutinib has been shown to target glioblastoma cell-derived pericytes—and not normal pericytes—to increase BTB permeability and chemotherapeutic efficacy [Citation44]. Thus, combining ibrutinib with traditionally brain-impermeant chemotherapeutics may represent a novel means of tumor-targeted drug delivery. Various other methods to improve drug penetration across the BBB have also been developed, such as receptor-mediated transcytosis (RMT) and adsorptive-mediated transcytosis (AMT). Although RMT has been a widely researched method, historically, its efficacy is limited by ligand competition and receptor saturation. In contrast, AMT is not hindered by these limiting factors. In one form of AMT, molecules can be conjugated to cell-penetrating peptides, small (<30 amino acid) peptides that are capable of crossing the BBB without significant membrane damage. For example, Angiopep (a 19-amino acid peptide) conjugated particles are based on modification with a ligand of the low density lipoprotein receptor related protein 1 (LRP1). These agents take advantage of the ability of LRP1 to mediate endocytosis of amyloid-beta peptides across the BBB [Citation8]. This technique has been utilized to facilitate delivery of doxorubicin in a mouse model of glioma, which was associated with improved median survival and low toxicity [Citation9]. In a study of mice implanted with breast tumor xenografts, intra-arterially administered Angiopep-2 conjugated to an anti-HER2 mAb was associated with an increased survival. Importantly, 60% of the administered dose was localized to the brain [Citation60].
Neurosurgical approaches have also been utilized to bypass the BBB and BTB. These include intracavitary drug injection through implanted devices such as the Ommaya reservoir and direct local delivery, such as Gliadel wafers [Citation5,Citation6].
Thermal therapy
The concept of treating malignancy with hyperthermia predates modern medicine, fueled by early anecdotal reports of tumor regression after systemic fever or infection from nineteenth century physicians. Preliminary studies on the use of hyperthermia as early as the 1890s relied on administration of pyrogenic toxins to elevate whole body temperature [Citation61]. Unfortunately these and other methods that rely on whole body hyperthermia are limited as temperature elevation remains modest, well below the critical 43 °C thermal death threshold for neoplastic cells, to prevent injury to normal tissue. Nonetheless, attempts to utilize whole body hyperthermia continued well into the twentieth century. Importantly, this led to the discovery of the synergistic impact of hyperthermia on BBB-impermeant chemotherapeutic agents, leading to the hypothesis that hyperthermia may disrupt the BBB [Citation62]. Unfortunately potentiation of chemotherapeutics in the setting of whole body hyperthermia was not localized to the CNS, resulting in increased toxicity to normal organs and tissue.
To overcome the limitations of whole-body hyperthermia, the use of targeted hyperthermia for treatment of glioblastoma was explored by Salcmann and Samaras in the early 1980s. In their pioneering work, human subjects with glioblastoma were implanted with a flexible wire microwave radiator that was connected transcutaneously to a 2450 MHz microwave generator to deliver heat up to a temperature of 45 °C to the tumor [Citation63,Citation64]. Although they were able to safely deliver treatments, the impact on survival was modest at best. Also, at the time, specifically delivering thermal therapy to tumor tissue without injuring the surrounding parenchyma was not technologically possible.
In 1983 Bown described LITT in brain tumor models using a neodymium:yttrium-aluminum-garnet (Nd:YAG) laser as another means of creating targeted thermal lesions [Citation14, Citation65–67]. Unfortunately, the technique was limited by inadequate cooling of the laser probe and the inability to accurately measure brain temperature to guide ablation.
The modern LITT procedure relies on several technologies that have since been developed to overcome these challenges. In the United States, two LITT systems are available: the NeuroBlate® System (Monteris Medical, Winnipeg, Manitoba, Canada) and the Visualase® Thermal Therapy System (Visualase Inc., Houston, Texas, USA). Although the two systems have several technical differences, the underlying biological principles are similar [Citation11,Citation68–70].
Litt
Energy from photons emitted by lasers applied intracranially has a variety of effects depending on the way in which it is applied. For example, using two-photon laser techniques, lesions as small as 15 µm have been created in the parenchyma to study the microglial response to intracranial injury and in the wall of CNS microvasculature to study hemorrhagic and ischemic stroke [Citation71,Citation72]. LITT is a clinical application of laser energy to create thermal lesions as large as 3 cm through conduction of heat to tissues distant from the laser probe. LITT is widely utilized in neuro-oncology due to its ability to effectively kill tumor cells. Photons emitted by the laser are absorbed by tumor cell chromophores, resulting in chromophore excitation followed by release of thermal energy and heating of surrounding tissue [Citation65,Citation73]. In order to achieve cell death, a minimum temperature must be maintained for a certain duration of time. Although heat capacity may vary between cell types and target organs, a specific thermal dose results in cellular necrosis and tissue coagulation. Thermal treatment of tumors takes advantage of the fact that tumor cells are more sensitive to thermal injury than normal cells. However, this therapeutic window is narrow, and accurate targeting and temperature measurement is therefore critical. In the case of CNS tumors, antineoplastic effects become apparent at 42 °C, while normal neurons become damaged at 43 °C [Citation74].
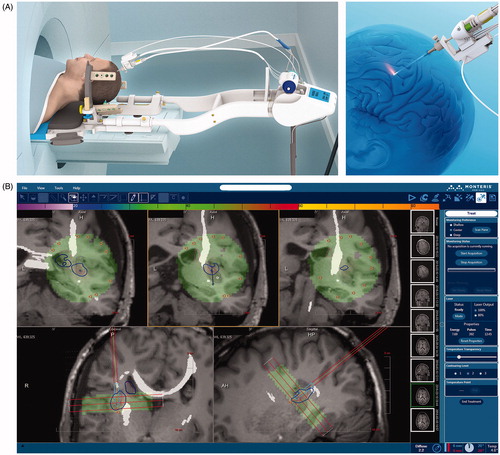
A LITT procedure is performed in conjunction with intraoperative MRI. Once the patient is placed under general anesthesia and intubated, the head is fixed in a rigid skull clamp and registered to a frameless stereotactic navigation system to plan the burr hole and trajectory of the laser probe. A skin incision and bur hole are made, after which the laser probe is inserted using stereotactic navigation. The patient’s head is then placed within the MRI bore with the probe in place (). An MRI scan is performed, allowing for monitoring of the position of the probe, which can be advanced or withdrawn during the procedure. Additionally, firing of the laser probe is interleaved with acquisition of MR images in real time. This information is integrated by a computer connected to the MRI scanner, allowing for measurement of tissue temperature using MR thermometry (). Using this technique, tissue temperature can be measured to within 1 °C with a spatial resolution of 1–2 mm [Citation15,Citation65]. Importantly, this allows for measurement of temperatures distant from the laser probe, allowing for ablation of the target lesion while sparing normal tissue. Using the Arrhenius equation, which predicts cell death as a function of tissue temperature and ablation time, the computer is able to display zones of tissue damage to ensure adequate ablation of the target lesion [Citation65,Citation70,Citation75].
Figure 2. Cranial laser interstitial thermal therapy. (A) Schematic depiction of the NeuroBlate® LITT delivery system (Monteris Medical, Winnipeg, Manitoba, Canada). The laser probe is stereotactically inserted through an approximately 0.5 cm skin incision and bur hole in the skull. The patient is then placed in the MRI scanner and the laser is activated remotely, with pulses interleaved with MRI acquisition for temperature monitoring. (B) Computer interface of the NeuroBlate® LITT delivery system (Monteris Medical, Winnipeg, Manitoba, Canada). MRI scans acquired while the probe is firing are overlaid in the computer interface with thermal damage threshold lines (blue TDT line shown here represents 43 °C for 10 min). As shown here, intraoperative MRI sequences can also be merged with previously acquired diffusion tensor MR imaging to highlight critical white matter tracts such as the corticospinal tract.
Although LITT is less invasive than open craniotomy and tumor debulking, it is not without risk. Reported complications of LITT include arterial injury, seizures, transient or permanent neurologic deficit, cerebrospinal fluid leak, and infection [Citation76,Citation77]. In a recent series of 102 patients treated with MR-guided LITT, 13.7% (n = 14) developed new deficits, 64.3% (n = 9) of which had complete resolution within 1 month post-op, 7.1% (n = 1) had partial resolution of symptoms, 14.3% (n = 2) did not have resolution of symptoms at the most recent follow-up, and 14.3% (n = 2) died without resolution of symptoms [Citation78]. Strategies to mitigate complications include the use of trajectories that avoid eloquent structures, staged procedures for larger lesions, and the use of steroids to reduce post-operative edema.
Impact of LITT on the blood-brain and blood-tumor barriers
Beyond tissue ablation, little is known about the biological impact of LITT on a cellular or molecular level in the CNS. Early work investigating the impact of diffuse thermal injury provides some insight into the biological impact of LITT on the BBB. In a rabbit model, hyperthermia to 42.5 °C was found to increase permeability to Trypan blue [Citation79]. Subsequently, in a rat model, Oscar et al. found increased permeability to inulin and D-mannitol, which are normally brain-impermeant, for up to 4 h after microwave hyperthermic therapy [Citation80]. In rats, whole head hyperthermia creates acute lesions in regions of the brain more susceptible to hyperthermia. Using this model, Urakawa et al. studied the structure of acute thermal lesions with electron microscopy. Like the thermal injury pattern produced by LITT, the authors identified 3 concentric regions of thermal lesions: a necrotic center, a surrounding reactive zone and a rim of viable brain tissue. In both the reactive zone and viable rim, pial and cortical vessels were permeable to intravenous horse radish peroxidase, a BBB tracer, and astrocyte end-feet were observed to be swollen. Importantly, increased BBB permeability was observed up to 3 days after treatment, indicating thermal therapy may create a therapeutic window of time for delivery of adjuvant therapies [Citation81].
More recently, Leuthardt et al. found elevated serum levels of brain specific enolase, which is normally restricted to the CNS, after laser ablation in patients with recurrent glioblastoma, suggesting that CNS permeability is indeed increased by LITT. Importantly, peak permeability appears to occur 1–2 weeks after ablation and resolves by 4–6 weeks. Additionally, post-LITT MRI findings suggest that the region of increased permeability includes a rim of parenchyma surrounding the treated lesion [Citation10]. Thus LITT may create a window of time during which drugs may be administered to treat residual, surrounding infiltrative disease. The release of proteins normally confined to the CNS, such as brain specific enolase, into the systemic circulation after LITT may also have implications for the immune response after laser ablation that remain to be investigated.
Our data from a LITT mouse model also suggests that laser ablation transiently increases BBB/BTB permeability, with peak permeability occurring within 1 week and lasting up to 30 days after ablation. Importantly, molecules as large as human IgG (approximately 150 kDa) are able to enter the CNS after LITT. At the cellular level, increased permeability appears to be mediated by increased transcytosis and/or tight junction disruption. In vivo, LITT increased the brain permeability of intravenous doxorubicin, and LITT in combination with doxorubicin was associated with improved survival when compared to either treatment alone [Citation82]. Related to this finding, we have two ongoing clinical trials investigating the use of the combination of LITT and doxorubicin in adult and pediatric populations (NCT01851733 and NCT02372409, respectively).
Future directions
Several studies utilizing LITT in the treatment of brain lesions have demonstrated feasibility and potential complications, but future studies are needed with a larger number of patient cohorts to determine long-term clinical outcomes. The understanding of LITT to transiently affect the BBB or BTB is in its infancy. At this point, only small series that illustrate the spatiotemporal relationship of LITT with BBB/BTB disruption have been described, and mechanisms of this phenomenon are just beginning to be understood. Future directions may include investigating the relationship between thermal dosage and the spatiotemporal relationship of the diffusion of molecules various sizes across the BBB/BTB. Further understanding in this area could lead to enhancing the sensitivity and specificity of LITT on target tissue of interest to maximize efficacy and minimize complications.
Conclusion
The BBB and BTB are comprised primarily of pericytes, endothelial cells and astrocyte end-feet which combine to form a restrictive barrier to the CNS. Techniques to overcome the BBB and BTB, which prevent the effective delivery of drugs to the CNS, are an area of intense investigation. Laser ablation is a thermal therapy technique that may increase BBB and BTB permeability in a targeted fashion, opening the possibility of combination therapies that may more effectively treat CNS tumors.
Disclosure statement
AHK received research grants from Monteris Medical and Stryker.
Additional information
Funding
References
- Langen UH, Ayloo S, Gu C. Development and cell biology of the blood-brain barrier. Annu Rev Cell Dev Biol. 2019;35:591–613.
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507.
- Thomsen MS, Routhe LJ, Moos T. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab. 2017;37(10):3300–3317.
- Rapoport SI. Osmotic opening of the blood–brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol. 2000;20(2):217–230.
- Sandberg DI, Bilsky MH, Souweidane MM, et al. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery. 2000;47(1):49–54. discussion 54–45
- Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (bcnu) wafers (gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88.
- Menei P, Jadaud E, Faisant N, et al. Stereotaxic implantation of 5-fluorouracil-releasing microspheres in malignant glioma. Cancer. 2004;100(2):405–410.
- Demeule M, Regina A, Che C, et al. Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther. 2008;324(3):1064–1072.
- Ren J, Shen S, Wang D, et al. The targeted delivery of anticancer drugs to brain glioma by pegylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33(11):3324–3333.
- Leuthardt EC, Duan C, Kim MJ, et al. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):e0148613
- LaRiviere MJ, Gross RE. Stereotactic laser ablation for medically intractable epilepsy: the next generation of minimally invasive epilepsy surgery. Front Surg. 2016;3:64
- Salehi A, Kamath AA, Leuthardt EC, et al. Management of intracranial metastatic disease with laser interstitial thermal therapy. Front Oncol. 2018;8:499.
- Kamath AA, Friedman DD, Akbari SHA, et al. Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: safety, efficacy, and outcomes. Neurosurgery. 2019;84(4):836–843.
- Bown SG. Phototherapy in tumors. World J Surg. 1983;7(6):700–709.
- De Poorter J, De Wagter C, De Deene Y, et al. Noninvasive mri thermometry with the proton resonance frequency (prf) method: in vivo results in human muscle. Magn Reson Med. 1995;33(1):74–81.
- Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25.
- O'Brown NM, Pfau SJ, Gu C. Bridging barriers: a comparative look at the blood-brain barrier across organisms. Genes Dev. 2018;32(7-8):466–478.
- Wolburg H, Wolburg-Buchholz K, Kraus J, et al. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105(6):586–592.
- Hamm S, Dehouck B, Kraus J, et al. Astrocyte mediated modulation of blood-brain barrier permeability does not correlate with a loss of tight junction proteins from the cellular contacts. Cell Tissue Res. 2004;315(2):157–166.
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185.
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2:5.
- Attwell D, Mishra A, Hall CN, et al. What is a pericyte? J Cereb Blood Flow Metab. 2016;36(2):451–455.
- Vanlandewijck M, He L, Mae MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554(7693):475–480.
- Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566.
- Wong AD, Ye M, Levy AF, et al. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7
- Liu X, Tu M, Kelly RS, et al. Development of a computational approach to predict blood-brain barrier permeability. Drug Metab Dispos. 2004;32(1):132–139.
- Nag S, Begley D. Blood-brain barrier, exchange of metabolites and gases. Pathol Genet. Cerebrovas Dise. 2005:22–29.
- Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58(2):140–161.
- Zhao Z, Nelson AR, Betsholtz C, et al. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163(5):1064–1078.
- Mohri M, Nitta H, Yamashita J. Expression of multidrug resistance-associated protein (mrp) in human gliomas. J Neurooncol. 2000;49(2):105–115.
- Abe T, Mori T, Wakabayashi Y, et al. Expression of multidrug resistance protein gene in patients with glioma after chemotherapy. J Neurooncol. 1998;40(1):11–18.
- Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109(2):181–190.
- Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the cns. Nat Immunol. 2017;18(2):123–131.
- Konsman JP, Drukarch B, Van Dam A-M. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin Sci. 2007;112(1):1–25.
- Karman J, Ling CY, Sandor M, et al. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173(4):2353–2361.
- Sarkaria JN, Hu LS, Parney IF, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-oncology. 2018;20(2):184–191.
- Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Primers. 2019;5(1):5
- Watkins S, Robel S, Kimbrough IF, et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014;5:4196
- Liebner S, Fischmann A, Rascher G, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100(3):323–331.
- Noell S, Ritz R, Wolburg-Buchholz K, et al. An allograft glioma model reveals the dependence of aquaporin-4 expression on the brain microenvironment. PLoS One. 2012;7(5):e36555
- Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622.
- Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341.
- Santarosa C, Castellano A, Conte GM, et al. Dynamic contrast-enhanced and dynamic susceptibility contrast perfusion mr imaging for glioma grading: preliminary comparison of vessel compartment and permeability parameters using hotspot and histogram analysis. Eur J Radiol. 2016;85(6):1147–1156.
- Zhou W, Chen C, Shi Y, et al. Targeting glioma stem cell-derived pericytes disrupts the blood-tumor barrier and improves chemotherapeutic efficacy. Cell Stem Cell. 2017;21(5):591–603 e594.
- Lyle LT, Lockman PR, Adkins CE, et al. Alterations in pericyte subpopulations are associated with elevated Blood-Tumor Barrier Permeability in Experimental Brain Metastasis of Breast Cancer. Clin Cancer Res. 2016;22(21):5287–5299.
- Phoenix TN, Patmore DM, Boop S, et al. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell. 2016;29(4):508–522.
- Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood-brain barrier: chemical modifications of drugs or drug-nanoparticles? Drug Discov Today. 2008;13(23–24):1099–1106.
- la Fougere C, Suchorska B, Bartenstein P, et al. Molecular imaging of gliomas with pet: opportunities and limitations. Neuro-oncology. 2011;13(8):806–819.
- Van Tellingen O, Yetkin-Arik B, De Gooijer M, et al. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12.
- Siegal T, Rubinstein R, Bokstein F, et al. In vivo assessment of the window of barrier opening after osmotic blood-brain barrier disruption in humans. J Neurosurg. 2000;92(4):599–605.
- Gonzales-Portillo GS, Sanberg PR, Franzblau M, et al. Mannitol-enhanced delivery of stem cells and their growth factors across the blood-brain barrier. Cell Transplant. 2014;23(4–5):531–539.
- Choi C, Kim HM, Shon J, et al. Additional increased effects of mannitol-temozolomide combined treatment on blood-brain barrier permeability. Biochem Biophys Res Commun. 2018;497(2):769–775.
- Williams PC, Henner WD, Roman-Goldstein S, et al. Toxicity and efficacy of carboplatin and etoposide in conjunction with disruption of the blood-brain tumor barrier in the treatment of intracranial neoplasms. Neurosurgery. 1995;37(1):17–27. discussion 27–18
- Hynynen K. Hyperthermia-induced drug delivery in humans. Nat Biomed Eng. 2018;2(9):637–639.
- Abrahao A, Meng Y, Llinas M, et al. First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using mr-guided focused ultrasound. Nat Commun. 2019;10(1):4373
- Sboros V. Response of contrast agents to ultrasound. Adv Drug Deliv Rev. 2008;60(10):1117–1136.
- Mitragotri S. Innovation - healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4(3):255–260.
- Carpentier A, Canney M, Vignot A, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8:343re342.
- Regina A, Demeule M, Tripathy S, et al. Ang4043, a novel brain-penetrant peptide-mab conjugate, is efficacious against her2-positive intracranial tumors in mice. Mol Cancer Ther. 2015;14(1):129–140.
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. 1. Am J Med Sci (1827–1924). 1893;105(5):487–510.
- Hahn GM. Potential for therapy of drugs and hyperthermia. Cancer Res. 1979;39(6 Pt 2):2264–2268.
- Salcman M, Samaras GM. Hyperthermia for brain tumors: biophysical rationale. Neurosurgery. 1981;9(3):327–335.
- Salcman M, Samaras GM. Interstitial microwave hyperthermia for brain tumors. Results of a phase-1 clinical trial. J Neurooncol. 1983;1(3):225–236.
- Rahmathulla G, Recinos PF, Kamian K, et al. Mri-guided laser interstitial thermal therapy in neuro-oncology: a review of its current clinical applications. Oncology. 2014;87(2):67–82.
- Hawasli AH, Kim AH, Dunn GP, et al. Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus. 2014;37(6):E1.
- Mohammadi AM, Hawasli AH, Rodriguez A, et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med. 2014;3(4):971–979.
- Hawasli AH, Ray WZ, Murphy RK, et al. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for subinsular metastatic adenocarcinoma: technical case report. Oper Neurosurg. 2011;70:onsE332-onsE338.
- Torres-Reveron J, Tomasiewicz HC, Shetty A, et al. Stereotactic laser induced thermotherapy (litt): a novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol. 2013;113(3):495–503.
- Mohammadi AM, Schroeder JL. Laser interstitial thermal therapy in treatment of brain tumors-the NeuroBlate System. Expert Rev Med Devices. 2014;11(2):109–119.
- Davalos D, Grutzendler J, Yang G, et al. Atp mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758.
- Nishimura N, Schaffer CB, Friedman B, et al. Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nat Methods. 2006;3(2):99–108.
- Stafford RJ, Fuentes D, Elliott AA, et al. Laser-induced thermal therapy for tumor ablation. Crit Rev Biomed Eng. 2010;38(1):79–100.
- Pettigrew RT, Galt JM, Ludgate CM, et al. Circulatory and biochemical effects of whole body hyperthermia. Br J Surg. 1974;61(9):727–730.
- Laidler KJ. The development of the arrhenius equation. J Chem Educ. 1984;61(6):494–498.
- Pruitt R, Gamble A, Black K, et al. Complication avoidance in laser interstitial thermal therapy: lessons learned. J Neurosurg. 2017;126(4):1238–1245.
- Salem U, Kumar VA, Madewell JE, et al. Neurosurgical applications of mri guided laser interstitial thermal therapy (litt). Cancer Imaging. 2019;19(1):65.
- Patel P, Patel NV, Danish SF. Intracranial mr-guided laser-induced thermal therapy: single-center experience with the visualase thermal therapy system. J Neurosurg. 2016;125(4):853–860.
- Allen IV. Cerebral changes in experimental heatstroke. Ir J Med Sci. 1964;465:413–422.
- Oscar KJ, Hawkins TD. Microwave alteration of the blood-brain barrier system of rats. Brain Res. 1977;126(2):281–293.
- Urakawa M, Yamaguchi K, Tsuchida E, et al. Blood-brain barrier disturbance following localized hyperthermia in rats. Int J Hyperthermia. 1995;11(5):709–718.
- Salehi A, Paturu MR, Patel B, et al. Therapeutic enhancement of blood-brain and blood-tumor barrier permeability by laser interstitial thermal therapy. Neuro Oncol Adv. 2020;2.
- Lee EJ, Fomenko A, Lozano AM. Magnetic resonance-guided focused Ultrasound: current status and future perspectives in thermal ablation and blood-brain barrier opening. J Korean Neurosurg Soc. 2019;62(1):10–26.
