Abstract
Objective
To evaluate complications after consecutive 100 sessions of cone-beam computed tomography (CBCT)-guided radiofrequency ablation (RFA) of lung tumors
Materials and methods
A retrospective study was conducted from January 2016 and October 2018. All procedures were performed using a CBCT virtual navigation guidance system, combining three-dimentional CBCT, needle planning software, and real-time fluoroscopy. Complications were evaluated for each RFA session in 63 consecutive patients (31 male, 32 female; mean age 58.0 years) with 121 lung tumors who underwent 100 sessions of CBCT-guided lung ablation with an internally cooled RFA system. Complications were recorded using the Common Terminology Criteria of Adverse Events (CTCAE) 5.0. A major complication was defined as a grade 3 or 4 adverse event.
Results
There was no postprocedural mortality. The major and minor complication rates were 5% and 28%, respectively. The major complications were significant pulmonary hemorrhage (1%), large hemothorax requiring drainage (1%), pneumonia treated with antibiotics (2%), and delayed bronchopleural fistula (1%). The minor complications were pneumothorax (15%), hemoptysis (11%), and subcutaneous emphysema (2%). Of the 15 pneumothoraces, percutaneous catheter drainage was required in six sessions. Pneumothorax was more likely to occur if RFA was performed on two or more tumors at one session. Immediate, periprocedural and delayed complications were 23%, 9%, and 1%, respectively.
Conclusion
CBCT-guided RFA of lung tumors is a relatively safe procedure with acceptable morbidity.
Introduction
Lung radiofrequency ablation (RFA) is a safe and effective minimally invasive treatment option for primary and metastatic lung tumors [Citation1–3]. It is especially useful for poor surgical candidates due to poor physiologic or oncologic conditions. The most widely accepted imaging modality for guiding lung RFA has been conventional multi-detector computed tomography (MDCT) [Citation4,Citation5]. However, there are several limitations to the MDCT-guided procedure, including a lack of real-time monitoring and the physical and spatial factors caused by the CT gantry [Citation6]. Cone-beam CT (CBCT) is an image guidance modality, which is well-suited to the interventional procedure, and provides both real-time fluoroscopy and three-dimensional CT-like imaging [Citation7]. In addition, a new state-of-the-art CBCT virtual navigation guidance system merges CBCT with dedicated needle planning software and real-time fluoroscopy. It provides a virtual needle pathway to the target nodule, helping operators navigate the needle into the target after the operator determines the skin entry site and destination target based on preprocedural CBCT data [Citation8].
CBCT has been used for guiding transthoracic procedures such as biopsy and thermal ablation [Citation8–14]. Some studies reported that there was no significant difference in complications between the CBCT group and MDCT or MDCT-fluoroscopy group for percutaneous transthoracic lung biopsy [Citation10,Citation11]. Complications associated with lung RFA have been reported in many studies, thereby leading to major limitations with this technique [Citation15–18]. However, the reported studies mainly addressed complications of lung RFA using conventional MDCT or MDCT-fluoroscopy. There exist only a few studies on lung RFA under CBCT guidance, and these studies were limited to specific groups or involved small samples [Citation12–14]. Therefore, it is difficult to establish a clear true complication rate of the CBCT-guided lung RFA.
In our hospital, lung RFA service was set up in 2016, and since then, lung RFA was performed under CBCT guidance. We retrospectively reviewed the incidence and characteristics of the complications after 100 sessions of CBCT-guided lung RFA.
Materials and methods
Patients
This single-center retrospective study was performed between January 2016 and October 2018 and included all consecutive patients with primary or metastatic lung tumors referred for percutaneous RFA. Our institutional review board approved this study. Before lung ablation, all patients were routinely clinically staged by physical examination, laboratory studies, and radiologic imaging studies. Primary lung cancers were diagnosed from biopsy and metastatic lung tumors were diagnosed from either imaging findings or biopsy. The procedure was performed on patients with unresectable tumors due to previous pulmonary resection, poor cardiopulmonary function, multiple tumors, or refusal to undergo surgery. The patients who were considered unsuitable for RFA included the ones with active infection, coagulation abnormalities, lung failure, and an Eastern Cooperative Oncology Group performance score >2.
Procedure
All ablations were performed on an inpatient basis under a combination of sedation and analgesia with midazolam and fentanyl. General anesthesia was not used in any case. Local anesthesia (1% lidocaine hydrochloride injection) was used for the procedure. Prophylactic antibiotics were not routinely administered. The patient was positioned prone or supine, depending on the location of the tumor. Although lung RFA has been performed at our hospital since 2016, the procedures were performed by a single interventional radiologist with more than 10 years of experience in percutaneous RFA of abdominal organ and percutaneous transthoracic needle biopsy.
Lung RFA was performed with a CBCT virtual navigation guidance system (CBCT system with virtual guiding software: XperCT and XperGuide software, Allura Xper FD20; Philips Healthcare, Best NL). CBCT acquisition protocols were used with the C-arm in propeller/head position. Applied parameters of image acquisition were rotation speed 55°/s; rotation duration 4.1 s; total arc trajectory range, 240°; matrix size 1024 × 1024; and automatic exposure control. Acquisition frames were transferred to the 3D angiography workstation. Each 3D image covered a volume of around 250 × 200 × 200 mm.
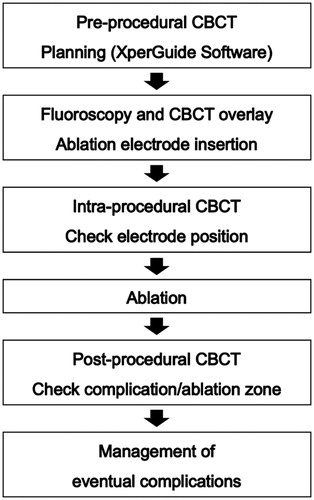
The pre-procedural CBCT was done for adequate planning of the lung RFA. Using the virtual guiding software (XperGuide), the operator established the target lesion (‘target point’) and decided the needle entry point at the skin surface. These two points thus defined a virtual pathway to the center of the tumor. On the basis of this planning, a live fusion image of fluoroscopy and the relevant double oblique slice of the CBCT with the virtual pathway was created, with appropriate C-arm angulation for an ‘entry-point view’ and a ‘progression view’ [Citation19]. The ablation electrode was then inserted to the right depth under real-time fluoroscopy and the CBCT slice overlay, first in an ‘entry-point view’ and then in an orthogonal projection. During the progression of the electrode, the patient was asked to stop breathing when the outline of the diaphragm or the rib cage on the fluoroscopy overlay matches the CBCT slice. After electrode positioning, a CBCT scan was performed to check the correct placement of the electrode ().
Figure 1. CBCT-guided lung RFA with virtual guidance in a 62-year-old woman. (a) CT image shows an 8-mm nodule (arrow) in the left lower lobe. (b) Virtual-guided planning for a safe needle route (green line) to the target lesion was performed. (c) When CBCT moves automatically until the entry-point view (i.e., vertical alignment from the entry site [red circle] to the target lesion [green circle] with virtual color), spots can be seen on the fluoroscopic image. (d) CBCT was performed after electrode placement to confirm the exact location of the needle tip.
![Figure 1. CBCT-guided lung RFA with virtual guidance in a 62-year-old woman. (a) CT image shows an 8-mm nodule (arrow) in the left lower lobe. (b) Virtual-guided planning for a safe needle route (green line) to the target lesion was performed. (c) When CBCT moves automatically until the entry-point view (i.e., vertical alignment from the entry site [red circle] to the target lesion [green circle] with virtual color), spots can be seen on the fluoroscopic image. (d) CBCT was performed after electrode placement to confirm the exact location of the needle tip.](/cms/asset/89049946-3be7-498b-91d9-e4121e61bfdb/ihyt_a_1784472_f0001_c.jpg)
We used a RF 200 W generator (VIVA RF generator, STARmed, Korea) and internally cooled 17-gauge electrodes (Octopus, STARmed, Korea) with a 2.5 cm or 3 cm active tip. Initially, the RF power was set at 30 W and then gradually increased to obtain a temperature of 90 °C. The ablation time was 10–14 min at each site of the tumor. When the target tumor was completely covered with ground-glass opacity (representing the ablation zone) on intra-procedural CBCT, the ablation was terminated by removing the electrode. Post-procedural CBCT was performed immediately and one day after the RFA procedure to evaluate the ablation zone and procedural complications such as pneumothorax, hemothorax, or parenchymal hemorrhage. Technical success was defined as satisfactory electrode placement, completion of ablation protocol, and complete coverage of the target tumor with ablation zone on post-procedural CBCT. The minimal ablation margin was evaluated based on pre-procedural CBCT obtained on the day of ablation and post-procedural CBCT obtained on the day after ablation. The distances between the tumor and reliable anatomic landmarks were measured on pre and post-procedural CBCT [Citation20]. The subtracted distance was used to assess the ablation margin and the smallest value was considered to be the minimal ablation margin. The minimal ablation margin size was categorized as either <5 mm or ≥5 mm. Each step is described in .
Complications
Complications were defined according to the Common Terminology Criteria for Adverse Events version 5.0 [Citation21]. Any patient death within 30 days of imaging-guided tumor ablation was considered a grade 5 adverse event. The grade 3 or 4 adverse events were defined as a major complication. Grade 1 or 2 adverse events were defined as minor complications.
Follow-up
All patients were followed up at 1, 3, 6, and 12 months, and thereafter at 6-month intervals with contrast-enhanced chest CT. The diagnostic criteria of local tumor progression was similar to those that have previously been described in the literature [Citation22]. In short, evidence of local tumor progression was considered to be present if the ablation zone was circumferentially enlarged or there was the appearance of a nodular or eccentric enhancement focus in the ablation zone.
Statistical analysis
All statistical analyses were performed with descriptive statistics using SPSS software version 18.0 (SPSS, Chicago, IL, USA). Between the groups comparisons were made using the chi-square test and p-values of less than 0.05 were considered statistically significant.
Results
From January 2016 to October 2018, 63 consecutive patients (31 men and 32 women) with a mean age of 58.0 years (range 31–81) and with a total of 121 tumors underwent percutaneous RFA in 100 ablation sessions. The mean tumor size was 12.8 mm (range, 4–50 mm). Of the ablated tumors, 26 tumors (21.5%) were primary lung cancers (all non-small cell carcinomas), whereas 95 tumors (78.5%) were lung metastases from various organs (colorectal, head and neck, kidney, liver, uterus, ovary, and stomach). There were 17 cases of two lesions ablated at one session. There were 2 cases of three lesions ablated at one session and both of these patients had metastatic tumors from head and neck cancer. Of the 100 sessions, 69 sessions had at least one invisible target tumor on fluoroscopy alone. Multiple electrode ablation or overlapping ablation was performed in 21 ablation sessions. Complications occurred in 33 of 100 sessions. Of the 69 sessions with fluoroscopy-invisible tumors and the remaining 31 sessions, there were 22 (31.9%) and 11 (39.3%) sessions with complications, respectively (p = 0.723). Among the multiple electrode ablation procedures, 4 complications occurred, all of which were grade 1 complications (1 pneumothorax, 3 hemoptysis). Immediate termination of the ablation session before complete ablation was decided in three cases because of intra-procedural complication such as severe pulmonary hemorrhage (n = 1) or large pneumothorax (n = 2). The data are summarized in .
Table 1. Characteristics of the 100 RFA sessions in 63 patients with a total of 121 tumors.
Major complications
Major complications occurred in 5 of all 100 RFA sessions. These included one case of pulmonary parenchymal hemorrhage treated in the intensive care unit (ICU), one case of hemothorax requiring percutaneous catheter drainage, two cases of pneumonia treated with antibiotics, and one case of delayed bronchopleural fistula treated with surgical repair. All the major complications were grade 3 and no procedure-related death occurred ().
Table 2. Major Complications in 100 Lung Radiofrequency Ablation (RFA) Sessions.
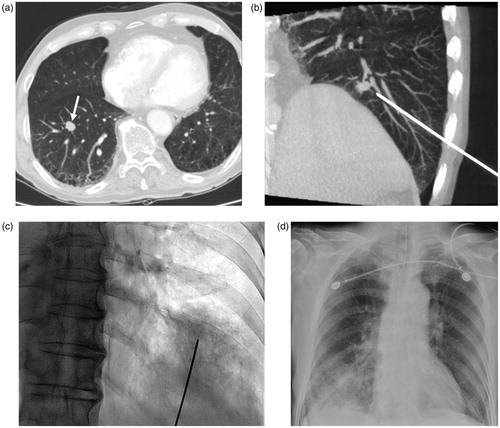
In the case of pulmonary hemorrhage, the patient presented with excessive hemoptysis, hypoxia, and decreased systolic blood pressure (below 80 mmHg) during the procedure. Immediate termination of the ablation session was decided and the patient was admitted to the ICU. Plain chest radiography revealed consolidation due to pulmonary hemorrhage at the site of the procedure. The pulmonary hemorrhage eventually recovered after about 9 days of conservative treatment. Subsequently, the patient demonstrated clinical improvement and was discharged ().
Figure 3. An 81-year-old man underwent RF ablation of pulmonary metastasis from colorectal carcinoma in the anterior basal segment of the right lower lobe. (a) Chest CT obtained before RFA shows the tumor (arrow), which demonstrates the intimate relation of central pulmonary lesions with the blood vessels, thereby increasing the risk of pulmonary hemorrhage. (b) CBCT image during insertion of RF electrode. Notice traversing the intervening pulmonary vessels by the needle electrode. (c) C-arm fluoroscopy image showed the extent of intra-parenchymal hemorrhage after the insertion of the electrode. Immediate termination of the ablation session was decided because of excessive hemoptysis and deterioration of the clinical condition of the patient. The patient was treated in the intensive care unit. (d) After 3 h, progressive increase in pulmonary hemorrhage was observed in chest plain radiography.
The patient who developed hemothorax was readmitted after 8 days of the lung ablation. Percutaneous catheter drainage (PCD) was performed on the hemothorax and more than 1000 cc of blood was drained. After 5 days of PCD, the catheter was removed and the patient was discharged.
The two patients who developed pneumonia had a fever higher than 39 °C and were treated by prolonged administration of antibiotics. These patients received about 2 weeks of prolonged hospital stay for this complication and were discharged without obvious sequelae.
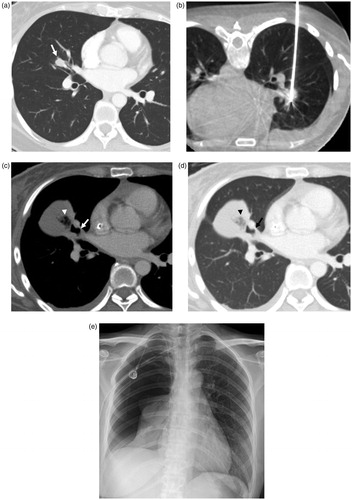
In the case of delayed bronchopleural fistula, the tumor was centrally located in the right middle lobe. In the supine position, it was difficult to avoid bronchovascular structure and breast tissue near the nipple. Therefore, the procedure was performed in prone position; the RF electrode traversed the major fissure. The patient had large pneumothorax due to delayed bronchopleural fistula 82 days after the procedure and eventually underwent surgical repair ().
Figure 4. Bronchopleural fistula developed after lung radiofrequency ablation in a 34-year-old woman with an intrapulmonary metastasis from ovarian cancer. (a) Chest CT obtained before RFA demonstrates the tumor (arrow) in the right middle lobe. (b) There is an electrode placement through the tumor under CBCT guidance. (c, d) Computed tomography image obtained 51 days after ablation shows collection of fluid and air (arrowhead) in the right major fissure and adjacent bronchus (arrow), suggesting the possibility of bronchopleural fistula. (e) Plain chest radiograph obtained 82 days after ablation shows large pneumothorax. The bronchopleural fistula was closed after surgical repair.
Minor complications
In the 100 RFA sessions, minor complications developed after 28 sessions (). Pneumothorax was the most common complication and occurred in 15 cases. Pneumothorax was divided into large and small based on >2 cm or ≤2 cm between the lung margin and the chest wall at the level of the hilum [Citation23]. In the case of small pneumothorax, conservative treatment including oxygen administration was given. In patients with a large pneumothorax, percutaneous catheter drainage was performed. Of the 15 pneumothoraces, percutaneous catheter drainage was required in six sessions. In two cases requiring PCD, large pneumothorax developed during the ablation procedure and the lung partly collapsed, so the procedure was terminated before complete ablation of the tumor. In six of the 15 pneumothorax cases (40%) and 13 of the 85 non-pneumothorax cases (15.3%), RFA was performed on two or more tumors at one session. This difference was statistically significant (p = 0.025). Eleven pneumothorax cases occurred in the first 50 ablation sessions. There was one case with pulmonary emphysema of all pneumothorax cases. There was no case of traversal of the major pulmonary fissure by an electrode. The visibility of the target tumor on fluoroscopy did not show a significant relationship with the incidence of pneumothorax. (12 cases [17.4%] of 69 sessions with fluoroscopy-invisible tumors vs. 3 cases [9.7%] of the remaining 31 sessions, p = 0.318)
Table 3. Minor Complications in 100 Lung Radiofrequency Ablation (RFA) Sessions.
Hemoptysis was the next most common complication and occurred in 11 cases. Ten cases of hemoptysis were self-limiting and lasted only for 3 days at the most. One patient who was re-admitted for hemoptysis exhibited improvement with conservative treatment such as adequate hydration and continuous monitoring of the vital signs. Seven cases of hemoptysis occurred in a lesion with the electrode traversing the lung tissue by >3 cm where this lesion was closer to the hilum. In three cases, the electrode tract penetrating the lung tissue was less than 2.5 cm, but the tumor was surrounded by blood vessels larger than 2 mm in diameter. Two cases of more than one lesion were ablated at one session. Five cases of hemoptysis occurred in the first 50 ablation sessions.
Subcutaneous emphysema occurred after two ablation sessions. Both the patients were clinically asymptomatic and treatment was not considered as essential.
Ablation margin and efficacy
Initial technical success was observed in 118 of 121 lesions on CBCT obtained during and the day after ablation procedure, except for three cases in which RFA was terminated before complete ablation due to complications. Minimal ablation margin of at least 5 mm was achieved in 92/118 (78%) of the ablation zones and 26 (22%) had an ablation margin of less than 5 mm. All patients survived at 6 months and 5 out of 118 lesions (4.2%) showed local recurrence at the ablation site during the first 6 months. All five lesions with local recurrence had minimal ablation margin less than 5 mm. Of these, 3 lesions locally recurred at the site of the ablation margin less than 5 mm, and these ablation margins were located at the tumor edge adjacent to the blood vessel.
Discussion
In our study, the major complication rate was 5% and minor complication rate was 28%. Moreover, 75% (21 cases) of minor complications were mild/grade 1 complications with clinical observation alone. In a retrospective study of 1000 lung RFA sessions, Kashima et al. reported 9.8% of major complications and 59.8% of minor complications [Citation17]. The mortality rate within 30 days of their study was 0.4%. According to a recent meta-analysis of lung RFA, the pooled rates of major and minor complications were 6% (95%CIs: 3%–8%) and 27% (95%CIs: 14%–41%), respectively [Citation24]. In light of these studies, lung RFA is considered as a safe modality for treating lung tumors.
Pneumothorax is the most frequent complication after percutaneous lung RFA, with incidences ranging from 11% to 52% [Citation16–18,Citation25–28]. A recent meta-analysis by Kennedy et al. stated that there are several risk factors for pneumothorax, including increased age, male gender, no history of lung surgery, number of tumors ablated, and increased length of electrode depth [Citation29]. In addition, several studies have reported in common that pulmonary emphysema was a risk factor for pneumothorax [Citation16,Citation26–28]. In our study, pneumothorax occurred in 15% of the patients and six of these cases required percutaneous catheter drainage. These results compare favorably with recent large-scale studies and meta-analysis suggesting that pneumothorax occurs in 37% to 38.4% of the procedure, with the need for aspiration or chest tube drainage in 29% to 31.9% [Citation18,Citation29]. The number of tumors ablated at one session was significantly associated with pneumothorax in our study. Moreover, about 73% of the pneumothorax cases occurred in first 50 ablation sessions, indicating that there may be a learning curve to reduce this complication.
The incidence of hemorrhagic complications of lung RFA is reported up to 18%, and these complications are manifested as self-limiting alveolar hemorrhage, hemoptysis or hemothorax [Citation16,Citation25,Citation30]. Although most of the hemoptysis typically resolves spontaneously, the hemorrhages can rarely be massive and even fatal [Citation30,Citation31]. Therefore, practitioners of lung ablation should be aware of the risk of pulmonary hemorrhage. Risk factors for intra-parenchymal hemorrhage include lesions <1.5 cm in diameter, basal and middle lung zone lesions, the needle track traversing the lung parenchyma by >2.5 cm, traversing pulmonary vessels in the track of ablation, and the use of multi-tined electrodes [Citation30]. In our study, there were 13% of hemorrhagic complications, most of which were self-limiting, but there was one case of massive hemoptysis and another case of large hemothorax. In eleven cases, including the massive hemoptysis case, the tumor was located close to the hilum or pulmonary vessels larger than 2 mm in diameter. To reduce the risk of pulmonary hemorrhage, the electrode should be inserted parallel to the blood vessels to avoid traversing the blood vessels. In addition, physicians should be aware of hemothorax caused by the intercostal arterial injury.
The incidence of post-ablation pneumonia ranged from 1.8% to 5.7% in the previous studies for lung RFA with a patient series of more than 100 [Citation15,Citation17,Citation18,Citation32]. In our study, we obtained a similar result with a rate of 2%. If the body temperature is higher than 38.5 °C for 5 days after the procedure, pneumonia should be suspected. According to the sputum or blood culture results or clinical findings, antibiotics may be needed and such treatment should be done carefully and early to prevent abscess.
Bronchopleural fistula is a rare but severe complication of lung RFA [Citation17,Citation33,Citation34]. According to the pathogenetic mechanism of spontaneous pneumomediasinum described by Macklin, free air can leak from ruptured alveoli due to increased air pressure applied to alveoli [Citation35]. This causes emphysema to spread from the bronchovascular tissue sheath to the mediastinum. Similarly, bronchopleural fistula occurs when RFA-induced coagulation necrosis, represented by ground-glass opacity, is adjacent to visceral pleura and necrotic tissue is sloughed. Management of the bronchopleural fistula was challenging and required pleurodesis, endobronchial management, or surgical repair.
MDCT is the most commonly used guiding modality for lung ablation, but does not provide real-time guidance. MDCT-fluoroscopy has an optimal technique for real-time monitoring, and several studies on the lung RFA have reported the use of this modality [Citation17,Citation36,Citation37]. However, the limitations of MDCT-fluoroscopy include closed CT gantry and limited imaging plane orientation [Citation38]. In contrast, CBCT can provide a greater working space for needle placement with wide range of angulation and rotation, so double oblique approaches are easier to perform. Another limitation of MDCT-fluoroscopy is that the needle path can only be observed through a single axial imaging plane during needle insertion and greater patient’s cooperation is required to control breathing [Citation38]. In particular, targeting lung tumor in lower lobe can be technically difficult due to the constant movement. The CBCT virtual navigation system offers advanced needle planning under real-time guidance, using combination of CBCT imaging, 2 D real-time fluoroscopy, and virtual needle pathway [Citation8,Citation19]. The advantage of CBCT guidance is the effective control of patient breathing with real-time fluoroscopy so that the depth of respiration matches the CBCT slice overlay on fusion imaging system. The constant control of the full length of the electrode using fluoroscopy may reduce complications such as pneumothorax. Although the number of samples was small, the frequency of pneumothorax was 11.6% to 12.5% in recent studies on CBCT-guided lung RFA [Citation13,Citation14].
Despite the above advantages, there are some limitations of CBCT guidance. CBCT offers less soft tissue contrast than MDCT, so there may be limitations in distinguishing tumor heterogeneity. In addition, CBCT has a longer scanning time than MDCT (CBCT: 4–10 s vs. MDCT: 0.5 s), resulting in higher susceptibility to motion artifacts. In particular, CBCT guidance can be problematic when a moving target tumor is displaced by the pneumothorax or needle [Citation39]. If the lesion location is challenging, such as metastasis near the mediastinum, an artificial pneumothorax or tumor displacement using deployable RF needle may help to perform the procedure safely [Citation39,Citation40]. In these cases, MDCT guidance with faster acquisition and fewer artifacts seems more appropriate. In our study, two procedures failed due to a large pneumothorax. This seems to be due to the aforementioned limitations of CBCT or lack of operator experience. Several studies have shown that an ablation margin of less than 5 mm is a risk factor for local tumor recurrence [Citation20,Citation22]. In our study, all tumors that recurred locally within 6 months had an ablation margin of less than 5 mm on post-procedural CBCT. We performed intra-procedural CBCT to evaluate the ablation zone before removing the electrode, and additional ablation or multiple electrode approach was performed whenever needed to provide the sufficient ablation margin. Nevertheless, the ablation margin of less than 5 mm was observed in 22% of the ablation zone on post-procedural CBCT. The use of CBCT for evaluation of the ablation zone before electrode removal can be limited due to tissue contraction, architectural distortion, and needle streak artifact.
The present study has several limitations. First, lung RFA was performed by a single operator at a single center, which could have biased the complication rates. It is also possible that the operator’s learning curve was reflected in efficacy and complications, since this is the operator’s initial experience with lung RFA. Second, because this study had a retrospective design, the true incidence of complications may have been underestimated. Third, our study design did not include a comparison between the complication of CBCT and that of MDCT guided lung RFA. However, in our hospital, conventional MDCT is used for diagnosis and screening, and there are many patients scheduled for diagnostic CT scan. Therefore, we always use CBCT in the angio-suite for lung RFA, which can simplify scheduling for patients requiring diagnostic CT scans and can be economical in terms of asset utilization.
In conclusion, our first 100 procedures have demonstrated that CBCT-guided RFA for lung tumors is a relatively safe procedure with acceptable morbidity. Although lung RFA can induce serious major complications, most are usually manageable and these complications should not limit the indications for lung RFA. In hospitals where MDCT is not readily available due to tight diagnostic CT workflow, CBCT provides a valuable alternative to MDCT for lung RFA.
Acknowledgments
Our study was conducted without any financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- de Baere T, Palussiere J, Auperin A, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology. 2006;240(2):587–596.
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9(7):621–628.
- de Baere T, Auperin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol. 2015;26(5):987–991.
- Alexander ES, Dupuy DE. Lung cancer ablation: technologies and techniques. Semin Intervent Radiol. 2013;30(2):141–150.
- Mouli SK, Kurilova I, Sofocleous CT, et al. The role of percutaneous image-guided thermal ablation for the treatment of pulmonary malignancies. AJR Am J Roentgenol. 2017;209(4):740–751.
- Carlson SK, Felmlee JP, Bender CE, et al. CT fluoroscopy-guided biopsy of the lung or upper abdomen with a breath-hold monitoring and feedback system: a prospective randomized controlled clinical trial. Radiology. 2005;237(2):701–708.
- Orth RC, Wallace MJ, Kuo MD. C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol. 2008;19(6):814–820.
- Choo JY, Park CM, Lee NK, et al. Percutaneous transthoracic needle biopsy of small (≤1 cm) lung nodules under C-arm cone-beam CT virtual navigation guidance. Eur Radiol. 2013;23(3):712–719.
- Lee SM, Park CM, Lee KH, et al. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of lung nodules: clinical experience in 1108 patients. Radiology. 2014;271(1):291–300.
- Rotolo N, Floridi C, Imperatori A, et al. Comparison of cone-beam CT-guided and CT fluoroscopy-guided transthoracic needle biopsy of lung nodules. Eur Radiol. 2016;26(2):381–389.
- Cheng YC, Tsai SH, Cheng Y, et al. Percutaneous transthoracic lung biopsy: comparison between C-arm cone-beam CT and conventional CT guidance. Transl Oncol. 2015;8(4):258–264.
- Cazzato RL, Battistuzzi JB, Catena V, et al. Cone-Beam Computed Tomography (CBCT) versus CT in lung ablation procedure: which is faster? Cardiovasc Intervent Radiol. 2015;38(5):1231–1236.
- Amouyal G, Pernot S, Dean C, et al. Percutaneous radiofrequency ablation of lung metastases from colorectal carcinoma under C-arm cone beam CT guidance. Diagn Interv Imaging. 2017;98(11):793–799.
- Xiang ZJ, Wang Y, Du EF, et al. The value of cone-beam CT-guided radiofrequency ablation in the treatment of pulmonary malignancies (≤3 cm). Biosci Rep. 2019;39(2):pii:BSR20181230.
- Zhu JC, Yan TD, Glenn D, et al. Radiofrequency ablation of lung tumors: feasibility and safety. Ann Thorac Surg. 2009;87(4):1023–1028.
- Okuma T, Matsuoka T, Yamamoto A, et al. Frequency and risk factors of various complications after computed tomography-guided radiofrequency ablation of lung tumors. Cardiovasc Intervent Radiol. 2008;31(1):122–130.
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center’s experiences. AJR Am J Roentgenol. 2011;197(4):W576–580.
- Welch BT, Brinjikji W, Schmit GD, et al. A national analysis of the complications, cost, and mortality of percutaneous lung ablation. J Vasc Interv Radiol. 2015;26(6):787–791.
- Braak SJ, van Strijen MJ, van Leersum M, et al. Real-time 3D fluoroscopy guidance during needle interventions: technique, accuracy, and feasibility. AJR Am J Roentgenol. 2010;194(5):W445–451.
- Kurilova I, Gonzalez-Aguirre A, Beets-Tan RG, et al. Microwave ablation in the management of colorectal cancer pulmonary metastases. Cardiovasc Intervent Radiol. 2018;41(10):1530–1544.
- Cho J, Yoon J, Kim Y, et al. Linguistic validation of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events in Korean. J Glob Oncol. 2019;5:1–10.
- Yang Q, Qi H, Zhang R, et al. Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors: evaluation based on a review of 147 tumors. J Vasc Interv Radiol. 2017;28(4):481–489.
- MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii18–31.
- Li G, Xue M, Chen W, et al. Efficacy and safety of radiofrequency ablation for lung cancers: a systematic review and meta-analysis. Eur J Radiol. 2018;100:92–98.
- Hiraki T, Gobara H, Fujiwara H, et al. Lung cancer ablation: complications. Semin Intervent Radiol. 2013;30(02):169–175.
- Nour-Eldin NE, Naguib NN, Saeed AS, et al. Risk factors involved in the development of pneumothorax during radiofrequency ablation of lung neoplasms. AJR Am J Roentgenol. 2009;193(1):W43–48.
- Hiraki T, Tajiri N, Mimura H, et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: incidence and risk factors. Radiology. 2006;241(1):275–283.
- Yamagami T, Kato T, Hirota T, et al. Pneumothorax as a complication of percutaneous radiofrequency ablation for lung neoplasms. J Vasc Interv Radiol. 2006;17(10):1625–1629.
- Kennedy SA, Milovanovic L, Dao D, et al. Risk factors for pneumothorax complicating radiofrequency ablation for lung malignancy: a systematic review and meta-analysis. J Vasc Interv Radiol. 2014;25(11):1671–1681.e1671.
- Nour-Eldin NE, Naguib NN, Mack M, et al. Pulmonary hemorrhage complicating radiofrequency ablation, from mild hemoptysis to life-threatening pattern. Eur Radiol. 2011;21(1):197–204.
- Vaughn C, Mychaskiw G, 2nd, Sewell P. Massive hemorrhage during radiofrequency ablation of a pulmonary neoplasm. Anesth Analg. 2002;94(5):1149–1151.
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243(1):268–275.
- Sakurai J, Hiraki T, Mukai T, et al. Intractable pneumothorax due to bronchopleural fistula after radiofrequency ablation of lung tumors. J Vasc Interv Radiol. 2007;18(1 Pt 1):141–145.
- Cannella M, Cornelis F, Descat E, et al. Bronchopleural fistula after radiofrequency ablation of lung tumours. Cardiovasc Intervent Radiol. 2011;34(Suppl 2):S171–S174.
- Mackln CC. Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum. Arch Intern Med. 1939;64:913–926.
- Smith SL, Bowers D, Jennings P, et al. Pulmonary radiofrequency ablation in a district general hospital: is it a safe and effective treatment? Clin Radiol. 2016;71:939.e931.
- Aufranc V, Farouil G, Abdel-Rehim M, et al. Percutaneous thermal ablation of primary and secondary lung tumors: comparison between microwave and radiofrequency ablation. Diagn Interv Imaging. 2019;100(12):781–791.
- Jin KN, Park CM, Goo JM, et al. Initial experience of percutaneous transthoracic needle biopsy of lung nodules using C-arm cone-beam CT systems. Eur Radiol. 2010;20(9):2108–2115.
- de Baere T, Tselikas L, Catena V, et al. Percutaneous thermal ablation of primary lung cancer. Diagn Interv Imaging. 2016;97(10):1019–1024.
- Prud’homme C, Deschamps F, Moulin B, et al. Image-guided lung metastasis ablation: a literature review. Int J Hyperthermia. 2019;36(2):37–45.