Abstract
Purpose
To assess the use of optimized radiofrequency (RF) to achieve larger, spherical ablation volumes with short application duration for hepatocellular carcinoma (HCC).
Materials and methods
Twenty-two patients (M:F = 17:5, median age 69.6 year, range 63–88) with 28 HCCs due to HCV + liver cirrhosis underwent RFA. 20/28 (71.4%) were tumors ≤3cm diameter, and 8/28 (28.6%) ranged from 3.2 to 4.2 cm. RF was applied using up to 2500mA via an optimized pulsing algorithm with real-time ultrasound monitoring to detect hyperechogenic changes. Single insertions of an internally cooled electrode were performed using exposed tips of 2 or 3 cm for 13 HCCs and 4 cm for 15 HCCs. All patients were followed-up for a minimum of 5 years with contrast-enhanced computed tomography (CECT).
Results
Technical success was achieved without adverse events in all cases. The mean ablation time was 8.5 ± 2.6 min. In 21/28 (75%), ablation duration ranged from 3 to 9 min, with 12 min duration applied in only 7/28 (25%). Mean coagulation diameters were 2.4 ± 0.14, 3.3 ± 0.62, and 4.4 ± 1.0, for 2, 3 and 4 cm electrodes, respectively (p < 0.01). The sphericity index was 74.9 ± 12.8 for 4 cm electrodes and 81.9 ± 8.0 for shorter electrodes (p = 0.091). At 5-year follow-up, no tumor ≤3 cm had recurrence and only 2/8 (25%) >3 cm tumors developed local progression. One patient had multifocal disease with no local progression.
Conclusion
Efficient delivery of RF energy can considerably decrease the ablation time in many instances while achieving larger, relatively spherical, and reproducible areas of ablation with extremely low rates of local tumor progression and adverse events.
Introduction
The liver is the most common target for percutaneous thermal ablations, with decades of clinical experience and established efficacy earning this modality recognition as a first-line treatment option in both primary and secondary liver cancers [Citation1–4]. Largely due to its relatively early availability, radiofrequency (RF) ablation is the most commonly used ablation technique worldwide. Yet, despite the development of sequential technical improvements in device design (including internal cooling and multi-tined electrodes) [Citation5,Citation6] and energy delivery (including switching of electrode activation and pulsed energy delivery) [Citation7], RF has been associated with a number of limitations, including longer than desired ablation time, heat-sink effect adjacent to large blood vessels, and tissue charring around the electrode tip due to steep temperature gradient near the electrode [Citation8–10]. In addition, it is likely that suboptimal energy delivery of RF generators has historically limited effective ablation using electrode tip exposures of 2.5–3.0 cm, given the lack of sphericity occurring with longer exposures [Citation11,Citation12]. Thus, over the last decade, microwave ablation (MWA) has gained ever greater prominence for clinical use given a demonstrated capability of achieving larger ablation volumes in shorter operative times compared to clinically available RF systems, with some MWA systems producing more predictable shape of the ablation volume and significantly less heat sink effect [Citation13,Citation14]. Yet, to-date there is no clear clinical evidence documenting the superiority of MWA versus RFA for most clinical scenarios [Citation15,Citation16].
Recently, more thorough and systematic optimization of RF pulsing algorithms has been reported in ex vivo tissues [Citation17]. This optimization allows for the administration of a greater amount of current and provides dynamic energy deposition through an optimized pulsing algorithm using internally cooled electrode exposures of up to 5 cm. In the current study, our preliminary experience of percutaneous ablation in a small group of hepatocellular carcinomas (HCCs) using this method is reported. The aim of this single-center retrospective study of prospectively collected data is to assess whether this optimized RF energy delivery overcomes some of the classical limitations of RF ablation, specifically by achieving ablation in shorter times than the previously recommended (i.e., conventional) 12 min ablation duration or by creating larger ablative zones by increased energy application via longer 4 cm electrode tip exposures [Citation8].
Materials and methods
The study was performed at a single tertiary referral center for patients with liver tumors with the approval of the local Institutional Ethics Committee. Written informed consent was obtained from all patients prior to treatment.
Patients population
From July 2011 to April 2015, a total of 22 patients (17 males and 5 females, median age 69.6 year, range 63–88 years) with 28 HCCs in a subset of HCV-related cirrhosis underwent RF ablation using the novel ablation system based upon the indication-to-treatment of a multidisciplinary team including liver surgeons, hepatologists, radiotherapists, oncologists, and interventional radiologists.
According to the BCLC classification [Citation18], only patients in very early (Stage 0: single nodule ≤2 cm, Child–Pugh A-B, ECOG PS 0) and early stage of the disease (Stage A: 1–3 nodules, Child–Pugh A-B, ECOG PS 0) were enrolled in this study. All the HCCs were diagnosed through a non-invasive radiological workup, following the European Association for the Study of the Liver (EASL) 2018 clinical practice guidelines [Citation2]. The HCCs were located in segment III (3 HCCs), IV (4), V (1), VI (4), VII (10), and VIII (6). Fifteen HCCs (53.6%) were peripherally located, thirteen (46.4%) were centrally located. Nine HCCs (32.1%) ranged from 1.0 to 2.0 cm in size, 11/28 (39.3%) from 2.1 to 3.0 cm, and the remaining 8/28 (28.6%) from 3.2 to 4.2 cm. The median size was 2.6 ± 0.87 cm. The median tumoral volume was 9.15 cm3 (range 0.5–38.8 cm3). Six patients had two nodules treated during the same session, while one patient was treated twice at different times for an HCC developed de novo. All the ablations were performed by two interventional radiologists with more than 15 years of experience performing thermal ablation.
Pretreatment diagnostic assessment
All patients were evaluated with hepatic baseline ultrasound (US), contrast-enhanced US (CEUS), and abdominal contrast-enhanced computed tomography (CECT). Both CEUS and CECT were achieved in the arterial, portal, and late phases. CEUS was performed after intravenous injection of 2.4 to 4.8 ml of second-generation contrast agent (SonoVue, Bracco, Milan, Italy). CT scans were acquired following injection of 110 ml of iopamidol (Iopamiro, Bracco, Milan, Italy) at 3–4 ml/s, using 5 mm slice thickness, 7 mm collimation, 1:1 pitch, 120 or 140 kVp, and 280–300 mA.
Radiofrequency ablation
An internally cooled electrode RF system (Cambridge Interventional CRF System, Burlington, MA) was used for all patients. This consisted of a high power RF generator producing up to 2500 mA output into 50 Ω, a fluid pump, tubing, cables, and four large grounding pads [Citation17]. To maximize the efficiency of energy deposition, the generator algorithm first endeavored to achieve a pre-set current based upon electrode tip length over 30 s [Citation17], and then pulsed the electrode current in response to changes in tissue impedance. While the impedance was stable, current was increased in 100 mA increments every 30 s. When impedance rose, current was halted for at least 20 s before it was resumed. When impedance rose so rapidly that a current could not be maintained for at least 10 s, the current was reduced by 100 mA when energy deposition resumed [Citation17].
For each ablation, a 17-gauge internally cooled electrode was introduced via coaxial 15-gauge electrically insulated metallic cannula with a tip exposure of 2, 3, or 4 cm. Refrigerated (4 °C) water circulating within the electrodes maintained the electrode temperature at or below 22 °C.
Treatment procedure
Treatments were performed under assisted ventilation during short-acting anesthesia using propofol (10 mg/ml) and alfentanil (0.5 mg/ml) or moderate sedation and analgesia with the association of neuroleptic (droperidol, 1.25–2.5 mg) and analgesic (fentanyl, 50–250 mcg) drugs, as decided by the anesthesiologist. Continuous hemodynamic monitoring was performed throughout the procedure.
Real-time US guidance was employed in all cases using a 3.5 MHz probe with a biopsy guide (MyLab Twice and MyLab 9, Esaote, Genoa, Italy). When the HCC nodule was not visible or had poor conspicuity at US, a navigation system (Virtual Navigator, Esaote, Genoa, Italy) was used, co-registering real-time US and pre-acquired CECT [Citation19,Citation20]. Additionally, CEUS was performed intraprocedurally in selected cases to improve target visibility and guide electrode insertion. The ablation was considered complete and energy application was stopped based upon real-time ultrasonographic findings when either the size of the hyperechoic area due to gas formation was stable and not increasing for 3 min or when hyperechogenicity appeared to have exceeded both the tumor margins plus an ablative margin of at least 5 mm in all the diameters for 3 min, or alternatively when a maximum of 12 min of energy (i.e., a conventional dose) were applied. CEUS was also routinely performed 5–7 min after electrode withdrawal once most of the acute hyperechoic changes in the ablation zone had dissipated. Adequate ablation was defined as lack of contrast enhancement throughout the tumor and at least 5 mm thick perinodular rim (ablative margin) [Citation1]. Moreover, ultrasound contrast could be used to guide immediate additional ablation(s) if the volume of the avascular zone is considered insufficient in comparison with the target nodule size and the desired 5-mm ablative margin [Citation21,Citation22], but this event did not occur in our group of cases that were always ablated with single insertions of single electrodes.
Post-procedural assessment
Based upon institutional protocol, following RF ablation, all patients were hospitalized for 1–2 days for clinical observation and procedure-related pain management.
Post-procedural CT scans were acquired 24 h following ablation using the same technique as before treatment, and subsequently at 3–4 months interval in the first 3 years and at 4–5 months in the following years [Citation23]. Post-ablation CT scans were always evaluated by two radiologists (L.S. and T.I.) with more than 10 years of experience in ablative treatments. All patients underwent clinical and radiological follow-up for a period ranging from a minimum of 5 years after RF ablation.
Assessment of treatment efficacy
Primary technical success was defined as the absence of contrast enhancement in the target tumor on CECT within 24 h from the end of the procedure [Citation1], while complete ablation was defined as the lack of contrast-enhancement at the 3-month follow-up CT scan and the following CT scans encompassing the HCC in all dimensions plus the presence of an ablative margin with a thickness of at least 5 mm [Citation24]. In cases where technical success on the immediate post-procedural CECT was thought to be achieved by visual inspection, but complete necrosis was not observed on subsequent follow-up studies, a novel software package (Ablation Fit, RAW s.r.l., Milan, Italy) was retrospectively applied to assess the true status of ablation by performing precise quantification of the incomplete treatment [Citation25]. Briefly, pre- and post-ablation CT scans in 3D were automatically segmented and non-rigidly co-registered to enable better definition of the desired ablative margin and accurately determine as to whether the tumor and the ablative margin were completely or only partially included into the necrotic volume.
Data analysis
Energy and heating parameters, and the diameters (perpendicular and along the electrode axis), and sphericity of the resultant ablation were analyzed by electrode tip length as previously described [Citation17]. The primary clinical endpoint of no evidence of local tumor progression was analyzed by tumor size (≤3 cm versus >3 cm) and time of ablation (12 min conventional standard versus shorter ablation times). Relevant comparisons were performed using 2-tailed T-tests and Chi-square analysis with statistical significance set at 0.05.
Results
Technical success was achieved in all cases and no intra- or periprocedural adverse events occurred.
All the ablations were performed with single electrodes with tip exposures of 2 cm (2 HCCs), 3 cm (11 HCCs), or 4 cm (15 HCCs). Four cm electrode tips were used either for HCCs with sizes ≥ 3 cm (10 cases) or for HCCs located in proximity to large blood vessels (5 cases) with an intent of decreasing the heat sink effect through the achievement of large ablation volumes [Citation26]. The maximum current applied and the minimum impedance values during RF output delivery were dependent upon electrode tip size and ranged from 1100 to 2200 mA and from 53 to 80 Ω (mean 61.1), respectively (p < 0.01 both comparisons; ). Likewise, the maximum temperature at the electrode after ablation increased with energy and tip size (p < 0.05). Ablation times ranged from 3 to 12 min (mean: 8.5 ± 2.6 min, median: 8 min). Here too, ablation time to achieve 3 min of a stable gas cloud was dependent on the tip size and energy with a 4.5 ± 2.1 min average time for 2 cm electrodes, 8.7 ± 2.5 min for 3 cm, and 8.9 ± 2.4 min for 4 cm electrode tips (p < 0.01 for 2 cm electrodes versus others).
Table 1. Radiofrequency ablation parameters by internally cooled electrode tip size using the optimized pulse algorithm.
When viewed from a clinical perspective, for the 20 HCCs up to 3 cm in size, the ablation time ranged from 3 to 12 min (mean: 8.2 ± 2.6). Only in 4/20 (20%) HCCs of this size group the ablation time reached 12 min, due to the slow and partial gas formation observed with real-time US during the treatment. For the 8 HCCs larger than 3 cm (3.2–4.2 cm) the ablation time ranged from 6 to 10 min in 5/8 (62.5%) cases and was 12 min in only 3/8 (37.5%).
Despite any reduction in ablation time, technical success was achieved in all cases, as confirmed by CEUS and CECT at the end of the procedure. The median volume of necrosis produced was 26.1 cm3 (range 5.6–95.7 cm3). Both the length and diameters of the ablation increased with electrode tip size with coagulation diameter measuring 2.4 ± 0.14, 3.3 ± 0.62, and 4.4 ± 1.0 cm, for 2, 3, and 4 cm electrode tips, respectively (p < 0.01) (). The size of the volumes of necrosis exceeded of 5 mm or more the maximum diameter of the ablated HCCs in all the three dimensions in 19/28 (67.9%) HCCs, including 14/20 (70%) nodules up to 3 cm and 5/8 (62.5%) HCCs ≥3.2 cm. The sphericity index [17)] of all the volumes of necrosis achieved ranged from 52.5% to 97.1% (mean: 78.1 ± 11.2), with no statistically significant difference between the group of 13 HCCs ablated with 2 and 3 cm exposed tips (mean: 81.9 ± 8.0) and the 15 HCCs treated with 4 cm exposed tips (mean: 74.9 ± 12.8) (p = 0.091). No significant difference in volume of necrosis, ablation time, and sphericity was found between centrally and peripherally located HCCs (p > 0.2).
At 1-year follow-up CECT, focal enhancement at the periphery of the necrotic volume consistent with local tumor progression (LTP) was identified in only 2/28 (7.1%) HCCs, both of which were originally larger than 3 cm. No additional local tumor progression was identified. Thus, all tumors ≤3 cm and 6/8 (75%) tumors >3 cm treated with shorter RFA duration were successfully treated to 5-year follow up ( and ).
Figure 1. Short course, large volume RF ablation with long term favorable outcome. (a) A 2.2 cm HCC at S8 (arrow) was ablated with a single insertion of a 4 cm exposed tip RF electrode in 6 min with 2000 mA current. (b) At 24-h, CT the dimensions of the necrosis volume achieved were 4.8 × 4.3 × 5.5 cm. At 2- (c) and 4-year (d) follow-up, ablation was complete with no LTP.
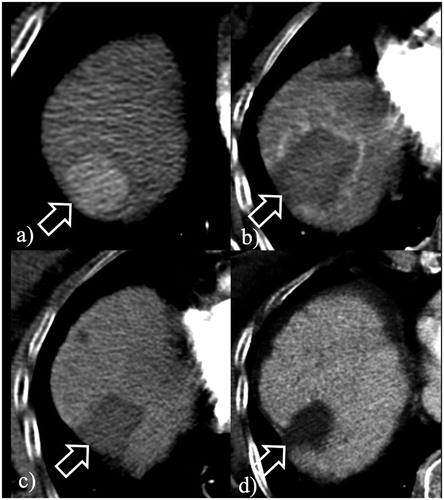
Figure 2. Large volume RF ablation from optimized current and longer electrodes. (a) A 4.2 cm HCC at S4 (arrow) was ablated with a single insertion of a 4 cm exposed tip RF electrode in 12 min with 2200 mA current. (b) At 24-h CT, the dimensions of the necrosis volume achieved were 5.2 × 4.1 × 4.4 cm. (c) At 2-year follow-up, ablation was complete with no LTP.
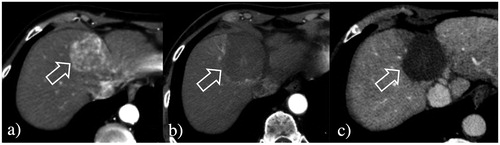
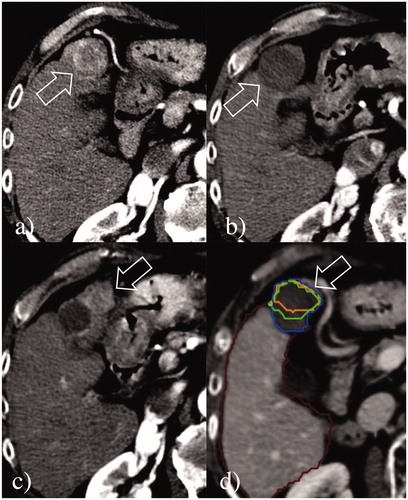
The two HCCs >3 cm that developed LTP had ablation times of 6 and 8 min, respectively, given the rapid and apparently large gas formation that exceeded the target ablative margins. For these two HCCs, the Ablation-fit software retrospectively applied to compare pre- and 24-h post-ablation CECT in 3D confirmed that despite an initial determination by visual inspection of an apparently successful treatment, in actuality, the achieved ablation zone did not exceed the target volume, which includes the tumor and a 5 mm ablative margin in all the three dimensions. Specifically, in one 3.3 cm tumor, although no residual viable tumor was detected post ablation, 15.4% of the 5-mm ablative margin was not ablated (). In the second 3.9 cm HCC, 5.2% of the tumor and 44.4% of the 5-mm ablative margin were not included in the post-ablation volume of necrosis, thus, indicating that the subsequent LTP had likely been mostly caused by imperfect tumor targeting and incomplete ablation. One additional patient developed multifocal HCC at the 2-year follow-up CT, but no LTP was detected in the 3.2 cm HCC ablated.
Figure 3. Local tumor progression post-RF ablation from incomplete treatment. (a) A 3.3 cm HCC at S4 (arrow) was ablated with a single insertion of a 3 cm exposed tip RF electrode in 6 min with 1600 mA current. (b) At 24-h CT, the dimensions of the necrosis volume achieved were 3.5 × 3.3 × 3.5 cm. (c) Although the treatment seemed to be complete, at 9-month follow-up CT, a large region of local tumor progression was noted in the anterior portion of the volume of necrosis (arrows). (d) Retrospective assessment of the pre- and post-ablation CT scans non-rigidly co-registered by Ablation-fit software (RAW s.r.l., Milan, Italy) showed that a small portion (15.4%) of the 5-mm ablative margin (green line) was not achieved in the anteromedial part of the HCC, suggesting incomplete treatment was due to an initial technical failure from sub-optimal electrode placement and ablation coverage. The margins of the volume of necrosis (blue line), of the HCC (orange line), and the ablative margin are exactly overlapping.
Discussion
Although strategies to optimize pulsing energy and thus achieve more efficient heat transfer and larger ablation volumes for RF ablation have been reported [Citation27,Citation28], these possible advantages have never been thoroughly investigated or implemented into clinically available systems. Thus, limited energy deposition provided by the majority of commercially available RF systems has made the clinical use of electrodes with exposures longer than 2.5–3.0 cm impractical, due to a marked increase in ablation length and only a modest increase in ablation diameter (i.e., oblong-shaped zones), with consequent loss of the needed sphericity. These limitations of RF favored the increasing clinical use of MWA, particularly for tumors larger than 2.0–2.5 cm and/or those adjacent to large blood vessels [Citation29]. Although larger tumor size (>2.5 cm) has been sporadically associated with improved outcomes for MWA compared to RFA [Citation30], other papers report comparable results in tumors <4 cm [Citation31]. For example, a recent meta-analysis reports similar therapeutic outcomes of percutaneous MWA and RFA for HCC in terms of complete ablation, local recurrence, disease-free survival, overall survival, and complication rate, even for tumors >3 cm [Citation32].
The outcomes achieved in our initial clinical study with this optimized RF technology confirm the results recently described in ex vivo bovine liver models and demonstrate the potential for changing the scenario of RF technology [Citation17]. Owing to an optimized RF generator that allowed to efficiently increase energy deposition and tissue heating, we demonstrated that it is possible to substantially decrease the ablation time while achieving good ablation outcomes. Only 4/20 HCCs ≤3 cm were ablated in the usual 12-min time recommended for all the RFA systems due to the slow and partial gas formation observed with real-time US during the treatment, while for all the remaining 16/20 HCCs ≤3 cm, the ablation time ranged from 3 to 8 min. In addition, 5/8 HCCs larger than 3 cm were successfully ablated with ablation time ranging from 6 to 10 min.
We also demonstrated that with this optimized RF output the use of electrodes with longer tip exposures is possible, with 4 cm electrode tips producing 4.4 cm mean coagulation diameter in 6–12 min, which is larger than previously reported coagulation diameters in the clinical setting [Citation33]. This was achieved without the typically encountered significant loss of sphericity of the volumes of necrosis of older systems. Indeed, the mean sphericity index achieved in the 15 HCCs ablated with 4 cm exposed tip electrodes (74.9 ± 12.8%) was comparable to that of the 13 HCCs ablated with either 2 and 3 cm exposed tips (81.9 ± 8.0%) (p = 0.09).
Despite the use of shorter ablative times and longer exposed tip electrodes, technical success was achieved in all cases. In addition, in 25/28 (89.3%) HCCs treated, the diameters of ablation volumes were greater than the maximum diameter of the tumors and 19/25 (76%) of them had an ablative margin larger than 5 mm from a single RF application. At 2-year follow-up, local tumor progression had occurred in only 2/28 (7.1%) HCCs, and both of these were initially larger than 3.0 cm. In both of these cases, the treatment failure was less likely due to the shorter ablation time, and more likely attributable to suboptimal targeting, as retrospectively demonstrated by Ablation-fit software [Citation25]. Indeed, it is conceivable that if such recently developed software had been available at the time of ablation, an additional short course of newly target ablation would have likely avoided the subsequent local progression.
We acknowledge that this study has some major limitations, most notably the small number of patients within the cohort, the small number of HCCs larger than the “conventional” threshold of 3 cm, and its retrospective nature. Moreover, additional study will be required for other tumor populations. Nevertheless, our series represents the first in vivo study describing an optimization of RF technology that could potentially have beneficial attributes for patient care. Finally, it is possible that more accurate measurement results could be obtained with newer generation automated measurement systems as they become more widely available [Citation34].
In conclusion, we demonstrate that efficient delivery of RF energy into liver tumors through a single internally cooled electrode can produce larger, relatively spherical, and reproducible volumes of necrosis by means of longer electrode tip exposures, and can reduce ablation time for shorter electrode tip exposures, with extremely low rates of local progression and adverse events. If the outcomes of this preliminary clinical experience are confirmed in future larger studies, limitations associated with RF ablation such as procedure duration and <3 cm ablation diameters will need to be reconsidered, as well as the associated comparisons between RF ablation and other ablative technologies such as microwave, that are based on historical suboptimal energy delivery.
Disclosure statement
All authors approved the manuscript and this submission, and did not receive any funds or other financial help for this publication. Eric Cosman is an employee of Cambridge Interventional; S. Nahum Goldberg is a paid consultant to Cambridge Interventional.
References
- Ahmed M, Solbiati L, Brace CL, Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology. 2014;273(1):241–260.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25(12):3438–3454.
- Lee MW, Kang D, Lim HK, et al. Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma < 3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol. 2020;30(4):2391–2400.
- Goldberg SN, Gazelle GS, Solbiati L, et al. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3(8):636–644.
- Goldberg SN, Solbiati L, Hahn PF, et al. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209(2):371–379.
- Laeseke PF, Sampson LA, Frey TM, et al. Multiple-electrode radiofrequency ablation: comparison with a conventional cluster electrode in an in vivo porcine kidney model. J Vasc Interv Radiol. 2007;18(8):1005–1010.
- Goldberg SN, Gazelle GS. Radiofrequency tissue ablation: physical principles and techniques for increasing coagulation necrosis. Hepatogastroenterology. 2001;48(38):359–367.
- Hong K, Georgiades C. Radiofrequency ablation: mechanism of action and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S179–S186.
- Ikeda K, Osaki Y, Nakanishi H, et al. Recent progress in radiofrequency ablation therapy for hepatocellular carcinoma. Oncology. 2014;87(Suppl 1):73–77.
- Nishikawa H, Kimura T, Kita R, et al. Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia. 2013;29(6):558–568.
- Rempp H, Mezger D, Voigtlaender M, et al. A comparison of internally water-perfused and cryogenically cooled monopolar and bipolar radiofrequency applicators in ex vivo liver samples. Acad Radiol. 2014;21(5):661–666.
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S192–S203.
- Amabile C, Ahmed M, Solbiati L, et al. Microwave ablation of primary and secondary liver tumours: ex vivo, in vivo, and clinical characterisation. Int J Hyperthermia. 2017;33(1):34–42.
- Glassberg MB, Ghosh S, Clymer JW, et al. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Onco Targets Ther. 2019;12:6407–6438.
- Kamal A, Elmoety AAA, Rostom YAM, et al. Percutaneous radiofrequency versus microwave ablation for management of hepatocellular carcinoma: a randomized controlled trial. J Gastrointest Oncol. 2019;10(3):562–571.
- Ben-David E, Nissenbaum I, Gurevich S, et al. Optimization and characterization of a novel internally-cooled radiofrequency ablation system with optimized pulsing algorithm in an ex-vivo bovine liver. Int J Hyperthermia. 2019;36(2):81–88.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314.
- Mauri G, Gennaro N, De Beni S, et al. Real-time US-18FDG-PET/CT image fusion for guidance of thermal ablation of 18FDG-PET-positive liver metastases: the added value of contrast enhancement. Cardiovasc Intervent Radiol. 2019;42(1):60–68.
- Muglia R, Solbiati L. New technological advancements for interventional oncology. Chin Clin Oncol. 2019;8(6):65–70.
- Francica G, Meloni MF, Riccardi L, et al. Ablation treatment of primary and secondary liver tumors under contrast-enhanced ultrasound guidance in field practice of interventional ultrasound centers. A multicenter study. Eur J Radiol. 2018;105:96–101.
- Ju J-X, Zeng Q-J, Xu E-J, et al. Intraprocedural contrast-enhanced ultrasound-CT/MR fusion imaging assessment in HCC thermal ablation to reduce local tumor progression: compared with routine contrast-enhanced ultrasound. Int J Hyperthermia. 2019;36(1):785–793.
- Young S, Taylor AJ, Sanghvi T. Post locoregional therapy treatment imaging in hepatocellular carcinoma patients: a literature-based review. J Clin Transl Hepatol. 2018;6(2):189–197.
- Jiang C, Liu B, Chen S, et al. Safety margin after radiofrequency ablation of hepatocellular carcinoma: precise assessment with a three-dimensional reconstruction technique using CT imaging. Int J Hyperthermia. 2018;34(8):1135–1141.
- Solbiati M, Muglia R, Goldberg SN, et al. A novel software platform for volumetric assessment of ablation completeness. Int J Hyperthermia. 2019;36:337–343.
- Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9(1):101–111.
- Solazzo SA, Ahmed M, Liu Z, et al. High-power generator for radiofrequency ablation: larger electrodes and pulsing algorithms in bovine ex vivo and porcine in vivo settings. Radiology. 2007;242(3):743–750.
- McGahan JP, Loh S, Boschini FJ, et al. Maximizing parameters for tissue ablation by using an internally cooled electrode. Radiology. 2010;256(2):397–405.
- van Tilborg A, Scheffer HJ, de Jong MC, et al. MWA versus RFA for perivascular and peribiliary CRLM: a retrospective patient- and lesion-based analysis of two historical cohorts. Cardiovasc Intervent Radiol. 2016;39(10):1438–1446.
- Chinnaratha MA, Chuang MA, Fraser RJL, et al. Percutaneous thermal ablation for primary hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(2):294–301.
- Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3(5):3:317–325.
- Tan W, Deng Q, Lin S, et al. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):264–272.
- Pereira PL, Trubenbach J, Schenk M, et al. Radiofrequency ablation: in vivo comparison of four commercially available devices in pig livers. Radiology. 2004;232(2):482–490.
- Vo Chieu VD, Wacker F, Rieder C, et al. Ablation zone geometry after CT-guided hepatic microwave ablation: evaluation of a semi-automatic software and comparison of two different ablation systems. Int J Hyperthermia. 2020;37(1):533–541.
