Abstract
Purpose
This study was conducted to prepare a novel tumor-biotargeting high-intensity focused ultrasound (HIFU) synergist for indirectly delivering lipid nanoparticles based on the targeting ability of Bifidobacterium longum to the hypoxic region of solid tumors. The effects of two different delivery methods on the imaging and treatment of solid tumors enhanced by lipid nanoparticles were compared.
Methods
Biotinylated lipid nanoparticles coated with PFH were prepared, cross-linked with B. longum in vitro using a streptavidin-conjugated B. longum antibody (SBA), and observed and detected by laser confocal microscopy and flow cytometry. Solid tumors were treated with HIFU and PFH/BL-NPs. The effects of different delivery methods on the tumor targeting and efficiency of retention of PFH/BL-NPs were observed using Small animal live imaging and frozen sections from small animals.
Results
The PFH/BL-NPs prepared in this study showed good biocompatibility and safety. PFH/BL-NPs and B. longum were cross-linked in a cluster-like manner (confocal laser scanning microscope) in vitro, with a cross-linking rate of 84 ± 6.23% (flow cytometry). The delivery of B. longum followed by that of PFH/BL-NPs not only enhanced the ability of PFH/BL-NPs to target solid tumors (small animal live imaging), but also increased the retention time of PFH/BL-NPs in the tumor (frozen slices), enhancing the effect of the HIFU synergist.
Conclusion
Delivery of B. longum followed by that of PFH/BL-NPs can enhance the imaging of solid tumors and effectively improve the efficiency of HIFU treatment of solid tumors, providing a basis for further clinical work.
Introduction
One of the challenges in targeted therapy of solid tumors is ensuring the efficient and safe delivery of therapeutic substances to the target area. Because of the complex microenvironment of tumors, including hypoxia, low pH, and resistance of cancer cells to drugs, targeted treatment of tumors is difficult [Citation1–5]. Many studies have shown that anaerobic bacteria can target the hypoxic zone of solid tumors and can carry a variety of therapeutic agents for treating these tumors. However, several bacterial strains have several limitations that preventing their use as biomedical microrobots, including complex incubation procedures, drug resistance against antibiotics, and pathogenicity in living animals [Citation6,Citation7]. As a probiotic, Bifidobacterium has been widely recognized for its biological safety [Citation8]. It not only targets solid tumors [Citation9], but also increases retention efficiency and prolongs the retention time of lipid microspheres in the target area [Citation10]. Therefore, Bifidobacterium shows potential as a tumor target-targeting agent.
High-intensity focused ultrasound (HIFU) has gained attention for the treatment of solid tumors [Citation11,Citation12]. The treatment principle of HIFU is the focusing of ultrasound waves at a point to generate higher energy through thermal, mechanical, and cavitation processes, which can lead to an instant rise in temperature to above 60 °C; this ‘burns’ the tumor without causing damage to surrounding normal tissue [Citation13]. However, there are several disadvantages of HIFU, limiting its efficiency and safety. Ultrasound energy decays with increasing distance, and abundant and rapid blood flow in the tumor can decrease ultrasound energy deposition [Citation14,Citation15], necessitating a longer treatment period. Even with real-time monitoring by magnetic resonance imaging or ultrasound images, prolonged treatment with HIFU can increase the incidence of side effects. Microbubble nanoparticles are currently considered as the safest and most effective HIFU synergist. In this study, we used perfluorohexane (PFH) encapsulated in lipid nanoparticles. PFH can improve the treatment efficiency of HIFU by enhancing the thermal and cavitation effect of ultrasound waves at the target tissue. When the temperature during treatment exceeds the boiling point of PFH (57 °C), the nanoparticles are more likely to become microbubbles and enhance nanoparticle rupture, thereby enhancing the treatment effects. Thus, PFH can be used to enhance HIFU treatment through ultrasound monitoring [Citation16,Citation17].
We encountered many difficulties in using our cross-linking method at an early stage of the study. In the electro-conversion method, the synergist was not successfully transferred into Bifidobacterium; this chemical bonding method exhibited a low success rate and could only be used for in vitro cross-linking and delivery. The cationic cross-linking method was effective in vitro but was less stable in vivo, and some studies have reported that cations have biotoxic side effects [Citation18,Citation19]. The biotin–avidin cross-linking method is very stable and has been successfully used to cross-link bacteria with liposomes [Citation20]. Therefore, in this study, we cross-linked Bifidobacterium and lipid nanoparticles using the biotin-avidin linking method.
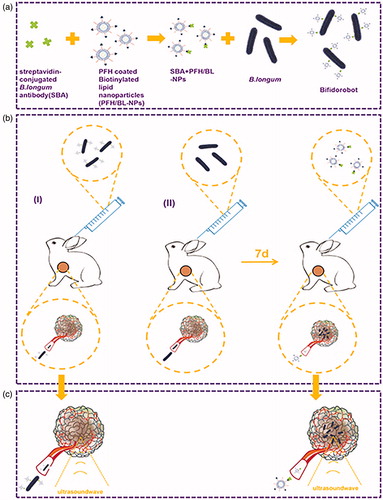
Most studies of bacterial targeting of tumors involved direct cross-linking of the bacteria to the delivery substance or recombination of genes and bacteria as an arch on a ‘bacterial robot’ to target the tumor [Citation21–23]. However, effective delivery of nanomedicines to tumor cells is difficult because of the complex tumor microenvironments, resulting in poor and uneven perfusion, poor vascular permeability, and a high density of stromal cells, as well as increased interstitial pressure [Citation24]. Therefore, in this study, we examined delivery efficiency using different delivery methods. The first delivery method included direct delivery of ‘Bifidorobots’, which were prepared by cross-linking Bifidobacterium longum (B. longum) with PFH-coated biotinylated lipid nanoparticles (PFH/BL-NPs) via a streptavidin-conjugated B. longum antibody (SBA) in vitro (). However, after the Bifidorobots were formed, their volume was increased, making tumor targeting difficult. Therefore, we also used a method in which B. longum was allowed to colonize the tumors for 7 days, and SBA and PFH/BL-NPs were cross-linked in vitro to prepare 'Antibodyobots' which were used to target B. longum in the tumor. (). Then compared the HIFU treatment efficiency of the two delivery methods ().
Materials and methods
Chemicals
All chemicals were used directly without further purification. Dipalmitoylphosphatidylcholine and Bio-DSPE-PEG (2000)-Amine were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Fluorescein isothiocyanate (FITC) and PFH were purchased from Beyotime Biotech Ltd. (Shanghai, China). Trichloromethane (CHCl3) was purchased from Chongqing East Chemical Industry Ltd., Co. (Chong Qing, China). DiI and DiR were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Streptavidin B. longum antibody was purchased from Wuhan AtaGenix Laboratories Ltd., Co. (Wuhan, China).
Synthesis of BL-NPs and PFH/BL-NPs
Briefly, BL-NPs (lipid composition: dipalmitoylphosphatidylcholine and DSPE-PEG-Bio; molar ratio of 91.2:8.8. If fluorescent staining was required, 1 mg DiI or DiR was also added) were dissolved in chloroform, and the solvent was evaporated. Next, 2 ml of phosphate-buffered saline (PBS) was added to dissolve the BL-NPs, after which 100 µL of PFH was added to the suspension. The suspension was emulsified in an ice bath using an ultrasonic cell disruptor (Sonics &Materials, Inc., Newtown, CT) at 130 W for 6 min (5 s on and 5 s off). The nanoemulsion was centrifuged at 4 °C for 5 min at 8228 ×g and washed three times with PBS to remove the dissociated lipids and PFH. The samples were stored at −4 °C.
Characterization of BL-NPs and PFH/BL-NPs
DiI labeled PFH/BL-NPs (excitation and emission wavelengths of 549 and 565 nm, respectively) were observed using a confocal laser scanning microscope (CLSM, Nikon A1, Tokyo, Japan). Microscopic manifestations of BL-NPs and PFH/BL-NPs were observed by transmission electron microscopy (TEM, Hitachi 7500, Hitachi Ltd., Tokyo, Japan). After adjusting the BL-NPs and PFH/BL-NPs with water, the particle size distribution and zeta potential were measured using a dynamic light scattering instrument (Malvern Instruments, Malvern, UK) at a wavelength of 633 nm, light scattering angle of 90°, and temperature of 25 °C.
Cross-linking of B. longum and PFH/BL-NPs in vitro
DiI-stained PFH/BL-NPs (150 µL, 0.5 mg/mL) were reacted with 400 µL of SBA (40 µg/mL) at 22 °C for 30 min to form Antibodyobots. The Antibodyobots were centrifuged (5142 ×g, 5 min) and then washed three times with PBS. To simulate the in vivo environment, the antibody was incubated in 10% fetal bovine serum at 37 °C for 48 h, followed by the addition of 200 µL of B. longum (FITC stained, 4 × 108 bacilli/mL) to the reaction for 30 min at 37 °C. The mixture was then centrifuged (1028 ×g, 5 min) and washed three times with PBS. Finally, cross-linking between B. longum and Antibodyobots was observed and detected by CLSM and flow cytometry (BDFACSVantage SE, BD Biosciences, Franklin Lakes, NJ). And the growth curves of B. longum and B. longum + Antibodyobots over a period of 50 h were compared: After cross-linking B. longum and Antibodyobot as described above, it was compared with B. longum without special treatment. Both were cultivated anaerobically in MRS broth medium. Every 4 h, the absorbance at 600 nm wavelength was measured with a spectrophotometer, each group was tested three times, and the bacterial growth characteristic curve was drawn within 50 h.
Targeting ability and retention capacity in vivo
Antibodyobots (0.5 mg/mL PFH/BL-NPs, 40 µg/mL SBA) were prepared at the same time as PFH/BL-NPs and SBA as described above. The concentration of B. longum was adjusted to 4 × 108 bacilli/mL, and 4 ml of Bifidorobots were prepared using 2 ml of B. longum and 2 ml of Antibodyobots as described above.
Small animal live imaging
Forty-two tumor-bearing rabbits were randomly divided into two groups with 21 rabbits in each group. Each rabbit in the Bifidorobot group was injected with 4 ml of Bifidorobots (2 ml B. longum + 2 ml Antibodyobots), whereas each rabbit in the B. longum + Antibodyobots group was first injected with 2 ml of B. longum, and then injected with 2 ml of Antibodyobots after 7 days (all PFH/BL-NPs were stained with DiR). Each rabbit in the two groups was sacrificed at different time points (before injection and after 2, 12, 24, 48, 72, or 96 h of injection). Tumors and important organs were removed and placed in a six-well plate (in the absence of light) for immediate observation of fluorescence. The average fluorescence value in each group of tumors at each time point was calculated.
Frozen section to observe the retention of PFH/BL-NPs in tumors
Eighteen tumor-bearing rabbits were randomly divided into three groups and each group of rabbits was injected with 2 ml of PFH/BL-NPs (0.5 mg/mL), 4 ml of Bifidorobots, or 2 ml of B. longum + 2 ml of Antibodyobots (all PFH/BL-NPs were stained with DiI). After 48 h, the rabbits were sacrificed, and the tumors were removed to prepare frozen sections. The tumor cells in the frozen sections were stained with DAPI and observed by CLSM.
In vivo HIFU ablation
A Model-JC200 Focused Ultrasound Tumor Therapeutic system (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China) was used for all HIFU experiments. The focal length, diameter, and operating frequency were 220 mm, 145 mm, and 0.94 MHz, respectively. A high-energy ultrasound beam was emitted from the therapeutic transducer, and the ultrasound grayscale changes in the targeted tissue were monitored using an ultrasound diagnostic transducer (Esaote, Italy, with a probe frequency range of 3.5–5.0 MHz) in real-time, and analyzed by Gray Val 1.0 software provided with the therapeutic system.
Fifty tumor-bearing rabbits were randomly divided into five groups with 10 rabbits in each group: Group I: HIFU + PBS, Group II: HIFU + B. longum, Group III: HIFU + PFH/BL-NPs, Group IV: HIFU + Bifidorobot, and Group V: HIFU + B. longum + Antibodyobot. First, the rabbits in Group V were injected intravenously with 2 ml of B. longum (4 × 108 bacilli/mL). Seven days later, the rabbits in Group I were injected intravenously with 2 ml PBS, whereas those in Group II were injected intravenously with 2 ml of B. longum (4 × 108 bacilli/mL). Rabbits in Group III were injected intravenously with 2 ml PFH/BL-NPs (0.5 mg/mL), those in Group IV were injected intravenously with 4 ml of Bifidorobots, and those in Group V were injected intravenously with 2 ml of Antibodyobots. In all rabbits, the treatment area was depilated the day before HIFU ablation. Before treatment, the sound power was calibrated with radiation pressure. The tumor-bearing rabbits were anesthetized with sodium pentobarbital (30 mg/kg) and placed on the HIFU treatment bed in a prone position. The tumor site was completely immersed in degassed water (for acoustic coupling). The path from the transducer to the tumor was aligned along the z-axis. All rabbits were treated with the same power in the HIFU ablation experiments for systematic evaluation (acoustic power 250 W, irradiation duration 5 s). After 24 h of HIFU ablation, all tumor-bearing rabbits were sacrificed. The tumor was completely dissected and cut longitudinally into two parts from the middle in the direction of HIFU treatment. One-half of the sections was stained with 2,3,5-triphenyltetrazolium chloride (TTC) solution at 37 °C for 30 min to determine the coagulative necrosis volume. The coagulative necrotic volume (V) was calculated as follows: V (mm3) = (π/6) × length × width × depth. The other tumor sections were used for hematoxylin–eosin (HE) staining.
Effect of B. longum on HIF-1α expression in tumors
Eighteen tumor-bearing rabbits were randomly divided into two groups with nine rabbits in each group. All rabbits in one group were injected with 2 ml of B. longum (4 × 108 bacilli/mL), whereas those in the other group were injected with 2 ml of PBS. The rabbits in both the groups were sacrificed at 1, 3, and 7 days after injection, and the tumors were removed and sectioned. Immunohistochemical staining was performed to detect the expression of hypoxia-inducible factor (HIF)-1α. Positive HIF-1α expression was indicated as tan, brownish yellow, or light yellow colors in the sections. For each section, five fields of view were randomly selected for analysis (200× magnification). Image-Pro Plus 6.0 software (Rockville, MD) was used to measure the integral optical density value and area of positive cells in the tumor section, and to calculate the mean optical density value. A larger mean optical density value indicated a stronger intensity of the positive reaction.
Statistical analysis
All data were analyzed with GraphPad Prism version 6.0 software (GraphPad, Inc., La Jolla, CA). Quantitative data were expressed as the mean ± standard deviation. One-way analysis of variance and multi-way analysis of variance was used for multiple-group comparisons. Student’s t-test was utilized to determine the significance of differences between two groups. p-values < .05 were considered as statistically significant (NS, not significant, *p < .05, **p < .01, ***p < .001, ****p < .0001)..
Results
Synthesis and characterization of BL-NPs and PFH/BL-NPs
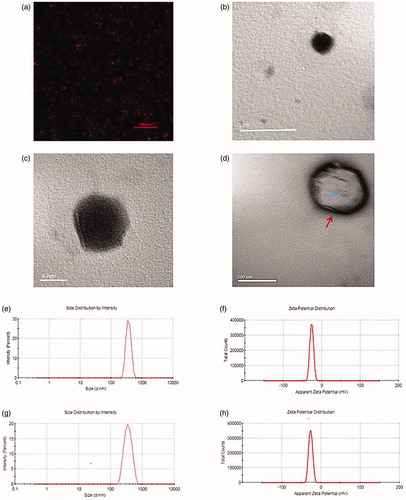
We obtained BL-NPs and PFH/BL-NPs by a single emulsification method. Both samples exhibited a uniform emulsion when observed visually. As shown in the CLSM and TEM images, the BL-NPs and PFH/BL-NPs were spherical in shape and uniform in size ().The average diameters of the BL-NPs and PFH/BL-NPs were 317.9 ± 11.64 nm (PDI = 0.049) and 364.4 ± 53.49 nm (PDI = 0.113), respectively (). The average zeta potentials of the BL-NPs and PFH/BL-NPs were-15.67 ± 2.04 mV and −23.1 ± 5.46 mV, respectively (), and the encapsulation efficiency of PFH was 58.51 ± 1.78%.
Figure 2. Morphology and characterization. (a) OM of PFH/BL-NPs. (b,c) TEM of PFH/BL-NPs. (d) TEM of BL-NPs, the red arrow refers to the liposome membrane and the blue arrow refers to the bubble. (e) Size distribution of BL-NPs (n = 3). (f) Zeta potentials of PFH/BL-NPs (n = 3). (g) Size distribution of PFH/BL-NPs (n = 3). (h) Zeta potentials of PFH/BL-NPs (n = 3).
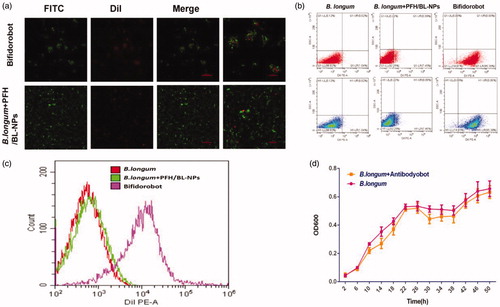
Cross-linking of PFH/BL-NPS and B. longum in vitro
To simulate the in vivo experimental environment, PFH/BL-NPs were cross-linked with SBA to prepare the antibody, which was incubated in serum for 48 h and then reacted with B. longum and ligated. CLSM showed that DiI-labeled red fluorescent PFH/BL-NPs and FITC-labeled green fluorescent B. longum were cross-linked in the B. longum and Antibodyobot reaction group, whereas no cross-linking was observed in the blank control group (). In addition, flow cytometry analysis showed that the cross-linking rate between B. longum and PFH/BL-NPs was 84 ± 6.23% (). The B. longum + Antibodyobot displayed a growth curve similar to that of B. longum (), indicating that the growth activity of B. longum was not affected by cross-linking with PFH/BL-NPs.
Figure 3. In vitro conjunction of PFH/BL-NPs and B. longum. (a) CLSM images of DiI-labeled PFH/BL-NPs, FITC-labeled B. longum and Bifidorobot. (b,c) FCM images of binding efficiencies of PFH/BL-NPs and B. longum (n = 3). (d) Growth characteristics of B. longum and B. longum + Antibodyobot in vitro (n = 3).
Targeting ability and retention capacity in vivo
Small animal live fluorescence imaging
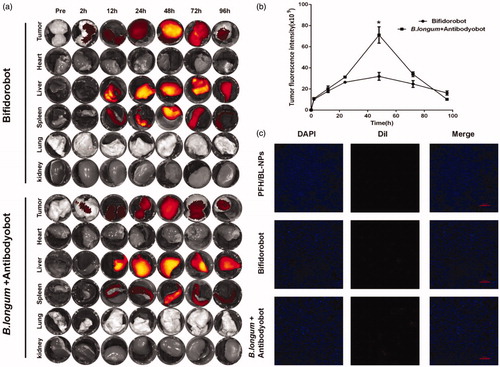
As described above, two groups of tumor-bearing rabbits were sacrificed at different time points (before injection and at 2, 12, 24, 48, 72, or 96 h after injection), and the tumor, heart, liver, spleen, lung, and kidney were removed for immediate observation via FLI. At 48 h, the fluorescence intensity of tumors in both groups reached a maximum; however, the fluorescence intensity of the B. longum + Antibodyobot group was significantly higher than that of the Bifidorobot group (*p < .05) (). Over time, the fluorescence intensity of the tumors in the two groups decreased and was decreased significantly after 96 h.
Figure 4. Targeting ability of Bifidorobot and Antibodyobot in vivo. (a) Fluorescence images of Bifidorobot and B. longum + Antibodyobot group at pre, 24 h, 48 h, 72 h, and 96 h of injection. (b) Quantification of fluorescence intensity at the tumor site (n = 10, *p < .05). (c) Frozen slice of PFH/BL-NPs, Bifidorobot and B. longum + Antibodyobot group at 48 h after injection (n = 6).
Retention of PFH/BL-NPs in frozen tumor sections
To determine the retention efficiency of PFH/BL-NPs in tumors using different delivery methods, we injected tumor-bearing rabbits with PFH/BL-NPs, Bifidorobot, and B. longum + Antibodyobot. At 48 h after injection, the fluorescence intensity of the DiI-labeled red fluorescent PFH/BL-NPs in the PFH/BL-NPs, Bifidorobot, and B. longum + Antibodyobot groups increased sequentially, indicating that the concentration of PFH/BL-NPs in the three groups of tumors increased sequentially (). These results agree with those of FLI.
In vivo HIFU ablation
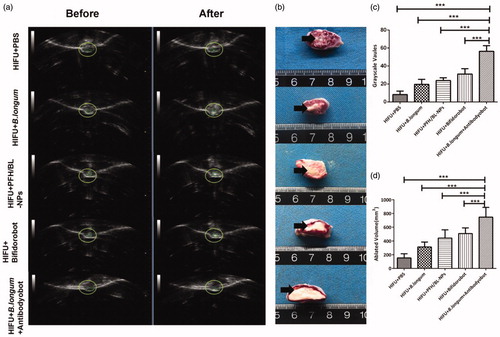
Based on the imaging results, we chose 48 h after nanoparticle injection as the time point for HIFU ablation. At this time point, the efficiency of retention of nanoparticles in the tumor was highest. In all groups, varying degrees of ultrasound grayscale changes were observed after ablation (). However, the ultrasound grayscale change of the B. longum + Antibodyobot group was the most obvious and significantly differed from that of other groups (***p < .001) (). After TTC staining, normal tissues appeared as red, whereas coagulated necrotic tissues appeared as white because of ablation (). Coagulation necrosis was observed in all groups after ablation; however the volume of coagulation necrosis was highest in the B. longum + Antibodyobot group and significantly differed from that in the other groups (***p < .001) ().
Figure 5. Synergistic HIFU treatment in vivo. (a) Ultrasonic gray scale changes before and after HIFU ablation. (b) Coagulative necrosis of tumors by TTC staining after HIFU therapy. (c) Quantitative analysis of ablated volume (n = 10, ***p < .001). (d) Quantitative analysis of gray scale values (n = 10,***p < .001).
Effect of B. longum on HIF-1α expression in tumors
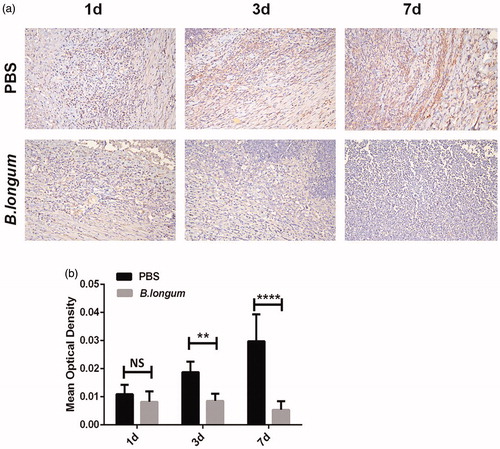
According to the results and statistical analysis, there was no significant difference in the number of HIF-1α-positive cells in the two groups of tumors at 1 day after injection. However, the number of HIF-1α-positive cells in tumors of the B. longum group was significantly lower at 1 day after injection than that in the control group at 3 and 7 days after injection (). The expression of HIF-1α in tumors of the B. longum group began decreasing progressively compared to that in the control group at 3 days after injection (**p < .01). At 7 days after injection, the expression of HIF-1α in the tumors of the B. longum group was significantly reduced compared to that in the control group (****p < .0001) ().
Figure 6. Bifidobacterium longum effects on HIF-1α expression in tumors. (a) Expression of HIF-1α in tumors at different times after injection of B. longum and PBS. (b) Quantitative analysis of average optical density value of HIF-1α-positive expression in each group (n = 3, **p < .001,****p < .0001).
Discussion
In this study, we developed a new approach for delivering PFH/BL-NPs into deeply embedded solid tumors in live animals based on the tumor-targeting ability of B. longum to enhance the treatment efficiency and safety of HIFU for solid tumors. We successfully synthesized PFH/BL-NPs. The liposomes prepared in this study were PEGylated, which reduced their rapid elimination by the mononuclear phagocytosis system in systemic circulation, thereby maximizing their accumulation in the target area [Citation25,Citation26]. In this study, the tumor-targeting ability of B. longum and safety of Antibodyobots were preliminarily verified (Figure S1–4). The in vitro cross-linking of B. longum and PFH/BL-NPs was observed by CLSM; considering that SBA and PFH/BL-NPs are cross-linked via biotin-avidin, one SBA can be linked with four PFH/BL-NPs. According to the agglomeration method shown in and considering that the B. longum antibody is an IgG antibody, the B. longum antibody may simultaneously bind to more than one B. longum. Moreover, before flow cytometry analysis, the antibody was placed in serum for 48 h and then reacted with and bound to B. longum, indicating that this cross-linking method is both efficient and stable. Comparison of the growth curves showed that cross-linking with PFH/BL-NPs did not affect the biological activity of B. longum.
Analysis of the targeting ability demonstrated that all delivery methods can deliver PFH/BL-NPs to solid tumors through blood circulation. The retention was highest at 48 h, which was determined to be the best time point for treating tumors. In addition, the results confirmed that B. longum cross-linked to PFH/BL-NPs has good tumor-targeting ability through the biotin-avidin interaction; however, the retention time of PFH/BL-NPs delivered through B. longum + Antibodyobot was much higher. Based on analysis of frozen sections, the retention capacity for nanoparticles in decreasing order is as follows: B. longum + Antibodyobot group > Bifidorobot group > PFH/BL-NPs group. Thus, first allowing colonization of B. longum followed by Antibodyobot delivery may lead to a larger number of nanoparticles in the target area compared to direct delivery of Bifidorobots.
Analysis of HIFU ablation indicated that the efficiency of HIFU treatment was the highest in the B. longum + Antibodyobot group, and previous experiments showed that this group retained a large number of nanoparticles. If the same therapeutic effect is desired, the treatment time should be reduced, which can also improve the safety of HIFU treatment. In contrast, the Bifidorobot group showed significantly different results from the first three groups; however, the gray change and tumor ablation volume were significantly weaker in the Bifidorobot group than in the B. longum + Antibodyobot group (***p < .001) (). Thus, this delivery method can improve the therapeutic effect to some extent but is weaker than the effects observed when B. longum is delivered first.
The results of HE staining (Figure S5) and measurements of the rabbit weight (Figure S6) after HIFU treatment demonstrated the safety of HIFU combined with B. longum and PFH/BL-NPs for treating solid tumors. The results of HE staining (tumor part) and measurements of tumor volume (Figure S7) after HIFU treatment indicated that the B. longum + Antibodyobot group had the best HIFU treatment effect. By comparing the ablation volume of HIFU in vitro (Figures S8 and S9) and in vivo, the accumulation of nanoparticle retention in tumors in the HIFU + Bifidorobot group and the HIFU + B. longum + Antibodyobot group was found to exceed 0.05 mg (Figure S10).
The reason why B. longum + Antibodyobot group has the best HIFU ablation effect may be because of agglomeration caused by the cross-linking method, as observed by CLSM in in vitro cross-linking analysis. The volume of Bifidorobot is larger than Antiboyobot, which may make it more difficult to enter the tumor. Second, the proliferation of B. longum in tumor tissues can activate macrophages to enhance their phagocytosis activity [Citation27]. Because endocytosis is the main mechanism by which liposomes enter cells, a larger number of activated macrophages may result in more PFH/BL-NP being engulfed, increasing the retention of PFH/BL-NP in tumors. Third, the proliferation of B. longum in tumors can increase the permeability and retention of blood vessels by increasing the level of nitric oxide [Citation28,Citation29]. This makes it easier for PFH/BL-NPs to penetrate the vascular endothelium and enter the tumor tissue. Finally, the hypoxic condition in the tumor is one cause of drug resistance [Citation8], and previous experiments have shown that B. longum can inhibit the hypoxic area of tumors; therefore, this result may also be related to decreased tumor resistance to PFH/BL-NPs due to the presence of B. longum.
Conclusion
This is the first study to report successful cross-linking of PFH/BL-NPs with B. longum through the biotin-avidin interaction and deliver the cross-linked product to living solid tumors. Two delivery methods were compared: (I) direct delivery of Bifidorobots; (II) colonization of tumor with B. longum for 7 days, followed by delivery of Antibodyobots to the colonized tumor. The latter delivery method provides better imaging and more effective tumor treatment, providing a basis for further clinical work.
Supplemental Material
Download PDF (1.6 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Brown JM. Tumor hypoxia in cancer therapy. Meth Enzymol. 2007;435:297–321.
- Xia Y, Choi HK, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem. 2012;49:24–40.
- Moeller BJ, Cao Y, Li CY, et al. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5(5):429–441.
- Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 2007;26(2):319–331.
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4(6):437–447.
- Lefevre CT, Bernadac A, Yu-Zhang K, et al. Isolation and characterization of a magnetotactic bacterial culture from the Mediterranean Sea. Environ Microbiol. 2009;11(7):1646–1657.
- Park SJ, Park SH, Cho S, et al. New paradigm for tumor theranostic methodology using bacteria-based microrobot. Sci Rep. 2013;3:3394.
- Taniguchi S, Fujimori M, Sasaki T, et al. Targeting solid tumors with non-pathogenic obligate anaerobic bacteria. Cancer Sci. 2010;101(9):1925–1932.
- Zhou H, He Z, Wang C, et al. Intravenous administration is an effective and safe route for cancer gene therapy using the Bifdobacterium mediated recombinant HSV-1 thymidine kinase and ganciclovir. IJMS. 2016;17(6):891.
- Gao X, Zou WJ, Jiang BL, et al. Experimental study of retention on the combination of Bifidobacterium with High-Intensity Focused Ultrasound (HIFU) synergistic substance in tumor tissues. Sci Rep. 2019;9(1):6423.
- Hwang JH, Crum LA. Crum current status of clinical high-intensity focused ultrasound. Conf Proc IEEE Eng Med Biol Soc. 2009;2009):130–133.
- Hsiao YH, Kuo SJ, Tsai HD, et al. Clinical application of High-intensity focused ultrasound in cancer therapy. J Cancer. 2016;7(3):225–231.
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5(4):321–327.
- Umemura S, Kawabata K, Sasaki K. In vivo acceleration of ultrasonic tissue heating by microbubble agent. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52(10):1690–1698.
- Zhou X, Wen L, He G, et al. SonoVue-enhanced high intensity focused ultrasound ablation on rabbit VX2 liver tumors. Ultrasound Med Biol. 2009;8:220–220.
- Luo W, Zhou X, Ren X, et al. Enhancing effects of SonoVue, a microbubble sonographic contrast agent, on high intensity focused ultrasound ablation in rabbit livers in vivo. Ultrasound Med. 2007;26(4):469–476.
- Li Q, Du J, Yu M, et al. Transmission electron microscopy of VX2 liver tumors after high-intensity focused ultrasound ablation enhanced with SonoVue. Adv Ther. 2009;26(1):117–125.
- Romoren K, Fjeld XT, Poleo AB, et al. Transfection efficiency and cytotoxicity of cationic liposomes in primary cultures of rainbow trout(Oncorhynchus mykiss) gill cells. Biochim Biophys Acta. 2005;1717(1):50–57.
- Soenen SJ, De Cuyper M. How to assess cytotoxicity of (iron oxide-based) nanoparticles: a technical note using cationic magnetoliposomes. Contrast Media Mol Imaging. 2011;6(3):153–164.
- Ektate K, Munteanu MC, Ashar H, et al. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots). Sci Rep. 2018;8(1): 13062.
- Luo Y, Xu D, Gao X, et al. Nanoparticles conjugated with bacteria targeting tumors for precision imaging and therapy. Biochem Biophys Res Commun. 2019;514(4):1147–1153.
- Luo CH, Huang CT, Su CT, et al. Bacteria-mediated hypoxia-specific delivery of nanoparticles for tumors imaging and therapy. Nano Lett. 2016;16(6):3493–3499.
- Wang L, Vuletic I, Deng D, et al. Bifidobacterium breve as a delivery vector of IL-24 gene therapy for head and neck squamous cell carcinoma in vivo. Gene Ther. 2017;24(11):699–705.
- Zhang B, Jiang T, Tuo YY, et al. Captopril improves tumor nanomedicine delivery by increasing tumor blood perfusion and enlarging endothelial gaps in tumor blood vessels. Cancer Lett. 2017;410:12–19.
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–651.
- Rapoport NY, Efros AL, Christensen DA, et al. Microbubble generation in phase-shift nanoemulsions used as anticancer drug carriers. Bubble Sci Eng Technol. 2009;1(1–2):31–39.
- Mokrozub VV, Lazarenko LM, Babenko LP, et al. Effect of probiotic strains of lacto- and bifdobacteria on the activity of macrophages and other parameters of immunity in cases of staphylococcosis. Mikrobiol Z. 2012;74:90–98.
- Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151.
- Maeda H, Fang J, Inutsuka T, et al. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol. 2003;3(3):319–328.