Abstract
Background
Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been shown to provide benefits in the management of peritoneal metastasis. Cisplatin (CDDP) is one of the most frequently used drugs for peritoneal infusion. A major restriction is that CDDP causes renal toxicity and acute renal failure, sometimes leading to chronic renal failure. The aim of the present study was to assess the impact of sodium thiosulfate (ST) in preventing renal impairment (RI) following HIPEC with CDDP.
Methods
This prospective study assessed the RI rates for all patients who underwent HIPEC with CDDP during two successive periods: without ST (nST Period; from November 2016 to September 2017) and with ST (ST Period; from October 2017 to March 2018). During the ST Period, patients received an ST infusion at 9 mg/m2 prior to HIPEC and at 12 mg/m2 at the end of the procedure. RI was defined by postoperative serum creatinine >1.6 times elevation of baseline value. The impact of ST treatment was evaluated by comparison of the RI rates between the two periods.
Results
During ST Period, none of 38 patients (0%) developed RI versus 11/35 patients (31.4%) during the nST Period (p < .005); 2 of whom required definitive hemodialysis. Baseline characteristics, background circumstances, indications and laboratory parameters before HIPEC were comparable between the two groups, as well as CDDP dose use during HIPEC.
Conclusion
ST appears to be an effective drug for the prevention of the renal toxicity of CDDP used for HIPEC and should be used for all such procedures.
Introduction
Cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy (HIPEC) has become an unavoidable therapy in the management of a number of peritoneal carcinomatoses. Cisplatin is one of the most frequently used chemotherapies during HIPEC; it is used for several indications such as peritoneal mesothelioma and peritoneal metastasis of ovarian, endometrial, or gastric origin. HIPEC with cisplatin (CDDP) has proven interesting in long-term survival for these conditions [Citation1–5].
The main side effect of CDDP is its acute and chronic nephrotoxicity, whether used systemically or intraperitoneally. First, CDDP induces early proximal renal tubular damage, leading to an acute or subacute tubular necrosis; subsequently acute renal failure can occur early during the first postoperative days following HIPEC with CDDP. Second, CDDP can induce a gradual and irreversible decrease in long-term glomerular filtration capacity, and therefore chronic renal failure. This toxicity is the main restriction to the use of intraperitoneal CDDP; acute renal failure rate after HIPEC with CDDP is reported to be up to 40.3% [Citation6–9], and the rate of severe chronic renal failure (grade 3 or 4, i.e. glomerular filtration rate <30 ml/min) is estimated to be up to 8.5% [Citation6–11]. These acute or chronic renal failures lead to long-term dialysis in up to 4.3% of patients, and may also compromise the subsequent administration of other anticancer agents for consolidation or in case of recurrence.
Sodium thiosulfate (ST) is a pharmacological agent that has been identified for many years to prevent and treat toxicities induced by the systemic use of platinum salts. The mechanism of action of ST is unclear, but its benefits in systemic CDDP chemotherapy are recognized [Citation12]; in particular, ST is used as a protective and curative agent [Citation13] for renal failure induced by a CDDP overdose [Citation14]. This led to the hypothesis that ST may also be of benefit in the prevention of renal failure induced by HIPEC with CDDP [Citation15,Citation16]. In their randomized trial, Van Driel et al. reported no increased frequency of renal failure with the use of Sodium Thiosulfate following HIPEC with CDDP [Citation5]. In France, as in some other countries, the use of this agent needs a specific and nominative request (ATU – Authorization for Temporary Use) to the national medicines agency (Agence nationale de sécurité du médicament et des produits de santé in France) and is not used.
The aim of this study was to assess the impact of ST in the prevention of renal impairment during HIPEC with CDDP from a prospective database.
Patients and methods
Protocol
In this single-center study, all consecutive patients who underwent cytoreductive surgery with HIPEC with CDDP between November 2016 and April 2018 at the Lyon-Sud Hospital were included. From October 2017 and April 2018, all consecutive patients received ST following an individual specific and nominative request (ATU) to the ANSM (the ST period). They were compared to consecutive patients treated from November 2016 to September 2017 (the nST period).
Treatment
All patients were managed perioperatively with a standardized and equivalent protocol: immunonutrition for 7 days, discontinuation of nephrotoxic treatments, preoperative hydratation for 48 h, and bowel preparation. Patients received HIPEC with CDDP after a complete (CC-0) or sub-complete (CC-1) cytoreductive surgery [Citation17]. The intraperitoneal infusion of CDDP was between 50 and 100 mg/m2, with a mesenteric temperature of 42 °C, for a duration of 60–90 min regarding the protocol used [Citation5,Citation7,Citation18]. Pre- and post-operative clinical and laboratory data were prospectively collected and recorded in an identified database. No patient was excluded. For administration of ST the protocol described by Van Driel et al. was used [Citation5]: patients received a first intravenous bolus of 9 mg/m2 initiated at the same time of the start of CDDP intraperitoneal perfusion, and delivered in 20 min; they then received a second infusion of 12 g/m2 for 6 h, initiated at the end of the HIPEC ().
Figure 1. Sodium Thiosulfate administration. Van Driel et al. [Citation5,p.230–240].
![Figure 1. Sodium Thiosulfate administration. Van Driel et al. [Citation5,p.230–240].](/cms/asset/a6528441-cff0-4ccb-b11a-c384a2ce9223/ihyt_a_1795277_f0001_c.jpg)
Objectives
In order to evaluate the impact of ST in terms of nephroprotection, the primary objective was to compare the rate of postoperative acute renal impairement between patients treated over the two periods. The occurrence of acute renal failure was defined by a postoperative increase in serum creatinine to more than 1.6 times elevation of the basal value (World Health Organization grade I toxicity) during the first postoperative month [Citation19–21]. The secondary objectives were to compare the chronic renal failure rate following the third postoperative month and the long-term dialysis rate between the two periods. Potential side effects of ST, metabolic acidosis and allergic reactions, were searched for and collected.
Data collection
Pre-operative data were collected for each patient: clinical data (baseline characteristics, etiology and indication) and laboratory data (baseline serum creatinine level, and glomerular filtration rate). The intraoperative data collected were: surgical data (extent of peritoneal carcinomatosis evaluated by the Peritoneal Carcinomatosis Index (PCI), dose of intra-peritoneal CDDP used, duration of HIPEC, duration of procedure, and blood loss); anesthesiological data and medical management during procedure (intraoperative diuresis (ml and ml/kg/h), intravenous solute and amount infused (ml/kg/h), potential transfusions of blood-derived products (U), hemodynamic instability, and the use of vasopressor amines during the procedure (mg)); post-operative data (daily serum creatinine level and glomerular filtration rate from day 0 to day +10, serum creatinine level and glomerular filtration rate at 1 month, 3 months and 6 months, as well as other standard post-operative biological data: daily hemoglobin level, pH and lactic acid at +12 h and +36 h); surgical complications as well as other medical complications.
Statistical analysis
The Chi2 test was used to evaluate the differences between the two periods. Qualitative variables were compared using the Chi2 test. Quantitative variables were compared using the Wilcoxon test. Statistical significance was set at p < .05 for all analyses that were performed using SSPS 17.0® (SPSS Inc, Chicago, IL, USA).
Results
A total of 35 patients underwent HIPEC with CDDP during the nST period (without the use of ST) and 38 patients did so during the ST period (with the use of ST). The baseline characteristics of patients were not significantly different between the two study periods (), nor were the variables pertaining to intraoperative medical data, and the rates of surgical and medical complications ().
Table 1. Baseline characteristics and indications.
Table 2. Surgical and medical data: intraoperative surgical data, intra-operative medical management and postoperative complications.
Acute renal failure
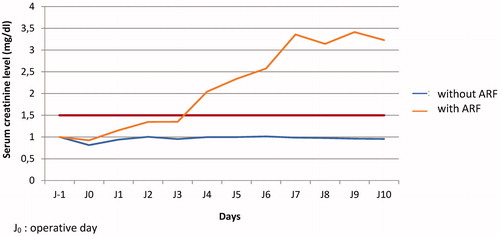
Eleven of the 35 patients (31.4%) who underwent an HIPEC with CDDP without the use of ST (nST Period) developed postoperative acute renal failure, compared to none of the 38 patients who received ST (0%) (TS period; p < .05). In the nTS period, the mean time to occurrence of acute renal failure was 4 days according to the WHO definition. A rise in serum creatinine was observed compared to the basal values on post-operative day 1 (median 1.154 mg/dl) for patients who evolved toward acute postoperative renal failure and those who did not. This difference was significant on post-operative day 2 (median 1.349, p < .005) and day 3 (median 1.352, p < .005); this was below the threshold of 1.6 times elevation of the basal value according to the WHO definition ().
Chronic renal impairment
Nine of the 11 patients who presented post-operative acute renal failure in the nST group (25.7%) subsequently evolved to chronic grade-3 or higher renal failure (glomerular filtration rate GFR <30%), following the third postoperative month, including 2 patients with grade-4/5 renal impairment (GFR <15%) and 2 patients needing a long-term dialysis (5.7%; ).
Table 3. Grade 3 or higher chronic renal impairment after HIPEC with cisplatin following the third postoperative month.
Side effects
No metabolic acidosis or allergic reaction was observed in any of the patients who received ST. There was no significant difference between the two groups for the pH level in the first 48 post-operative hours; mean pH was 7.29 (± 0.09) in the nST period versus 7.31 (± 0.07) in the ST period at + 12 h (p = .636), and 7.37 (± 0.14) versus 7.41 (± 0.92) at +36 h (p = .211). There was also no significant difference in post-operative lactic acid levels; mean arterial lactic acid concentration was 4.2 mmol/L (± 6.24) in the nTS period versus 4.6 mmol/L(± 2.06) in the ST period at + 12 h (p = .390), and 2.2 (± 0.80) versus 1.7 (± 0.69) at + 36 h (p = .133).
Discussion
In the present study, the use of ST during HIPEC with CDDP led to a lower of the rate of acute post-operative renal failure to zero. None of the potential side effects described or reported in the literature were observed. The results are consistent with those from other studies. On a similar type of use, Tilleman et al. have reported the effectiveness of ST in the prevention of the occurrence of renal failure after thoracic intracavitary hyperthermic chemotherapy during the surgical management of pleural mesothelioma [Citation22]; despite intrathoracic CDDP was used at a high dose (225 mg/m2), the use of ST reduced the renal toxicity of this procedure to 6%. This is higher than observed herein, but which could at least partly be explained by the higher doses of intracavitary CDDP used for the management of pleural mesothelioma. In addition, some teams already routinely use ST to prevent the nephrotoxicity induced by HIPEC with CDDP [Citation5,Citation15], and the rates of acute postoperative renal failure observed (<5%) are considerably lower than those described by some teams (up to more than 40%) [Citation6,Citation8–10,Citation23,Citation24].
As a nonspecific agent, ST has been used for many years in variable and heterogeneous indications. In nephrology, its chelation of cations led to it being used for the treatment of calciphylaxis occurring in patients under dialysis [Citation11]. Historically, it has been used for the treatment of ringworm or as an antifungal agent, and its use is also described in the management of cyanide poisoning [Citation25]. It has antioxidant properties related to its reaction with oxidized glutathione and reactive oxygen species leading to the formation of glutathione, a natural antioxidant; it would therefore restore the functions of endothelial cells. In addition, ST also interacts with various enzymes by transsulfurization producing hydrogen sulfite, a vasodilator at the microcirculatory level. The plasma concentration of ST increases linearly with the injected dose. The half-life of the plasma distribution phase is approximately 23 min. Regarding its elimination, some of the injected ST is oxidized into sulfite and then into sulfate at the hepatic level. Only a small fraction of thiosulfate is incorporated into the endogenous sulfur compounds. ST is then mainly eliminated by the renal route, by glomerular filtration, and secretion. In healthy subjects, renal clearance is approximately 1.86 ml/min/kg and non-renal clearance is 2.25 ml/min/kg, for an elimination half-life of approximately 16–80 minutesr. The reported side effects of ST are rare. Metabolic acidosis and a QT prolongation in patients with chronic renal impairment or dialysis patients were reported; furthermore possible anaphylactic reactions have been described, but none of these adverse effects were found in the present study.
A pharmacological peculiarity of ST, that is rarely discussed, is that it has a cationic chelating activity which equates it with an anti-alkylating agent. These anti-alkylating properties also make it use in direct subcutaneous instillation to prevent or treat tissue destruction induced by accidental extravasation of CDDP during systemic chemotherapy [Citation26]. From an oncological perspective, it therefore appears reasonable to question the use of a potentially anti-alkylant agent during the use of an alkylant chemotherapy such as CDDP. This has already been studied in a model of neuroblastoma, and it has been demonstrated that the use of ST does not compromise the antitumour effect of CDDP, both in vitro and in an animal model. This was confirmed in vitro and in murine models [Citation27,Citation28], and for endometrial and ovarian carcinomas [Citation28]. Although these studies appear reassuring as to a potential decrease in the effectiveness of chemotherapy, there is still no prospective study describing the impact of ST on clinical outcomes, in particularly long-term survival. However, data reported from the Dutch trial led by Van Driel et al. [Citation5], which assessed the impact of the addition of HIPEC with CDDP to interval cytoreductive surgery in the treatment of peritoneal metastases from ovarian origin do support the innocuousness of ST antitumoral activity of intraperitoneally administered CDDP; as ST was systematically used in this trial in the HIPEC group.
The main limitation of the present study concerns the comparison of two successive periods. However, no element of pre- or intra-operative management differed from one group to the other, and the groups were completely comparable. The only way to provide a higher level of proof would be a randomized study, but this would be of little interest.
Given the important renal toxicity of HIPEC with HIPEC and its potential serious consequences in the long term such as dialysis, and considering the results herein and those reported elsewhere, it appears not reasonable at this time not to use the weapon that is Sodium Thiosulfate to prevent the nephrotoxicity induced by HIPEC with HIPEC.
Conclusion
The use of ST appears to be a reliable and effective means to prevent the occurrence of acute and chronic renal failures induced by HIPEC with CDDP, and should be used for HIPEC and should be used for all such procedures.
Acknowledgements
We thank Mr Philip Robinson for the English language editing and review of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. JCO. 2009;27(36):6237–6242.
- Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116(24):5608–5618.
- Chia CS, You B, Decullier E, et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol. 2016;23(6):1971–1979.
- Bakrin N, Cotte E, Sayag-Beaujard A, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for the treatment of recurrent endometrial carcinoma confined to the peritoneal cavity. Int J Gynecol Cancer. 2010;20(5):809–814.
- van Driel WJ, Koole SN, Sonke GS. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(14):1363–1364.
- Antoun S, Meshaka P, Soltani D, et al. Complications and tolerance of heated intraperitoneal chemotherapy and cytoreductive surgery for peritoneal carcinomatosis: results of a phase I-II study of peritoneal carcinomatosis from different sources. Bull Cancer. 2000;87(9):665–670.
- Bakrin N, Bereder JM, Decullier E, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013;39(12):1435–1443.
- Roviello F, Marrelli D, Neri A, et al. Treatment of peritoneal carcinomatosis by cytoreductive surgery and intraperitoneal hyperthermic chemoperfusion (IHCP): postoperative outcome and risk factors for morbidity. World J Surg. 2006;30(11):2033–2040. discussion 2041-2032.
- Kusamura S, Baratti D, Younan R, et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550–2558.
- Sin EI, Chia CS, Tan G, Soo KC, et al. Acute kidney injury in ovarian cancer patients undergoing cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. Int J Hyperthermia. 2017;33(6):690–695.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43(6):1104–1108.
- Brock PR, Maibach R, Childs M, et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med. 2018;378(25):2376–2385.
- Dickey DT, Wu YJ, Muldoon LL, et al. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther. 2005;314(3):1052–1058.
- Selk N, Rodby RA. Unexpectedly severe metabolic acidosis associated with sodium thiosulfate therapy in a patient with calcific uremic arteriolopathy. Semin Dial. 2011;24(1):85–88.
- Guastalla JP, Vermorken JB, Wils JA, et al. Phase II trial for intraperitoneal cisplatin plus intravenous sodium thiosulphate in advanced ovarian carcinoma patients with minimal residual disease after cisplatin-based chemotherapy-a phase II study of the EORTC Gynaecological Cancer Cooperative Group. Eur J Cancer. 1994;30A(1):45–49.
- van Rijswijk RE, Hoekman K, Burger CW, et al. Experience with intraperitoneal cisplatin and etoposide and i.v. sodium thiosulphate protection in ovarian cancer patients with either pathologically complete response or minimal residual disease. Ann Oncol. 1997;8(12):1235–1241.
- Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5(4):219–228.
- Bakrin N, Classe JM, Pomel C, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. J Visc Surg. 2014;151(5):347–353.
- Ichai C, Vinsonneau C, Souweine B, et al. Acute kidney injury in the perioperative period and in intensive care units (excluding renal replacement therapies). Anaesth Crit Care Pain Med. 2016;35(2):151–165.
- Ichai C, Vinsonneau C, Souweine B, et al. Acute kidney injury in the perioperative period and in intensive care units (excluding renal replacement therapies). Ann Intensive Care. 2016;6(1):48
- McIlroy DR, Bellomo R, Billings FT, et al. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: renal endpoints. Br J Anaesth. 2018;121(5):1013–1024.
- Tilleman TR, Richards WG, Zellos L, et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg. 2009;138(2):405–411.
- Glehen O, Osinsky D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10(8):863–869.
- Hakeam HA, Breakiet M, Azzam A, et al. The incidence of cisplatin nephrotoxicity post hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery. Ren Fail. 2014;36(10):1486–1491.
- Hall AH, Dart R, Bogdan G. Sodium thiosulfate or hydroxocobalamin for the empiric treatment of cyanide poisoning? Ann Emerg Med. 2007;49(6):806–813.
- Goolsby TV, Lombardo FA. Extravasation of chemotherapeutic agents: prevention and treatment. Semin Oncol. 2006;33(1):139–143.
- Harned TM, Kalous O, Neuwelt A, et al. Sodium thiosulfate administered six hours after cisplatin does not compromise antineuroblastoma activity. Clin Cancer Res. 2008;14(2):533–540.
- Inoue M, Shimizu C, Shimizu H, et al. Neutralizing effect of sodium thiosulfate on antitumor efficacy of cisplatin for human carcinoma xenografts in nude mice. Gynecol Oncol. 1991;40(1):34–37.