Abstract
Immune checkpoint blockade (ICB) comprising monoclonal antibodies (mAbs) against immune ‘checkpoints’, such as CTLA-4 and the PD1/PDL1 axis have dramatically improved clinical outcomes for patients with cancer. However, ICB by itself has failed to provide benefit in a wide range of solid tumors, where recurrence still occurs with high incidence. These poor response rates may be due to the therapeutic shortcomings of ICB; namely, a lack of cancer-specific cytotoxicity and ability to debulk tumors. To overcome these limitations, effective ICB therapy may require the combination with other complementary therapeutic platforms. Here, we propose that photothermal therapy (PTT) is an ideal therapeutic modality for combination with ICB because it can generate both tumor-specific cytotoxicity and immunogenicity. PTT elicits these specific effects because it is a localized thermal ablation technique that utilizes light-responsive nanoparticles activated by a wavelength-matched laser. While ICB immunotherapy alone improves cancer immunogenicity but does not generate robust antitumor cytotoxicity, nanoparticle-based PTT elicits targeted and controlled cytotoxicity but sub-optimal long-term immunogenicity. Thus, the two platforms offer complementary and potentially synergistic antitumor effects, which will be detailed in this review. We highlight three classes of nanoparticles used as agents of PTT (i.e., metallic inorganic nanoparticles, carbon-based nanoparticles and organic dyes), and illustrate the potential for nanoparticle-based PTT to potentiate the effects of ICB in preclinical models. Through this discussion, we aim to present PTT combined with ICB as a potent synergistic combination treatment for diverse cancer types currently refractory to ICB as well as PTT monotherapies.
Introduction
Immune checkpoint blockade (ICB) has dramatically redefined cancer immunotherapy in the past decade with the advent of monoclonal antibodies (mAbs) targeting immune ‘checkpoints’ that unleash potent antitumor immune responses [Citation1]. Normal immune cell function relies on numerous checkpoint molecules expressed on their surface to regulate activation and/or suppression. The binding of an immune checkpoint molecule to its cognate ligand on another cell effectively suppresses the immune cell activity by signaling through an inhibitory pathway. This immune cell downregulation helps to prevent autoimmunity, graft rejection and overactive inflammatory responses, by damping the activation signal [Citation2–6]. Many cancer cells exploit this phenomenon by upregulating immune checkpoint ligands on their surface, such that when an immune checkpoint molecule binds to its cognate ligand on a cancer cell, the immune cell is ‘turned off’, thereby allowing the cancer cells to proliferate without inhibition by an immune response. Critically, ICB prevents this interaction by binding to either the immune checkpoint molecule (e.g., cytotoxic lymphocytes-associated antigen-4 [CLTA-4], programed cell death protein-1 [PD-1]) or the immune checkpoint ligand (e.g., programed death-ligand 1 (PD-L1)) to suppress the inhibitory pathway and enable immune cell activation. This reversal of immunosuppression is critical for a robust antitumor immune response and has emerged as a promising strategy to treat cancer.
Although other immune cells, such as B cells, NK cells and antigen-presenting cells (APCs) like dendritic cells (DCs) and macrophages play different and unique roles during cancer immune regulation [Citation7–9], T cell activation is a key phenomenon in generating an antitumor immune response, as T cells can generate antigen-specific cytotoxicity to effectively eliminate cellular targets [Citation10,Citation11]. CTLA-4 is a homolog to CD28 found on T cells, mainly on regulatory T cells (Tregs). Binding of CD28 to molecules of the B7 axis (B7-1/CD80 or B7-2/CD86) provides costimulatory signals to T cells, generating activation. Alternatively, binding of CTLA-4 directly to CD80 or CD86 generates inhibitory signals to T cells () [Citation12–16]. The functional effect of CTLA-4 on T cell activity is controversial; a comprehensive review of this debate was published recently by Walker and Sansom [Citation17]. On the other hand, the PD-1/PD-L1 axis involving T cells is well-described. PD-L1 is a transmembrane protein expressed on many cancer cell types that bind to PD-1 on the surface of T cells to cause T cell inhibition and apoptosis (). This binding also allows differentiation of Tregs and inhibits their apoptosis, shifting the balance toward immunosuppression [Citation18].
Figure 1. Representative mechanisms of action of monoclonal antibodies used in immune checkpoint blockade. (A) The binding of CTLA-4 to CD80/86 results in inhibition of T cell activity. An antibody to CTLA-4 (aCTLA-4) prevents this inhibitory interaction resulting in T cell activation. (B) The binding of PD-L1 to PD-1 on T cells results in inhibition of T cells. Monoclonal antibodies to PD-1 (aPD-1) and PD-L1 (aPD-L1) prevent this inhibitory interaction resulting in T cell activation.
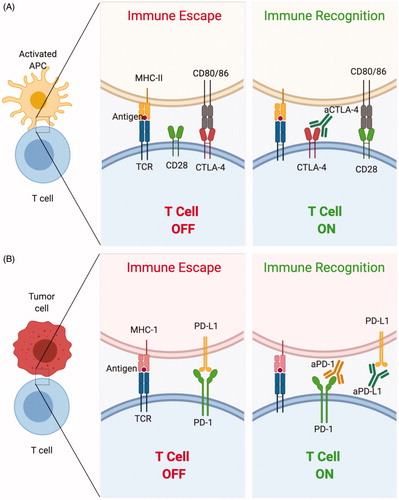
ICB comprising mAbs targeting CTLA-4, PD-1 or PD-L1 have shown promising outcomes in various malignancies. Consequently, several ICB mAbs have been US Food and Drug Administration (FDA)-approved for clinical use in diverse cancer types (). In 2018, it was reported that the percentage of US patients eligible for ICB immunotherapy increased from 1.5% in 2011 to 43.6% [Citation19]. However, these therapies are used on patients that do not respond to standard cancer therapies and typically as combination treatments. Further, most patients that underwent ICB monotherapy did not show long-term tumor remission and some did not respond to the treatment [Citation20,Citation21]. For instance, among the 43.6% patients eligible for ICB immunotherapy in 2018, the response rate was limited to 12.5% [Citation19]. In fact, the only successful clinical trials demonstrating long-term survival after ICB monotherapy used aPD-1 for the treatment of melanoma and small-cell lung carcinoma (Trial ID: NCT01295827, 2011–2019, completed) [Citation22]. Reflective of this, in almost all preclinical studies described in this review, while ICB could not effectively eradicate established tumors, it did enable improved immune responses. Additionally, ICB mAbs can generate dose-limiting and sometimes toxic immune-related adverse effects [Citation23,Citation24]. These limitations highlight the need for improving the response rates to ICB.
Table 1. US Food and Drug Administration approved immune checkpoint blockade.
An attractive strategy to boost response rates to ICB is combining them with other therapeutic modalities to improve treatment outcomes. Consequently, many groups have been investigating combination approaches with ICB that can provide ‘targets’ to reactivated immune cells with a goal of increasing tumor-specific responses, thus mitigating the limitations of ICB. ICB has been tested in combination with many standard-of-care cancer therapies including surgery, chemotherapy and radiation therapy [Citation25–30]. However, their combination with photothermal therapy (PTT) has been the focus of more recent studies.
PTT uses light-absorbing agents (primarily nanoparticles) and a wavelength-matched light source to generate heat, which can be exploited to cause thermal ablation of tumors, resulting in rapid tumor cell death and debulking [Citation31–34]. Temperatures achieved during PTT, which is a measure of thermal dose and resultant tissue ablation [Citation35–39], can be controlled based on the concentration of the PTT agents and/or the power of the incident light source. Noninvasive near infrared (NIR) wavelengths are most commonly used for PTT as these wavelengths can penetrate deeper into human tissue compared to other wavelengths [Citation40,Citation41].
Nanoparticle based-PTT has emerged as a promising cancer therapy, and it may offer several key advantages to ICB when used as a combination treatment strategy (). First, PTT is effective in generating cancer cell death. At varied thermal doses depending on cancer cell type, the heat generated during PTT can cause total tumor destruction, as shown in numerous in vitro studies and preclinical in vivo studies [Citation34,Citation42,Citation43]. However, in other models, PTT used as a monotherapy is inadequate in eliminating the tumor completely due to heterogeneous heat distributions (observed in larger tumors) and a suboptimal immune response, allowing for local recurrence and metastasis [Citation44]. This variability in PTT-based tumor eradication arises due to several factors, such as nanoparticle concentration, laser power density, thermal dose and irradiation time. Researchers are currently investigating the effects of each of these parameters on cancer cell outcomes, such as necrosis, apoptosis, immunogenicity and other cellular effects [Citation45–48]. Since ICB therapy generates excellent beneficial immune effects but requires additional help to achieve complete tumor eradication, combining PTT with ICB could work synergistically to overcome the limitations of each individual component; cancer cells are killed by PTT, followed by a systemic immune response by ICB, thereby eliminating local and distal disease.
Figure 2. Schematic representation of the advantages of combination of nanoparticle-based photothermal therapy (PTT; panel A) with immune checkpoint blockade (ICB; panel B). (A) PTT can cause 1. Total tumor ablation; 2. Release of tumor-specific antigens and adjuvants by cancer cells in the tumor microenvironment (TME) that can help recruit immune cells and 3. The nanoparticles used as PTT agents can capture the released antigens and adjuvants at the TME leading to a long-lasting and improved localized immune response. (B) Addition of immune checkpoint blockade (ICB), such as aPD-1, aPD-L1 and aCTLA-4 can increase systemic immune response and produce long-term memory. Together PTT and ICB can work synergistically as an effective combination therapy for rapid tumor debulking and improved long-term tumor protection, respectively.
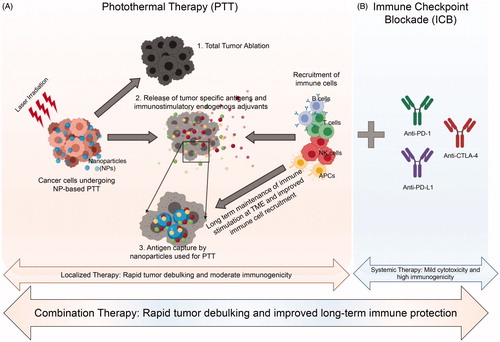
Second, PTT under certain thermal dose conditions and depending on the cancer cell type is immunostimulatory. To identify the thermal dose, a parameter called cumulative equivalent minutes at 43 °C (CEM43) is calculated by converting time-temperature profiles into a single normalized value [Citation49]. CEM43 values allow the direct comparison of heat exposure across different tissue types taking heating kinetics into account, and may enable the identification of a thermal dose that correlates with cancer-specific immunogenic cell death [Citation43,Citation50]. Several studies in different cancer models have demonstrated immunogenic effects of PTT, such as immunogenic cell death, maturation of APCs, increased tumor-infiltrating lymphocytes (TILs), increased pro-inflammatory cytokines in serum, increased effector T cells and decreased Tregs [Citation43,Citation50–54]. More recently, many researchers (including our laboratory) are exploring the antitumor immune effects of PTT to leverage an abscopal effect [Citation55–57], wherein a localized PTT intervention generates a systemic antitumor response that can eliminate untreated secondary or metastatic lesions [Citation58]. The immunostimulatory effects of PTT are (thus far) not robust enough alone to completely eradicate distal tumors or metastases but could improve the efficiency of ICB by providing complementary immunomodulation [Citation43,Citation50–54].
Lastly, recent studies are evaluating the ability of nanoparticle-based PTT to generate nanoparticle-mediated antigen capture. Since PTT triggers antigen release post-ablation [Citation59], locally residing nanoparticles can capture these released antigens, which in turn can improve immune recognition by APCs and T cells, thereby mediating an improved antitumor immune response [Citation60,Citation61]. This effect can complement ICB by providing antigen specificity to T cells; if T cells are directed to exert cytotoxicity specifically on the tumor cells while sparing antigen-poor normal cells, effective dosing and systemic toxicity may be improved.
Previous reviews have described the advantages of photoimmunotherapy using PTT and/or photodynamic therapy (PDT) with a wide range of immunostimulatory molecules including antibodies, vaccines, immune adjuvants and cytokines [Citation62–64]. Distinct from these earlier publications, in this review, we highlight the recent advances in nanoparticle-based PTT to potentiate ICB immunotherapy for treating cancer, summarize the state of the field and comment on the potential for its clinical translation. We specifically focus on metal-based inorganic nanoparticles, carbon-based nanoparticles and organic dyes as agents of PTT in combination with ICB because a significant body of literature exists for each of these platforms. Experimental details, such as nanoparticle sizes, routes of treatment administration, cell and mouse strains and other immune adjuvants included in the nanoparticle platform to boost the immune response, are listed in . Through the presented material, we seek to highlight the progress made in combining nanoparticle-based PTT with ICB, and its promise toward clinical translation.
Table 2. Information on nanoparticles formulation, immune checkpoint blockade, photothermal therapy, other immune adjuvants and targeted pre-clinical cancer models discussed in the review.
Methods
Search strategy
The databases used for systematically searching and organizing the articles included in this review were PubMed, Web of Science and Google Scholar websites. The keywords used in these search engines comprised the following terms: (PTT) AND (ICB OR immune checkpoint molecules OR CTLA-4 OR CTLA4 OR PD-1 OR PD1 OR PD-L1 OR PDL1) AND (cancer OR cancer therapy OR oncology) (nanoparticles OR Gold nanoparticles (GNPs) OR Iron oxide nanoparticles (IONPs) or Prussian blue nanoparticles (PBNPs) OR metal nanoparticle OR organic dyes) AND (PTT OR photothermal agents) AND (ICB OR immune checkpoint molecules OR CTLA-4 OR CTLA4 OR PD-1 OR PD1 OR PD-L1 OR PDL1). Data collection was done throughout the review process, thus no particular start or end date limit was set. The literature was continuously updated until April 2020. The search was restricted to combinations of PTT and immune checkpoints for cancer therapy applications.
Eligibility criteria
The studies included in this review were required to meet the following inclusion criteria: (1) Original research written in English; (2) Combines nanoparticle-based PTT and immune checkpoint molecules; (3) Restricted to the immune checkpoint molecules CTLA-4, PD-1 and/or PD-L1. A few review articles are included in general introduction to explain well-documented and extensively published topics.
Metal-based inorganic nanoparticles
Gold nanoparticles
GNPs are one of the most extensively investigated agents of PTT for cancer treatment [Citation32,Citation33,Citation62,Citation83–88]. When GNPs are excited by light of a specific wavelength, the collective oscillation of surface plasmons, a phenomenon known as surface plasmon resonance allows extinction of these surface plasmons, which is effectively dissipated as heat energy [Citation89,Citation33]. While GNP-based PTT (GNP-PTT) for preclinically treating cancer cells has been described in the literature over the past two decades, the combination of GNP-PTT with ICB antibodies has only been demonstrated in the past few years. In 2017, Liu et al. proposed a synergistic immuno photothermal nanotherapy (‘SYMPHONY’) treatment strategy for metastatic and unresectable MB49 bladder cancer. GNPs with sharp protrusions called gold nanostars (GNS) were used as agents of PTT (GNS-PTT) in combination with anti-PD-L1 monoclonal antibody (aPD-L1) in contralateral tumor-bearing C57BL/6 mice. GNS were injected intravenously and accumulated at tumor site via enhanced permeation and retention effect. One tumor was treated with GNS-PTT and the secondary tumor was left untreated. Mice treated with the combination of GNS-PTT with intravenously administered aPD-L1 exhibited complete tumor regression and long-term survival in 20% of the tumor-bearing mice, as compared with no long-term survival in tumor-bearing mice treated with aPD-L1 alone or GNS-PTT alone. Additionally, long-term-surviving GNS-PTT + aPD-L1-treated mice rejected tumor rechallenge, suggesting that antitumor immune memory had been generated. They also demonstrated an increased percentage of CD4+ and CD8+ T cells and B cells, and a decreased percentage of Tregs in the combination treatment group. This article represents the first proof-of-concept paper suggesting the synergistic antitumor immune effects of combining PTT with ICB using gold-based nanoparticles as PTT agents [Citation65].
Zhou et al. formulated bovine serum albumin (BSA)-bioinspired gold nanorods loaded with imiquimod (R837), a toll-like receptor 7 (TLR7) agonist as an immunoadjuvant (mPEG-GNRs@BSA/R837). DCs treated in vitro with mPEG-GNRs@BSA/R837 illustrated the platform’s ability to induce DC activation, a critical first step in engaging an antitumor immune response. In vivo PTT using intratumoral injection of mPEG-GNRs@BSA/R837 in combination with anti-PD-1 mAbs (aPD-1) in a B16-F10 melanoma model in C57BL/6 mice generated 80% survival compared to 70% survival in mice treated with mPEG-GNRs@BSA/R837 + PTT alone, and 10% survival in mice treated with aPD-1 alone. These data suggest that combining GNP-PTT (here, with another immunoadjuvant R837) with aPD-1 ICB can marginally improve survival of melanoma-bearing mice over PTT alone (10% increase), potentially reflecting an additive (rather than synergistic) effect of combining the individual therapies [Citation66].
While the aforementioned platforms delivered PTT agents and ICB antibodies separately, Luo et al. co-encapsulated aPD-1 peptide together with hollow gold nanoshells (HAuNS) within biodegradable poly (D, L-lactic-co-glycolide) (PLGA) nanoparticles (AA@PN). Synchronous tumor models were separately established for both colon cancer (CT26) and breast cancer (4T1) in BALB/c mice; primary tumors were intratumorally injected with the nanoparticle formulation containing both PTT agent and ICB and treated with PTT, and secondary tumors were left untreated. In both models, the primary tumors were eliminated when treated with AA@PN-PTT, and the secondary tumors exhibited delayed growth kinetics compared to controls, suggesting an abscopal antitumor immune effect. This effect was mechanistically illustrated by increased IFN-γ, CD4+ T cells and CD8+ T cells in the spleens, tumors and PBMCs of AA@PN-PTT-treated animals. Importantly AA@PN-PTT-treated animals survived significantly longer than aPD-1-peptide treated animals, suggesting the potential benefit in co-localizing a PTT agent and ICB molecule within a single platform [Citation67]. A subsequent paper from this group investigated the effects of administering Free CpG, a TLR agonist intravenously post intratumoral injection of AA@PN-PTT. Here, they described an even more potent antitumor immune response when CpG was added to AA@PN-PTT in a 4T1 spontaneous metastatic tumor model. Mice treated with AA@PN-PTT + CpG experienced complete regression of primary and secondary tumors, and minimal lung metastasis, suggesting added benefit of including an immunoadjuvant in the combination therapy comprising PTT and ICB. A similar demonstration was also shown in the CT26 metastatic tumor model [Citation68].
Iron oxide nanoparticles
In addition to GNPs, IONPs represent another class of nanoparticle used for PTT, although they are clinically used for magnetic fluid hyperthermia and as contrast agents in magnetic resonance imaging (MRI) [Citation90]. Despite successful translation as agents for magnetic hyperthermia, their application in PTT is not well documented, mainly due to the low molar absorption coefficient of IONPs at NIR wavelengths, leading to poor photothermal conversion efficiencies. Compared with GNPs and other NIR-responsive metal nanoparticles, a higher irradiation density (>1 W/cm2) or clustering of IONPs would be needed to ablate effectively a tumor site [Citation91,Citation92].
To overcome the sub-optimal photothermal conversion efficiency limiting IONPs as agents of PTT agents, Ge et al. fabricated spherical superparamagnetic particles of iron oxide (Fe3O4 SPs) that showed improved PTT characteristics [Citation93]. These SPs were encapsulated within a polymer nanoparticle along with imiquimod (R837) (Fe3O4-R837 SPs). Here, Fe3O4-R837 SPs were utilized for NIR wavelength PTT in combination with aPD-L1 ICB antibodies on 4T1 breast cancer in BALB/c mice. Analysis of lung metastasis after 24 d showed few or reduced metastatic masses in mice treated with aPD-L1, but the combination therapy elicited complete absence of metastatic nodules, indicating the effect of the combination therapy in eliminating metastasis. In a two tumor model (4T1), where following intravenous injection of the Fe3O4-R837 SPs, magnetic accumulation and PTT was performed on one tumor and the secondary tumor was left untreated, both treated and untreated tumor regressed showing a strong abscopal effect compared to no tumor regression in both primary and secondary tumors when treated with aPD-L1 alone [Citation69].
Recently, Chen et al. illustrated the role of Tregs in the efficacy of IONPs-PTT in combination with aCTLA-4 ICB in a 4T1 breast cancer model in BALB/c mice [Citation70]. Tregs are an important subset of TILs with higher expression of CTLA-4 [Citation94] and are often associated with poor prognosis in a wide variety of cancers [Citation95]. Chen et al. showed preferential depletion of Tregs post treatment with IONP-PTT + aCTLA-4 combination therapy in 4T1 breast cancer model in BALB/c mice, where aCTLA-4 immunotherapy by itself did not function effectively. Control tumors analyzed 12 d post-inoculation showed higher levels of infiltrating Tregs, whereas two cycles of IONPs-PTT on consecutive days showed 3.5-fold depletion in Tregs. In a delayed two-tumor model, when three intravenous injections of aCTLA-4 were given every 3 d and 2× PTT on consecutive days where performed only on primary tumor, 24 h post 1× intravenous injection of IONP, an abscopal effect was observed, with 100% distal tumor rejection. On the contrary, there was no decrease in tumor growth (similar growth as untreated tumors) with aCTLA-4 treatment alone. This combination treatment strategy also offered long-term immunological memory as illustrated by 66.6% tumor-free survival (8/12 animals) of rechallenged animals, demonstrating successful combination of PTT and ICB therapy [Citation70].
To investigate the effect of aPD-1 in combination with PTT in melanoma, Zhang et al. synthesized nanoparticles comprising aPD-1, iron oxide and perfluoropentane in a PLGA shell modified with PEG and a targeting moiety (GOP@aPD1). Both IONPs and perfluoropentane served as agents of PTT. In single B16-F10 tumor-bearing C57BL/6 mice, GOP@aPD1 injected intravenously triggered release of aPD-1 post PTT and induced toxicity on B16-F10 melanoma cells, as well as increased serum levels of IL-6, TNF-α and IFN-γ compared to PTT. Importantly, GOP@aPD-1-PTT-treated mice exhibited complete tumor regression compared to equivalent amounts of IONP-loaded NPs + PTT (no aPD-1) and aPD1-loaded NPs + PTT (no IONPs), demonstrating the synergistic effect of the combination treatment [Citation71].
Prussian blue nanoparticles
PBNPs are FDA-approved for use as sequestering agents for radioactive poisoning of cesium ions [Citation96,Citation97], but also have properties making them excellent agents for PTT. PBNPs have an absorption peak in the NIR range which allows them to absorb NIR light and convert it into heat, which can be utilized to kill cancer cells. We have shown that PBNPs can biodegrade at a mildly basic pH, mitigating concerns related to long-term persistence and toxicity of the nanoparticles in the body, and that PBNP-PTT elicits immunogenic cell death under certain thermal dose conditions [Citation43]. We investigated the combination of PBNP-PTT with aCTLA-4 ICB in a Neuro2A neuroblastoma model in A/J mice. Neuroblastoma is an aggressive pediatric cancer that is considered to be immunologically ‘cold’; that is, they express lower amounts of cancer-specific antigens and have lower tumor-infiltrating immune cells making then less responsive to ICB alone [Citation98,Citation99]. PBNP-PTT generated increased infiltration of CD45+ leukocytes in treated tumors in comparison to untreated tumors, suggesting the immunostimulatory effect. Mice treated with intertumoral injection of PBNP-PTT + intraperitoneal injection of aCTLA-4 showed complete tumor regression with significantly improved disease-free survival (56%) compared to its individual components of PBNP alone, aCTLA-4 alone, PTT alone and untreated controls. Interestingly, depleting CD4+ and CD8+ T cells using depletion antibodies completely abrogated the benefit seen with PBNP-PTT + aCTLA-4, with no improved survival compared to untreated animals, demonstrating the role of T cells in eliciting an immune response after the combination therapy [Citation72].
Other metal-based PTT agents
Other metal-based agents investigated for PTT in combination with ICB are discussed in this section. Song et al. introduced the first hollow PTT agent, bismuth selenide nanocages (Bi2Se3-NC), which encapsulated resiquimod (R848), a TLR7/8 agonist, as an immune adjuvant. The Bi2Se3-NCs were used for NIR wavelength PTT [Citation73,Citation100,Citation101] that triggered the release of R848 in a synchronous 4T1 model of breast cancer. In a two-tumor model, one 4T1 tumor was irradiated with PTT 24 h after intravenous administration of Bi2Se3-NCs. In combination with intravenous aPD-L1 ICB, Bi2Se3-NC/R848-PTT generated 90% disease-free survival compared to the NP formulation and aPD-L1 without PTT. Interestingly, 80% survival was seen in the combination treatment group (in the absence of the R848 adjuvant) demonstrating improved synergy obtained from combining aPD-L1 with PTT, creating a vaccine-like immune response even without the additional immunoadjuvant (R484), which resulted a marginal additional survival benefit [Citation102]. These effects were correlated with increased DC maturation, T cell infiltration, and systemic TNF-α and IFN-γ, suggesting an antitumor immune response generated by the PTT-ICB combination therapy.
Black phosphorous (BP) is a dichalcogenide semi-conducting transition metal with high photothermal conversion efficiency and biodegradability [Citation103,Citation104]. Due to these properties, they have been used as PTT agent for malignancies [Citation105–107]. Liang et al. [Citation74] designed a nanovehicle platform of red blood cell membrane (RM)-coated BP quantum dots (BPQD-RMNVs) as RMs have been used in decorating nanoparticles to increase systemic circulation [Citation108–110]. BPQD-RMNVs injected intravenously were combined with aPD-1 ICB in a 4T1 delayed two-tumor model in BALB/c mice, wherein a primary tumor was irradiated by NIR laser followed by intravenous administration aPD-1. While the immunotherapy or PTT alone improved survival days of the treated animals by 30 d, the combination treatment completely eliminated the primary tumor and prevented the formation of secondary tumor showing vaccine-like immune effects with 80% long-term disease-free survival and a promising preclinical treatment strategy for triple-negative breast cancer [Citation74].
Copper sulfide nanoparticles (CuS NPs) are p-type semiconductors with excellent optical properties enabling their use as PTT agents using 808 and 980 nm lasers [Citation111,Citation112]. Wang et al. used CuS NPs combined with aPD-L1 ICB for eradicating breast cancer. CuS NPs were used as agents of PTT and for antigen capture. They studied various polymer modifications on CuS NPs and found maleimide-PEG (PEG-Mal) coating to potentiate maximum antigen capture. A two-tumor model was developed using two different cell numbers of 4T1 cells. CuS NPs-PEG-Mal was intratumorally injected into the primary (largest) tumor and subjected to PTT on days 1, 3, 5 and 7. aPD-L1 was given intraperitoneally on days 1, 4 and 7. Mice treated with the combination of CuS NPs-PEG-Mal-PTT + aPD-L1 experienced total primary tumor eradication and significantly delayed secondary tumor growth compared to mice treated with the single therapies (aPD-L1 or CuS NPs-PEG-Mal-PTT). The combination treatment significantly increased serum TNF-α, INF-γ, IL-2 and IL-6 levels and also increased CD3 + CD45+ cells and CD8+ T cells compared to aPD-L1 and CuS NPs-PEG-Mal-PTT, once again illustrating the synergistic effects of PTT-ICB combination therapy [Citation75].
Carbon-based nanoparticles
Apart from their extensive investigation in biomedical applications, carbon-based nanoparticles are gaining interest as PTT agents due to their excellent optical properties, broad absorption range from UV to NIR wavelengths, large excitation coefficients and band-to-band transition in the NIR region [Citation113]. They demonstrate excellent drug loading efficiency, thus their potency as PTT agent is widely studied in combination with chemotherapy [Citation44,Citation114,Citation115]. Thus, immunotherapy in combination with carbon nanoparticle-based PTT can combine the positive aspects of both treatment strategies. Few studies have described the combination of ICB with carbon nanoparticle-based PTT; here, we highlight graphene oxide (GO) and carbon nanotubes (CNTs).
Indoleamine-2,3-dioxygenase (IDO) is an immunosuppressive molecule involved in tryptophan metabolism that can decrease T cell activity; thus, IDO inhibitors (IDOis) been investigated for their ability to reverse immunosuppression similarly to ICB [Citation116,Citation117]. Few IDOis (e.g., INCB24360 and NLG919) have been investigated in combination with PTT [Citation118,Citation119]. Yan et al. combined reduced GO (rGO) with aPD-L1 ICB and epacadostat, an IDOi. Epacadostat was encapsulated in rGO and coated with PEG and folic acid (PEG-rGO-FA-IDOi) for improving circulation time and specific accumulation in the tumor site. Two CT26 colon tumors with varying cell numbers were subcutaneously inoculated simultaneously, followed by intravenous injection of PEG-rGO-FA-IDOi, 12 h after which only the primary (larger) tumor was treated with PTT; aPD-L1 injections were administered intraperitoneally on day 1, 3 and 5 post-PTT. This combination treatment generated complete elimination of primary tumors and significantly delayed growth of distal tumors, with high levels of CD45+ leukocyte infiltration and increased proportions of effector and helper T cells to Tregs within secondary tumors as well as high IFN-γ level in serum. These beneficial antitumor effects were not observed for immunotherapy alone. In fact, aPD-L1 in combination with IDOi failed to suppress both primary and secondary tumors, whereas the PTT-treated group without immunotherapy effectively suppressed the treated primary tumor but failed to delay the secondary tumor growth. Thus, these three agents showed synergistic enhancement of antitumor immunity, again demonstrating the advantage of the PTT-ICB combination therapy over each monotherapy [Citation76].
CNTs, both single (SWCNTs) and multi-walled, have been also explored as agents of PTT in addition to other biomedical applications [Citation120,Citation121]. CNTs have high optical absorption in the NIR spectrum that makes them suitable for use as PTT agents. Studies investigating various coatings on CNTs have demonstrated to reduce their well-known cytotoxicity, a factor limiting their use for biological purposes [Citation122–124]. Wang et al. were the first to combine nanoparticle-based PTT with ICB. As the first ever proof-of-concept, they performed intratumoral SWCNT-PTT on primary orthotopic 4T1 breast tumors in BALB/c mice combined with intravenous aCTLA-4 ICB, which generated elevated pro-inflammatory cytokines TNF-α IL-12P70, IL-6 and IL-1β in blood sera and DC maturation. This correlated with the ability of SWCNT-PTT to generate maturation of bone marrow-derived DCs in vitro, suggesting its role in immunostimulation. In a delayed two-tumor model, the combination treatment of SWCNT-PTT and aCTLA-4 generated complete regression of both tumors, decreased the amount of Tregs, and increased the effector T cells in the secondary tumors compared to surgery + aCTLA-4, surgery alone or aCTLA-4 alone. To establish a lung metastasis model, 4T1 cells were first injected orthotopically in the mouse breast pad to establish primary tumor, followed by intravenous injection of 4T1 cells after a week to establish lung metastases. In this model, the SWCNT-PTT of the primary tumor combined with intravenous injection of aCTLA-4 caused low to no lung metastasis, illustrating the ability of the combination therapy to trigger the adaptive immune response and elicit antitumor immunity [Citation77].
Organic dyes
Many organic dyes undergo photoexcitation followed by non-radioactive relaxation of the excited species. This non-radioactive relaxation can generate heat that is utilized for killing cancer cells during PTT. Various organic dyes such as cyanine-based dyes (e.g., indocyanine green (ICG)) including heptamethine cyanines (e.g., IR825, IR780, IR808, IR2) and phthalocyanines (e.g., metallo-naphthalocyanines), diketopyrrolopyrrole-based agents, croconaine-based dyes, porphyrin-based dyes, polymer-based dyes (e.g., polypyrrole) have been used for PTT either as free agents or within nanoparticles. These formulations have been used alone and in combination with chemotherapy, radiation therapy, immunotherapies or therapies targeting epigenetic modulation [Citation50,Citation125,Citation126]. Here, we review the latest studies using the organic dyes ICG, polydopamine and IR820 as PTT agents in combination with ICB.
Indocyanine green
Chen et al. used PLGA nanoparticles encapsulating ICG as a PTT agent and R837, a TLR-7 agonist as adjuvant immunotherapy (PLGA-ICG-R837), in combination with aCTLA-4 ICB in 4T1 breast and CT26 colon cancer models. Without PTT or ICB, PLGA-ICG-R837 alone was able to increase DC maturation (CD11 + CD80 + CD86+) in lymph nodes, and increase IL-6, IL-12p70 and TNF-α in the sera of 4T1- and CT26-bearing mice over individual components. The combination PLGA-ICG-R837-PTT (intravenous injection of PLGA-ICG-R837 followed by PTT after 24 h) + aCTLA-4 therapy was investigated in a metastatic breast cancer model, wherein a primary tumor was inoculated in the breast pad and 4T1 cells were injected intravenously to seed metastases. Primary tumors were either treated with PTT or removed by surgery, and aCTLA-4 injections were subsequently administered intravenously. Mice treated with PLGA-ICG-R837-PTT + aCTLA-4 showed complete reduction in tumor volume and a significant increase in disease-free survival, correlating with increased T cell infiltration and decreased levels of Tregs in secondary tumors compared to surgery + aCTLA-4 or any other individual therapy. These results suggest the synergistic immune functions of PTT, R837 and aCTLA-4. Similar trends were observed in the synchronous CT26 colon cancer model [Citation78].
A study by Wang et al. explored the potential of nanoparticles to act as a platform for antigen capture during a combination of PDT, PTT and ICB therapy. A core/shell upconversion nanoparticle (UCNP) was used to assemble ICG as an agent for PTT, rose Bengal (RB) as an agent for PDT and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol) (DSPE-PEG-mal) lipid molecule as an agent for antigen capture. They demonstrated that UCNP/ICG/RB-mal generated heat (from PTT) and reactive oxygen species (from PDT), and immunogenic cell death in 4T1 breast cancer cells upon NIR laser irradiation in vitro. Released antigens from the in vitro treatment were incubated with the nanoparticles, illustrating effective antigen capture by measuring changes in size, charge, and protein content on nanoparticles. In an in vivo study using a 4T1 orthotopic model in BALB/c mice, intratumoral injection of UCNP/ICG/RB-mal + PTT with aCTLA-4 ICB therapy demonstrated improved therapeutic effects, increasing survival rate to 83% compared to UCNP/ICG/RB-mal + PTT alone with no aCTLA-4 (67%). Interestingly, aCTLA-4 alone did not result in any survival benefit or tumor suppression compared to control. In a rechallenge study, 50% of the combination treatment group rejected the tumor showing it induces durable antitumor immunity [Citation61]. These results illustrate the potential for nanoparticle-based PTT to improve ICB therapy in combination with other modalities (i.e., PDT, antigen capture).
Polydopamine
Polydopamine is a major pigment of naturally occurring melanin and possesses optical properties similar to melanin, enabling its excitation with 808 NIR lasers. Polydopamine is also biocompatible [Citation127]. Tian et al. demonstrated the use of polydopamine nanoparticles (PDMNs) with an immune checkpoint inhibitor, JQ1. According to them, this was the first demonstration of using a non-antibody-based immune checkpoint inhibitor with PTT [Citation79]. JQ1 is a bromodomain and extra-terminal (BET) inhibitor that disrupts interaction between BET proteins and acetylated histones leading to inhibition of transcription of tumorigenic genes like c-MYC. Recently, JQ1 has been proven to down-regulate PD-L1 by reducing c-MYC transcription [Citation128]. One main disadvantage of JQ-1 is that it is hydrophobic, thereby limiting its bioavailability. Thus, encapsulating it within a nanoparticle platform can promote bioavailability [Citation125]. PDMNs were used as both agents of PTT and as a biodegradable delivery platform for JQ1. In vitro analysis showed decreased BRD 4, c-MYC and PD-L1 expression on 4T1 cells exposed to JQ1 and JQ1 loaded PDMN (PDMN-JQ1). Addition of JQ1 did not change the heating efficiency of PDMN. An in vivo study using 4T1 single tumor model, where the established tumor in the flank of the animals was intratumorally injected with nanoparticle formulation, showed delayed growth of tumors treated with PDMN-JQ1-PTT. Analysis of tumors post-PTT showed reduced PD-L1 levels for JQ1 containing groups (JQ1-PTT and PDMN-JQ1-PTT), but increased lymphocyte infiltration was seen only for PDMN containing groups after thermal therapy (PDMN-PTT and PDMN-JQ1-PTT). PDMN-PTT also increased CD8+ T cells infiltration, which was further increased by the presence of JQ1 in PDMN-JQ1-PTT. A similar trend was observed for TNF-α, INF-γ and IL-2 levels in serum. In a delayed 4T1 two-tumor model, the combination therapy on primary tumor significantly delayed the growth of secondary untreated tumor and also increased systemic CD8+ central memory T cells [Citation79].
Another interesting study was conducted by Chen et al. using polydopamine-coated with Salmonella VNP20009 bacteria (pDA-VNP) as a PTT agent, in combination with a PD-1 antagonist, AUNP-12 peptide, embedded within phospholipid-based phase separation gel (P-AUNP). The bacteria provided deep penetration of the polydopamine (PTT agent), and the phase separation gel helped slow and sustained release of AUNP-12 for up to 42 d. In a B16-F10 melanoma model, mice treated with the combination therapy were intravenously injected with pDA-VNP, and P-AUNP was injected subcutaneously surrounding the established tumor. For groups treated with laser irradiation, PTT was conducted 3 d post pDA-VNP and P-AUNP injection. Mice treated with pDA-VNP + P-AUNP + PTT showed complete remission of tumor and a median survival of 70 d as compared to 55 and 14 d for mice treated with pDA-VPN + PTT and P-AUNP, respectively. Furthermore, the triple therapy group also showed significantly increased tumor infiltration of CD8+ T cells, tumor INF-γ and TNF-α, compared to the P-AUNP-treated group. The AUNP-12 peptide containing groups (P-AUNP and pDA-VNP + P-AUNP) showed significant decreases in PD-L1 expression on B16-F10 tumor cells and PD-1+ T cells within the tumors, suggesting a robust immunopermissive environment. The triple therapy also increased CD8+ T cells in spleen and serum IgG, IgG1, IgG2a, INF-γ and TNF-α levels compared to P-AUNP group, illustrating a strong systemic immune response. These studies suggest the potential for combined biotherapy, PTT and ICB for the elimination of melanoma [Citation80].
A multi-modal theragnostic platform using polydopamine as the PTT agent and carbon dots (CDs) as imaging probes was designed by Lu et al. [Citation81]. CDs have photothermal efficiency and thus can also be considered as agents of PTT. PEG-coated PDMNs were coated with CDs and immune adjuvant R848 (PDA-PEG-R848-CDs) and combined with aPD-L1 ICB to target 4T1 breast cancer. The release of R848 was facilitated by the heat produced during PTT. Biodistribution studies showed accumulation of intravenously injected PDA-PEG-R848-CDs in liver, kidneys and tumor compared to free CDs showing improved accumulation and retention properties of nanoparticles compared to free small molecules [Citation81]. Single 4T1 tumor-bearing mice treated with PDA-PEG-R848-CDs + PTT (three intravenous injections and three PTT cycles) showed an 80% survival benefit, prompting an efficacy study in a two-tumor model combining with aPD-L1 to improve immune benefits. In the delayed two-tumor model, Lu et al. injected PDA-PEG-R848-CDs intravenously and irradiated only the primary tumor after 24 h. Multiple doses of aPD-L1 were administered intravenously on days 1, 3 and 5 post NP injection. PDA-PEG-R848-CDs + PTT with no aPD-L1 caused complete primary tumor eradication but failed to delay the growth of secondary tumor. PDA-PEG-R848-CDs + PD-L1 but with no PTT intervention failed to suppress both primary and secondary tumor. The combination treatment successfully suppressed the growth of both primary and secondary tumors and showed increased tumor infiltration of CTLs and increased serum TNF-a and INF-g levels, again illustrating the promising combined effects of PTT-ICB photoimmunotherapy [Citation81].
IR820
Huang et al. designed a liquid gel (LG) depot made of soybean phosphatidylcholine and glycerol dioleate loaded with IR820 and aPD-L1 (aPD-L1/I@LG). IR820 was used as an agent of PTT to generate hyperthermia, causing the LG to undergo a gel-to-solid phase transition, thereby melting the construct and releasing the encapsulated aPD-L1. They termed the treatment scheme symbiotic mild photothermal-sensitized immunotherapy (SMPAI). They found that this mild PTT can increase infiltration of immune cells in the tumor microenvironment (TME) but can also generate upregulation of PD-L1 on tumor cells, thereby shifting the balance toward immunosuppression in the absence of aPD-L1 mAbs. This effect can ‘prime’ the tumor for targeting by aPD-L1 present in the combination therapy. To validate that SMPAI could generate an antitumor immune response, PTT was performed on non-immunogenic (poor in TILs) 4T1 breast tumors intratumorally injected with aPD-L1/I@LG, resulting in complete regression of the treated tumor. Interestingly, IR820 + PTT with no aPD-L1 ICB was ineffective, potentially due to the immunosuppression created by PD-L1 upregulation on tumor cells post-mild PTT. Post-treatment analysis revealed DC maturation in inguinal lymph nodes, increased CD4+ and CD8+ T cells and lower levels of Tregs and myeloid-derived suppressor cells (MDSCs) in the spleen and tumor, and increased levels of TNF-α and IFN-γ in the sera of SMPAI-treated mice compared to aPD-L1/I@LG without PTT, suggesting that local PTT sensitizes the tumor for aPD-L1 therapy. SMPAI was also able to generate an abscopal effect in mice bearing synchronous 4T1 tumors, and a systemic effect in mice bearing metastatic 4T1 tumors, suggesting its applicability in many stages of disease. Similar results were also obtained in B16-F10 melanoma, another non-immunogenic tumor model [Citation82].
Discussion and future perspective
Both ICB immunotherapy and PTT are promising platforms for cancer therapy, but limitations still exist for their use as monotherapies. ICB is effective in generating immune responses, but is often associated with immune-related adverse events at doses used in clinic [Citation23,Citation24]. PTT, on the other hand, generates excellent cytotoxicity (thermal ablation) of treated tumors, but may only exhibit antitumor immunogenicity at a very specific thermal dose (that is not sufficiently cytotoxic) [Citation43]. In this review, we have highlighted studies synergistically combining ICB and nanoparticle-based PTT. These studies demonstrate that nanoparticle-based PTT can potentiate the promising antitumor effects of ICB immunotherapy observed both preclinically and clinically.
Interestingly, nanoparticle-based PTT was shown to impact immunogenicity of tumor cells under certain conditions, albeit with suboptimal responses. In particular, PTT alone (without additional immunotherapy) increased PD-1 expression on CD4+ and CD8+ T cells [Citation65], significantly depleted tumor-infiltrating Tregs [Citation70], and recruited APCs to the TME [Citation99]. A critical consideration in optimizing nanoparticle-based PTT for combination with ICB is finding the appropriate thermal dose. We have demonstrated that immunogenic cell death occurs at a specific thermal window, depending on the cancer type [Citation43,Citation50,Citation52]. presents the temperatures attained during PTT in different tumor models reviewed here. Unfortunately, most of the studies we highlight do not discuss thermal dose, nor explain their rationale for choosing a particular temperature. Even fewer studies provide details about the effect of PTT on immunogenic cell death. We propose that choosing an effective cancer-specific thermal dose is an important parameter for maximizing the antitumor immunological effects of PTT for use in combination with ICB immunotherapy. The dosing strategy also appears to be a significant factor in driving the success of combining PTT with ICB. Most studies discussed in this review applied a single nanoparticle-based PTT approach, with multiple doses of ICB following the PTT treatment, but some differed; for example, four doses of mild PTT on consecutive days [Citation82], 10 cycles of PTT 24 h after intravenous NP injection [Citation67], two doses of PTT on consecutive days [Citation70]. A thorough investigation of the different PTT dosing strategies can help describe the optimal dose for each tumor type. Another trend observed in the presented studies is that while nanoparticle-based PTT alone can cause complete tumor regression of treated tumors, it fails to maintain long-term systemic immunity, allowing secondary or distal tumors to progress.
Based on these observations in the published literature, we hypothesize the following mechanism of action driving the robust responses to nanoparticle-based PTT in combination with ICB. Thermal ablation of an accessible tumor by PTT leads to production of tumor-specific antigens in the TME. Antigen-presenting cells in the vicinity of the PTT-irradiated region take up these antigens, migrate, mature and present them to the T cells in draining lymph nodes. The T cells, after recognizing these antigens, differentiate into cytotoxic CD8+ T cells and are recruited to the TME causing tumor regression. Furthermore, these cells can differentiate into memory T cells, which is important to maintain long-term memory to prevent recurrence and help eliminate distal metastasis. On the other hand, CD4+ T cells can also directly recognize the tumor-specific antigens and release cytokines to trigger a clonal expansion of cytotoxic CD8+ T cells, attacking remaining tumor cells. Complementary to this, ICB antibodies (e.g., aPD-1/aPD-L1, aCTLA-4) function by blocking immunosuppressive signaling pathways on T cells [Citation13,Citation18]. Thus, the antigens produced after PTT by the dying cells act as a vaccine and hence combining it with ICB can further activate an immune response. Overall, PTT in combination with ICB may lead to a strong antitumor response with long-term immune memory for effectively eradicating primary and metastasized tumors.
As many ICB antibodies are FDA-approved for clinically treating cancer, successful clinical translation of combination PTT and ICB will first rely on the clinical translation of PTT. We propose that PTT may be seamlessly incorporated into existing operating room infrastructure (e.g., clinical-grade lasers, syringes and needles to administer nanoparticles, existing anesthetic or analgesics, etc.); however, each nanoparticle formulation must individually be approved as agents of PTT. We envision the administration of PTT and ICB as an outpatient procedure (for accessible tumors) or in an intra-operative setting (for deeper tumors or tumors requiring additional surgery). For deep-seated tumors, researchers are developing laser instrumentation for interstitial PTT (iPTT) to administer PTT within the tumor tissue rather than superficially. Ongoing research seeks to optimize iPTT to apply the beneficial effects of PTT (with and without ICB) for a variety of tumor types in the clinic [Citation129–132].
To conclude, the combination of nanoparticle-based PTT with ICB immunotherapy has immense potential to function as a successful cancer therapy platform, especially because they mitigate each other’s limitations, working synergistically to improve tumor reduction and long-term disease-free survival benefits. Ongoing studies will help identify the appropriate thermal dose and dosing strategy for nanoparticle-based PTT to prime an optimal antitumor immune response, which can potentiate the benefits of ICB immunotherapy across several tumor types. These studies along with potential iPTT to target multiple tumor types are expected to play an important role in facilitating the clinical translation of this novel therapeutic combination for cancer.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Martins GA, Tadokoro CE, Silva RB, et al. CTLA-4 blockage increases resistance to infection with the intracellular protozoan Trypanosoma cruzi. J Immunol. 2004;172(8):4893–4901.
- Walker L. CTLA-4 and autoimmunity: new twists in the tale. Trends Immunol. 2015;36(12):760–762.
- Paterson AM, Lovitch SB, Sage PT, et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J Exp Med. 2015;212(10):1603–1621.
- Ariyan C, Salvalaggio P, Fecteau S, et al. Cutting edge: transplantation tolerance through enhanced CTLA-4 expression. J Immunol. 2003;171(11):5673–5677.
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19(1):225–252.
- Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–2715.
- Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16(1):7–19.
- Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol. 2015;15(3):149–159.
- Borst J, Ahrends T, Bąbała N, et al. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18(10):635–647.
- Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211(1):214–224.
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–310.
- Scheipers P, Reiser H. Role of the CTLA-4 receptor in t cell activation and immunity. Physiologic function of the CTLA-4 receptor. Immunol Res. 1998;18(2):103–115.
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–465.
- Linsley PS, Greene JL, Brady W, et al. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1(9):793–801.
- Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603.
- Walker LSK, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol. 2015;36(2):63–70.
- Salmaninejad A, Valilou SF, Shabgah AG, et al. PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234(10):16824–16837.
- Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535.
- Darvin P, Toor SM, Sasidharan Nair V, et al. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11.
- Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37(5):473–484.
- Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–588.
- Kaehler KC, Piel S, Livingstone E, et al. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol. 2010;37(5):485–498.
- Bajwa R, Cheema A, Khan T, et al. Adverse effects of immune checkpoint inhibitors (programmed death-1 inhibitors and cytotoxic T-lymphocyte-associated protein-4 inhibitors): results of a retrospective study. J Clin Med Res. 2019;11(4):225–236.
- Bakos O, Lawson C, Rouleau S, et al. Combining surgery and immunotherapy: turning an immunosuppressive effect into a therapeutic opportunity. J Immunother Cancer. 2018;6(1):86.
- Wang C, Sun W, Ye Y, et al. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat Biomed Eng. 2017;1(2):0011.
- Pfirschke C, Engblom C, Rickelt S, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44(2):343–354.
- Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–443.
- Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3(4):345–355.
- Derer A, Frey B, Fietkau R, et al. Immune-modulating properties of ionizing radiation: rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol Immunother. 2016;65(7):779–786.
- Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci. 2003;100(23):13549–13554.
- O’Neal DP, Hirsch LR, Halas NJ, et al. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209(2):171–176.
- Huang X, Jain PK, El-Sayed IH, et al. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23(3):217–228.
- Dickerson EB, Dreaden EC, Huang X, et al. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269(1):57–66.
- de Bruijne M, Holt B, van Rhoon GC, et al. Evaluation of CEM43°CT90 thermal dose in superficial hyperthermia. Strahlenther Onkol. 2010;186(8):436–443.
- P. D. Maguire TVSLRPELJGLRBPLWLDMBS. A phase II trial testing the thermal dose parameter CEM43° T90 as a predictor of response in soft tissue sarcomas treated with pre-operative thermoradiotherapy. Int J Hyperthermia. 2001;17(4):283–290.
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800.
- van Rhoon GC, Aleman A, Kelfkens G, et al. Health council of the Netherlands: no need to change from SAR to time-temperature relation in electromagnetic fields exposure limits. Int J Hyperthermia. 2011;27(4):399–404.
- Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia. 2011;27(4):320–343.
- Sordillo LA, Pu Y, Pratavieira S, et al. Deep optical imaging of tissue using the second and third near-infrared spectral windows. J Biomed Opt. 2014;19(5):056004.
- Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19(4):316–317.
- Burke A, Ding X, Singh R, et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc Natl Acad Sci USA. 2009;106(31):12897–12902.
- Sweeney EE, Cano-Mejia J, Fernandes R. Photothermal therapy generates a thermal window of immunogenic cell death in neuroblastoma. Small. 2018;14(20):1800678.
- Nam J, Son S, Ochyl LJ, et al. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9(1):1074.
- Sweeney EE, Burga RA, Li C, et al. Photothermal therapy improves the efficacy of a MEK inhibitor in neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Sci Rep. 2016;6(1):37035.
- Zhang Y, Zhan X, Xiong J, et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci Rep. 2018;8(1):8720.
- Perez-Hernandez M, Del Pino P, Mitchell SG, et al. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano. 2015;9(1):52–61.
- Ren Y, Qi H, Chen Q, et al. Thermal dosage investigation for optimal temperature distribution in gold nanoparticle enhanced photothermal therapy. Int J Heat Mass Transf. 2017;106:212–221.
- van Rhoon GC, Samaras T, Yarmolenko PS, et al. CEM43 °C thermal dose thresholds: a potential guide for magnetic resonance radiofrequency exposure levels? Eur Radiol. 2013;23(8):2215–2227.
- Ledezma DK, Balakrishnan PB, Cano-Mejia J, et al. Indocyanine green-nexturastat A-PLGA nanoparticles combine photothermal and epigenetic therapy for melanoma. Nanomaterials. 2020;10(1):161.
- Kepp O, Senovilla L, Vitale I, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3(9):e955691.
- Cano-Mejia J, Bookstaver ML, Sweeney EE, et al. Prussian blue nanoparticle-based antigenicity and adjuvanticity trigger robust antitumor immune responses against neuroblastoma. Biomater Sci. 2019;7(5):1875–1887.
- Zhou F, Yang J, Zhang Y, et al. Local phototherapy synergizes with immunoadjuvant for treatment of pancreatic cancer through induced immunogenic tumor vaccine. Clin Cancer Res. 2018;24(21):5335–5346.
- Bear AS, Kennedy LC, Young JK, et al. Elimination of metastatic melanoma using gold nanoshell-enabled photothermal therapy and adoptive T cell transfer. PLoS One. 2013;8(7):e69073.
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26(305):234–241.
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931.
- Abdo J, Cornell DL, Mittal SK, et al. Immunotherapy plus cryotherapy: potential augmented abscopal effect for advanced cancers. Front Oncol. 2018;8:85.
- Cano-Mejia J, Shukla A, Ledezma DK, et al. CpG-coated Prussian blue nanoparticles-based photothermal therapy combined with anti-CTLA-4 immune checkpoint blockade triggers a robust abscopal effect against neuroblastoma. Transl Oncol. 2020;13(10):100823.
- Chen Q, Hu Q, Dukhovlinova E, et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv Mater. 2019;31(23):1900192.
- Min Y, Roche KC, Tian S, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12(9):877–882.
- Wang M, Song J, Zhou F, et al. NIR-triggered phototherapy and immunotherapy via an antigen-capturing nanoplatform for metastatic cancer treatment. Adv Sci. 2019;6(10):1802157.
- Zhou F, Nordquist RE, Chen WR. Photonics immunotherapy — A novel strategy for cancer treatment. J Innov Opt Health Sci. 2016;09(01):1630001.
- Hou X, Tao Y, Pang Y, et al. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int J Cancer. 2018;143(12):3050–3060.
- Liu Y, Crawford BM, Vo-Dinh T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy. 2018;10(13):1175–1188.
- Liu Y, Maccarini P, Palmer GM, et al. Synergistic immuno photothermal nanotherapy (SYMPHONY) for the treatment of unresectable and metastatic cancers. Sci Rep. 2017;7(1):8606.
- Zhou B, Song J, Wang M, et al. BSA-bioinspired gold nanorods loaded with immunoadjuvant for the treatment of melanoma by combined photothermal therapy and immunotherapy. Nanoscale. 2018;10(46):21640–21647.
- Luo L, Yang J, Zhu C, et al. Sustained release of anti-PD-1 peptide for perdurable immunotherapy together with photothermal ablation against primary and distant tumors. J Control Release. 2018;278:87–99.
- Luo L, Zhu C, Yin H, et al. Laser immunotherapy in combination with perdurable PD-1 blocking for the treatment of metastatic tumors. ACS Nano. 2018;12(8):7647–7662.
- Ge R, Liu C, Zhang X, et al. Photothermal-activatable Fe3O4 superparticle nanodrug carriers with PD-L1 immune checkpoint blockade for anti-metastatic cancer immunotherapy. ACS Appl Mater Interfaces. 2018;10(24):20342–20355.
- Chen H, Luan X, Paholak HJ, et al. Depleting tumor-associated Tregs via nanoparticle-mediated hyperthermia to enhance anti-CTLA-4 immunotherapy. Nanomedicine. 2020;15(1):77–92.
- Zhang N, Song J, Liu Y, et al. Photothermal therapy mediated by phase-transformation nanoparticles facilitates delivery of anti-PD1 antibody and synergizes with antitumor immunotherapy for melanoma. J Control Release. 2019;306:15–28.
- Cano-Mejia J, Burga RA, Sweeney EE, et al. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomedicine. 2017;13(2):771–781.
- Olson TY, Schwartzberg AM, Orme CA, et al. Hollow gold − silver double-shell nanospheres: structure, optical absorption, and surface-enhanced Raman scattering. J Phys Chem C. 2008;112(16):6319–6329.
- Liang X, Ye X, Wang C, et al. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J Control Release. 2019;296:150–161.
- Wang R, He Z, Cai P, et al. Surface-functionalized modified copper sulfide nanoparticles enhance checkpoint blockade tumor immunotherapy by photothermal therapy and antigen capturing. ACS Appl Mater Interfaces. 2019;11(15):13964–13972.
- Yan M, Liu Y, Zhu X, et al. Nanoscale reduced graphene oxide-mediated photothermal therapy together with IDO inhibition and PD-L1 blockade synergistically promote antitumor immunity. ACS Appl Mater Interfaces. 2019;11(2):1876–1885.
- Wang C, Xu L, Liang C, et al. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv Mater Weinheim. 2014;26(48):8154–8162.
- Chen Q, Xu L, Liang C, et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7(1):13193.
- Tian Y, Wang X, Zhao S, et al. JQ1-loaded polydopamine nanoplatform inhibits c-MYC/programmed cell death ligand 1 to enhance photothermal therapy for triple-negative breast cancer. ACS Appl Mater Interfaces. 2019;11(50):46626–46636.
- Chen W, Guo Z, Zhu Y, et al. Combination of bacterial-photothermal therapy with an anti-PD-1 peptide depot for enhanced immunity against advanced cancer. Adv Funct Mater. 2020;30(1):1906623.
- Lu Q, Qi S, Li P, et al. Photothermally activatable PDA immune nanomedicine combined with PD-L1 checkpoint blockade for antimetastatic cancer photoimmunotherapy. J Mater Chem B. 2019;7(15):2499–2511.
- Huang L, Li Y, Du Y, et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat Commun. 2019;10(1):4871.
- Bardhan R, Lal S, Joshi A, et al. Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc Chem Res. 2011;44(10):936–946.
- Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc Chem Res. 2008;41(12):1842–1851.
- Loo C, Lin A, Hirsch L, et al. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat. 2004;3(1):33–40.
- Cheng X, Sun R, Yin L, et al. Light-triggered assembly of gold nanoparticles for photothermal therapy and photoacoustic imaging of tumors in vivo. Adv Mater. 2017;29(6):1604894.
- Vines JB, Yoon JH, Ryu NE, et al. Gold nanoparticles for photothermal cancer therapy. Front Chem. 2019;7:167.
- Riley RS, Day ES. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(4):.
- Kim H, Lee D. Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers-Basel. 2018;10(9):961.
- van Landeghem FKH, Maier-Hauff K, Jordan A, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30(1):52–57.
- Shen S, Wang S, Zheng R, et al. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials. 2015;39:67–74.
- Estelrich J, Busquets M. Iron oxide nanoparticles in photothermal therapy. Molecules. 2018;23(7):1567.
- Ge R, Li X, Lin M, et al. Fe3O4@polydopamine composite theranostic superparticles employing preassembled Fe3O4 nanoparticles as the core. ACS Appl Mater Interfaces. 2016;8(35):22942–22952.
- Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–118.
- Chao JL, Savage PA. Unlocking the complexities of tumor-associated regulatory T cells. J Immunol. 2018;200(2):415–421.
- Thompson DF, Church CO. Prussian blue for treatment of radiocesium poisoning. Pharmacotherapy. 2001;21(11):1364–1367.
- Delchet C, Tokarev A, Dumail X, et al. Extraction of radioactive cesium using innovative functionalized porous materials. RSC Adv. 2012;2(13):5707–5716.
- Maleki Vareki S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer. 2018;6(1):157.
- Bonaventura P, Shekarian T, Alcazer V, et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168.
- Li M, Wang Y, Lin H, et al. Hollow CuS nanocube as nanocarrier for synergetic chemo/photothermal/photodynamic therapy. Mater Sci Eng C Mater Biol Appl. 2019;96:591–598.
- Jiang L, Han J, Li J, et al. Photothermal therapy of cancer cells using novel hollow gold nanoflowers. Int J Nanomedicine. 2014;9:517.
- Song Y, Wang Y, Wang S, et al. Immune-adjuvant loaded Bi2Se3 nanocage for photothermal-improved PD-L1 checkpoint blockade immune-tumor metastasis therapy. Nano Res. 2019;12(8):1770–1780.
- Shao J, Xie H, Huang H, et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat Commun. 2016;7(1):12967.
- Li Y, Liu Z, Hou Y, et al. Multifunctional nanoplatform based on black phosphorus quantum dots for bioimaging and photodynamic/photothermal synergistic cancer therapy. ACS Appl Mater Interfaces. 2017;9(30):25098–25106.
- Chen W, Ouyang J, Liu H, et al. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv Mater. 2017;29(5):1603864.
- Lee HU, Park SY, Lee SC, et al. Black phosphorus (BP) nanodots for potential biomedical applications. Small. 2016;12(2):214–219.
- Guo T, Wu Y, Lin Y, et al. Black phosphorus quantum dots with renal clearance property for efficient photodynamic therapy. Small. 2018;14(4):1702815.
- Qi H, Liu C, Long L, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10(3):3323–3333.
- Li SY, Qiu WX, Cheng H, et al. A versatile plasma membrane engineered cell vehicle for contact-cell-enhanced photodynamic therapy. Adv Funct Mater. 2017;27(12):1604916.
- Gao W, Hu CM, Fang RH, et al. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater Weinheim. 2013;25(26):3549–3553.
- Tian Q, Jiang F, Zou R, et al. Hydrophilic Cu9S5 nanocrystals: a photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano. 2011;5(12):9761–9771.
- Tian Q, Tang M, Sun Y, et al. Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv Mater Weinheim. 2011;23(31):3542–3547.
- Patel KD, Singh RK, Kim HW. Carbon-based nanomaterials as an emerging platform for theranostics. Mater Horiz. 2019;6(3):434–469.
- Zeng Y, Yang Z, Li H, et al. Multifunctional nanographene oxide for targeted gene-mediated thermochemotherapy of drug-resistant tumour. Sci Rep. 2017;7(1):43506.
- Chen YW, Chen PJ, Hu SH, et al. NIR-triggered synergic photo-chemothermal therapy delivered by reduced graphene oxide/carbon/mesoporous silica nanocookies. Adv Funct Mater. 2014;24(4):451–459.
- Prendergast GC, Malachowski WP, Duhadaway JB, et al. Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res. 2017;77(24):6795–6811.
- Labadie BW, Bao R, Luke JJ. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan-kynurenine-aryl hydrocarbon axis. Clin Cancer Res. 2019;25(5):1462–1471.
- Peng J, Xiao Y, Li W, et al. Combined photothermal therapy and immunotherapy: photosensitizer micelles together with IDO inhibitor enhance cancer photothermal therapy and immunotherapy (Adv. Sci. 5/2018). Adv Sci. 2018;5(5):1870031.
- Xing L, Gong JH, Wang Y, et al. Hypoxia alleviation-triggered enhanced photodynamic therapy in combination with IDO inhibitor for preferable cancer therapy. Biomaterials. 2019;206:170–182.
- Li X, Fan Y, Watari F. Current investigations into carbon nanotubes for biomedical application. Biomed Mater. 2010;5(2):22001.
- Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotechnol. 2009;4(10):627–633.
- Firme CP, Bandaru PR. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomedicine. 2010;6(2):245–256.
- De La Zerda A, Zavaleta C, Keren S, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol. 2008;3(9):557–562.
- Vardharajula S, Ali SZ, Tiwari PM, et al. Functionalized carbon nanotubes: biomedical applications. Int J Nanomedicine. 2012;7:5361–5374.
- Zhou B, Li Y, Niu G, et al. Near-infrared organic dye-based nanoagent for the photothermal therapy of cancer. ACS Appl Mater Interfaces. 2016;8(44):29899–29905.
- Zheng M, Yue C, Ma Y, et al. Single-step assembly of DOX/ICG loaded lipid-polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano. 2013;7(3):2056–2067.
- Liu Y, Ai K, Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev. 2014;114(9):5057–5115.
- Casey SC, Tong L, Li Y, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352(6282):227–231.
- Bagley AF, Hill S, Rogers GS, et al. Plasmonic photothermal heating of intraperitoneal tumors through the use of an implanted near-infrared source. ACS Nano. 2013;7(9):8089–8097.
- He J, Wilson BC, Piao D, et al. Diffuse optical tomography to monitor the photocoagulation front during interstitial photothermal therapy: numerical simulations and measurements in tissue-simulating phantoms. Photon Laser Med. 2014;3(3):241–254.
- Kannadorai RK, Liu Q. Optimization in interstitial plasmonic photothermal therapy for treatment planning. Med Phys. 2013;40(10):103301.
- Liu S, Doughty A, West C, et al. Determination of temperature distribution in tissue for interstitial cancer photothermal therapy. Int J Hyperthermia. 2018;34(6):756–763.
