Abstract
Background
Disseminated peritoneal leiomyomatosis (DPL) is a rare, generally benign disorder. With the advent of laparoscopic surgery for uterine fibroids, the reported number of cases of DPL has significantly increased since the introduction of unconfined power morcellation. Morcellation and other procedures may be associated with DPL.
Methods
We present the case of a 48-year-old patient with DPL who underwent uterine artery embolization (UAE), laparoscopic myomectomy and high intensity focused ultrasound (HIFU) 11 years, 6 years, and 2 years before the final diagnosis of DPL was made. A subtotal hysterectomy with bilateral salpingo-oopherectomy was performed to remove the uterus, the fallopian tube and the ovaries. We carefully excised as many visible lesions as possible.
Results
After the surgical treatment performed in our center the patient became free of symptoms.
Conclusion
In our case, the occurrence of DPL is most likely associated with laparoscopic myomectomy using power morcellation. In addition, it should be noted whether some other factors played a role in stimulating the growth of the multiple nodules.
Introduction
Disseminated peritoneal leiomyomatosis (DPL) is a rare disorder characterized by the presence of multiple nodules composed of smooth muscle tissue scattered in peritoneal and subperitoneal spaces. The first case of DPL was reported by Wilson and Peale in 1952, and named by Taubert et al. in 1960s [Citation1,Citation2]. DPL is a benign fibrotic tumor occurring in reproductive age women whose etiology and pathophysiology remain unclear [Citation3]. The diagnosis of DPL is still a challenge because this disease is often asymptomatic; cases are usually detected incidentally. We present the case of a 48-year-old patient with DPL who underwent uterine artery embolization (UAE), laparoscopic myomectomy and high intensity focused ultrasound (HIFU) before the final diagnosis of DPL. After the complete surgical treatment performed in our center the patient became free of symptoms.
Case report
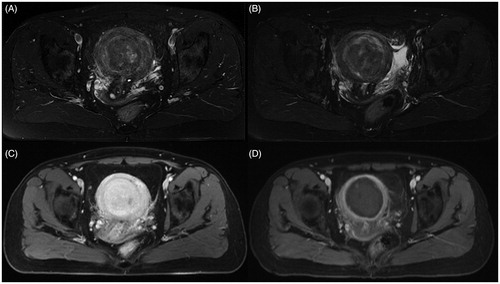
A 48-year-old patient presented to our hospital with a 6-month history of abdominal distention with recent worsening. She had undergone a previous UAE 11 years ago at a university hospital for menorrhagia due to multiple uterine fibroids with the largest one at 5.0 cm in diameter. She reported her heavy menstruation as slightly relieved after UAE. Six years ago, she went to the same university hospital due to the menstruation becoming heavier. An ultrasound scan revealed multiple intramural, submucosal uterine fibroids with the largest one at 7.8 cm in diameter, the blood test showed the hemoglobin lower than 10 g/L. She had undergone a laparoscopic myomectomy with unconfined power morcellation. The symptoms were relieved after the three dominant fibroids were removed. The post-operation pathological examination revealed leiomyomatic tissue with hyaline degeneration. Two years ago, the patient complained of heavy bleeding again and went to another external clinic. A magnetic resonance imaging (MRI) of the abdomen and pelvis was performed. The contrast-enhanced MRI showed multiple heterogeneously enhanced uterine fibroids, with the largest one at 6 cm protruding into the uterine cavity (). HIFU ablation was subsequently performed and the post-HIFU MRI showed the fibroid was completely ablated (). The heavy bleeding menstruation improved after HIFU.
Figure 1. Transverse view of MR images obtained before and 1 day after HIFU treatment. (A). A pre-HIFU T2 weighted image showed multiple nodules with the largest one at a 6 cm protruding into the uterine cavity; (B). A post-HIFU T2 weighted image showed the signal intensity of the largest fibroid decreased; (C). A pre-HIFU contrast MR image showed hyperenhancement of the fibroids; (D). A post-HIFU contrast MR image showed no perfusion in the largest fibroid. The largest fibroid was completely ablated.
Six months before the patient was admitted to our hospital, she developed abdominal distention. The patient denied menstrual pain, constipation and nausea. Initial ultrasonography of the pelvis revealed multiple uterine fibroids and multiple pelvic nodules of similar echogenicity as the uterine fibroids. An MRI was subsequently performed on the abdomen and pelvis for better characterization of these nodules. The MRI showed multiple lesions of different sizes with a similar appearance and intensity in the lower abdomen and pelvis. The size of the lesions ranged from 1 to 4 cm. The size of the uterine fibroid previously treated with HIFU decreased from 6 to 2 cm in diameter. Her physical examination was unremarkable and the laboratory blood tests, ECG and chest X-ray showed no anomalies. Based on her history of previous laparoscopic myomectomy with morcellation for uterine fibroids and the MRI findings, a diagnosis of DPL was highly suspected, so exploratory laparoscopic surgery was scheduled because she refused to have open surgery.
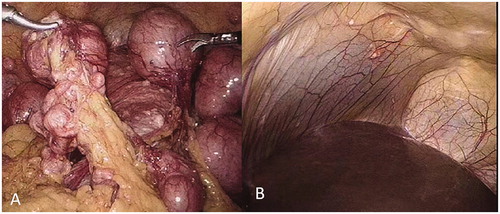
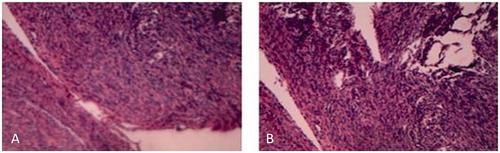
During laparoscopic surgery, extensive and dense multiple gray and white nodules were found on the surface of the uterus, broad ligaments, the fallopian tubes, the anterior and lateral abdominal wall, the vesicouterine pouch, the rectouterine pouch, the ovaries, liver, cecum, sigmoid colon and greater omentum (). The size of the uterus was about 5 months of gestation and the multiple nodules were ranged from 2 mm to 9 cm in size. The largest nodule of 9 cm in diameter was located at the fundus of the uterus, the other two bigger nodules, approximately 4 and 6 cm in size, respectively, were detected in the vesicouterine pouch and the rectouterine pouch. In addition, we found that the left broad ligament, the left fallopian tube, and the left ovary were surrounded by the intestinal tube, and were closely adhered to. So we first performed a laparoscopic subtotal hysterectomy with a bilateral salpingo-oophorectomy to remove the uterus, the fallopian tubes and the ovaries, then carefully excised the two bigger nodules in the vesicouterine pouch and the rectouterine pouch, as well as the visible small lesions on the liver (), partial omentectomy was also made. No intraoperative or postoperative complications occurred and the patient was discharged 3 days after surgery. The histological exam showed the multiple nodules with typical features of uterine fibroids. Neither atypia nor necrosis was detected in the specimen (). Based on the findings from laparoscopic surgery and the histological exam results, a diagnosis of DPL was established in this case. In April 2020, the six months follow-up after surgery, she reported no symptoms and the color Doppler ultrasound did not show any specific findings in the pelvic cavity.
Discussion
Uterine fibroids or leiomyomas are common benign tumors of the uterus. Generally, the prevalence is 20–40% among reproductive-age women [Citation4,Citation5]. Half of these patients have menorrhagia, lumbosacral pain, constipation and other symptoms, which can affect the quality of life and cause infertility5.
Over the last two decades, with the advent of laparoscopic surgery, the postoperative outcomes in terms of scar cosmesis and pain control in the surgical management of uterine fibroids have been greatly improved. However, this minimally invasive surgical technique has increased the risk of DPL, demonstrated by the significant increase in the number of DPL cases since the introduction of unconfined power morcellation [Citation6,Citation7]. In addition, in certain reported cases, the patients were pregnant or had a history of prolonged use of contraceptive pills, or had tumor secreting estrogens. Exposure to high levels of estrogen may be another risk factor associated with DPL [Citation8]. Moreover, a case of the familial clustering of DPL was identified [Citation9]. As in our case, she has no history of exposure to a high level of estrogens and also no family history of DPL; thus, an iatrogenic etiology is considered.
The previous reports showed that DPL takes 39–132 months to present after initial laparoscopic morcellation, with the number of lesions ranging from 1 to 16 at presentation [Citation10,Citation11]. In this case, DPL presented 11, 6, and 2 years after UAE, laparoscopic myomectomy, and HIFU respectively. The number of nodules was greater than 16 with the size varying from 2 mm to 9 cm in diameter in different locations. Based on her treatment history and laparoscopic surgery, we surmised that DPL in our case may be the consequence of unconfined laparoscopic morcellation in the surgical management of uterine fibroids. Recently, Batton et al. reported a case with no other contributor history who presented with DPL three years after UAE; therefore, we should note whether there is a causal relationship between UAE and DPL [Citation12]. However, we could not explain the causal relationship between UAE and DPL in this case because of the high number and varying sizes of the lesions. As a non-invasive treatment technique, HIFU can be safely used to ablate the uterine fibroid precisely without damaging to the surrounding structures [Citation13]. Although there was no evidence indicating that HIFU ablation for uterine fibroids may induce DPL, we must pay attention to the occurrence of DPL two years after HIFU because of the pre-HIFU and post-HIFU MRI did not show any remarkable nodules in the pelvic cavity. Therefore, it should be noted whether HIFU played a role in stimulating the growth of the multiple nodules in this case. However, we still cannot fully explain why the occurrence of DPL took 11, 6, and 2 years after UAE, laparoscopic myomectomy, and HIFU treatment respectively in this case. Also, it seems that the moment an incision is made into the uterine muscles to tackle the fibroid, fragments, or micro pieces would have discharged into the abdominal cavity to be implanted, but why some implants and grow and some do not? So we surmise the morcellation might only aid in the spread but not the cause of implantation and multiplication.
Although there is no consensus on the treatment of DPL, the management of DPL requires carefully excise all the visible lesions to achieve adequate clearance. In our case, it was impossible to remove all the visible nodules because there were too many small nodules. Considering her age, a subtotal hysterectomy with bilateral salpingo-oophorectomy was performed, and we removed the uterus, the fallopian tubes, and the ovaries, and carefully excised as many lesions as possible. However, we did not use gonadotropin-releasing hormone agonists as an adjuvant treatment for DPL following surgical excision. A regular clinical and radiological follow-up was recommended.
In conclusion, DPL is a rare, generally benign disorder. Morcellation and other procedures may be associated with DPL. In our case, the occurrence of DPL is most likely associated with laparoscopic myomectomy using power morcellation. In addition, it should be noted whether HIFU played a role in stimulating the growth of the multiple nodules.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Willson JR, Peale AR. Multiple peritoneal leiomyomas associated with a granulosa-cell tumor of the ovary. Am J Obstet Gynecol. 1952;64(1):204–208.
- Taubert HD, Wissner SE, Haskins AL. Leiomyomatosis peritonealis disseminata; an unusual complication of genital leiomyomata. Obstet Gynecol. 1965;25:561–574.
- Kurman RJ, Hedrick Ellenson L, Ronnett BM. Blaustein’s pathology of the female genital tract. 6th ed. New York (NY): Springer Science and Business Media LLC; 2011. p. 470–471.
- Wang J, Zhang G, Shi H, et al. Dextran uterine artery embolization to treat fibroids. Chin Med J. 2002;115(8):1132–1136.
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
- Tan HL, Koh YX, Chew MH, et al. Disseminated peritoneal leiomyomatosis: a devastating sequelae of unconfined laparoscopic morcellation. Singapore Med J. 2019;60(12):652–654.
- Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23(3):331–337.
- Wu C, Zhang X, Tao X, et al. Leiomyomatosis peritonealis disseminata: a case report and review of the literature. Mol Clin Oncol. 2016;4(6):957–958.
- Halama N, Grauling-Halama SA, Daboul I. Familial clustering of Leiomyomatosis peritonealis disseminata: an unknown genetic syndrome? BMC Gastroenterol. 2005;5:33.
- Lu B, Xu J, Pan Z. Iatrogenic parasitic leiomyoma and leiomyomatosis peritonealis disseminata following uterine morcellation. J Obstet Gynaecol Res. 2016;42(8):990–999.
- Chin H, Ong XH, Yam PK, et al. Extrauterine fibroids: a diagnostic challenge and a long-term battle. BMJ Case Rep. 2014;2014(1):bcr2014204928.
- Batton KA, Toskich BB, LeGout JD, et al. Disseminated peritoneal leiomyomatosis after uterine artery embolization. Cardiovasc Intervent Radiol. 2018;41(12):1972–1975.
- Zhang L, Chen WZ, Liu YJ, et al. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol. 2010;73(2):396–403.