Abstract
Background
The optimal treatment for colorectal cancer (CRC) with synchronous peritoneal carcinomatosis (PC) and liver metastases (LM) remains controversial. We aimed to investigate clinical outcomes in patients with CRC and concomitant PC and LM who had undergone curative surgery, including resections at both metastatic sites and synchronous intraabdominal chemotherapy.
Methods
We searched PubMed, EMBASE, and Web of Science databases for eligible studies. Studies focusing on the clinical effects of curative surgery and synchronous intraabdominal chemotherapy for patients with CRC and concomitant PC and LM were included. Meta-analysis results were recorded as hazard ratios (HRs), risk ratios (RRs) and mean differences.
Results
We included 9 of 998 identified studies in the meta-analysis, involving 746 patients (221 patients with PC + LM, 525 patients with PC). Overall survival (pooled HR 1.68, 95% confidence interval [CI] 1.33–2.13, p < 0.01) and disease-free survival (pooled HR 1.82, 95% CI 1.51–2.20, p < 0.01) were both lower in patients with PC + LM. A higher recurrence rate (RR 1.22, 95% CI 1.04–1.44, p = 0.02) and major postoperative morbidity (RR 1.47, 95% CI 1.19–1.82, p < 0.01) were also observed in patients with PC + LM.
Conclusion
Liver resection in combination with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for patients with CRC and synchronous hepatic and peritoneal metastases may be associated with worse survival and higher morbidity compared with patients with isolated PC. More restricted patient inclusion criteria should be established to facilitate an optimal prognosis for this patient group.
Introduction
In patients with colorectal cancer (CRC) and metastatic disease, the most common metastatic sites involve the liver and the peritoneum (55 and 25%, respectively) [Citation1,Citation2]. The presence of liver metastases (LM) or peritoneal carcinomatosis (PC) has previously been considered to be a terminal condition with a poor prognosis and has been typically treated with palliative systemic therapy. Over the past decade, radical surgery accompanied by chemotherapy has resulted in considerable improvements in metastatic CRC outcomes for patients. The 5-year survival rate for patients with colorectal LM has been reported to approach 50% after application of curative resection, with low postoperative morbidity and mortality [Citation3]. Recent studies have also reported that a combined treatment involving cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) could provide better survival outcomes for a well-selected group of patients with CRC and PC [Citation4].
Synchronous peritoneal and liver metastases (PC + LM) have been estimated to be present in approximately 8% of patients with metastatic CRC [Citation5]. Palliative treatment for these patients has resulted in a poor prognosis similar to that in patients with isolated PC. Using the Analysis and Research in Cancers of the Digestive System (ARCAD) database to retrospectively analyze data concerning 1375 patients with CRC and PC who had undergone systemic therapy, one study reported that the median overall survival (OS) for patients with isolated PC was 16.3 months (range, 13.5–18.8), and the median OS of patients with both PC and other organ involvement (74% presenting with LM) was 12.6 months (range, 12.0–13.1); however, the differences between the 2 groups were not statistically significant [Citation6]. It remains unclear whether a radical treatment strategy that combines liver resection with CRS and HIPEC would benefit patients with PC + LM in terms of improved long-term survival and acceptable morbidity. In 2008, at the Fifth International Workshop on Peritoneal Surface Malignancy in Milan, a consensus statement declared that patients with CRC with up to 3 small resectable parenchymal hepatic metastases could be considered as candidates for complete resection of all tumors during CRS [Citation7]. That consensus was largely based on expert opinion from the participating centers, and evidence-based studies to validate this consensus opinion are necessary. All subsequently available evidence to date has been derived from non-randomized comparative studies, most of which have compared the effect of complete resection and HIPEC therapy between patients with PC + LM and those with isolated PC, and the results of these studies sometimes conflict [Citation8–10].
Several prior meta-analyses have been conducted to summarize data from previous studies comparing the survival of patients with PC + LM and those with isolated PC. However, conclusions based on these studies remain controversial as there were conflicting results. In 2013, a meta-analysis conducted by de Cuba et al. had included 3 studies in its final analysis. The result showed a trend toward better survival in an isolated PC group, but without statistical significance [Citation11]. The conclusions drawn are likely to have been more susceptible to bias given the limited number of included studies, which also provided results with a relatively wide-ranging confidence interval; thus, weakening the power to distinguish real differences between the 2 patient groups. In 2016, Kwakman et al. considered these limitations and included 11 published studies. Their results indicated a better OS for patients with PC, and the findings were statistically significant [Citation12]. However, the validity of their results can be challenged in terms of their inclusion criteria, as 5 of the 11 included studies had been conducted at the same medical center, investigating similar populations, over a similar period. Furthermore, one study that reported findings concerning isolated PC versus isolated LM rather than PC + LM was also included in that meta-analysis. The most recent meta-analysis to analyze the effects of PC + LM was published in 2019 and included 7 studies, and no significant difference was found between PC + LM and PC [Citation13]. There was a duplication in 3 of the included articles as well, which was a limitation of this meta-analysis. Moreover, an accompanied I2 value of up to 85.3% was unacceptably high, while the source of such high heterogeneity was unclear.
Therefore, we undertook a systematic review of the relevant literature that included more recently published studies, in concordance with the PRISMA guidelines. This study aimed to compare the clinical outcomes for patients with CRC and PC, with or without LM, who had been treated with complete tumor cytoreduction and intraperitoneal chemotherapy. Since meta-analyses concerning recurrence, mortality, and morbidity rates for these patient populations had not been performed in prior studies, we also included these variables in the pooled analysis to facilitate a comprehensive evaluation of the efficacy and safety of this curative treatment strategy.
Materials and methods
Selection criteria
The inclusion criteria comprised of articles:
that compared the clinical outcomes for patients with CRC who presented with synchronous LM and PC (PC + LM group) with those presenting with isolated PC (PC group);
in which patients with PC had been treated with CRS and intraperitoneal chemotherapy, including HIPEC or early postoperative intraperitoneal chemotherapy (EPIC), and patients with LM had been treated with complete resection or alternative radical treatments, such as radiofrequency ablation (RFA) or cryoablation;
with sufficient data concerning survival outcomes that allowed for a comparison between patients in a PC + LM group and those in a PC group, and;
that reported both OS and disease-free survival (DFS) rates.
Exclusion criteria were as follows:
non-English reports, reviews, abstracts, letters, editorials, and case reports;
duplicated articles (when studies overlapped or had been duplicated, only the report providing most complete survival data was retained);
articles reporting multiple types of malignancy, where differentiation between patients with CRC and those with other types of tumors was not possible, and;
articles in which survival outcomes had not clearly been reported, or failure to extract survival data comparing PC + LM groups with PC groups.
Search strategy
We conducted a literature search in November 2019. Combinations of the following search terms were used to identify relevant studies in the PubMed, Web of Science, and EMBASE databases: ‘colon’, ‘rectal’, ‘colorectal’, ‘cancer’, ‘carcinoma’, ‘intraperitoneal chemotherapy’, ‘intraabdominal chemotherapy’, ‘HIPEC’, ‘EPIC’, ‘liver metastases’, ‘liver resection’, ‘hepatectomy’, ‘peritoneal carcinomatosis’, and ‘peritoneal metastases’. Manual screening of reference lists from selected studies or relevant review articles was performed to identify additional studies that were potentially eligible for quantitative synthesis.
Data extraction
The primary endpoints in this study were OS and DFS. Data concerning sites of recurrence, morbidity, mortality, operative time, reoperation rate, and duration of hospitalization were also extracted from the included articles for further analysis.
Statistical analysis
A large number of the included studies could not provide sufficient data for calculating the mean value and standard deviations of survival rates. Therefore, hazard ratios (HR) with corresponding 95% confidence intervals (CI) were used as alternatives to compare OS and DFS between the 2 patient groups (PC + LM versus PC) in the meta-analysis, which could be transformed from Kaplan–Meier curves presented in the included studies using Tierney et al.’s previously published method [Citation14]. Operative time and duration of hospitalization were compared between groups in the form of mean differences. In terms of perioperative morbidity, mortality, sites of recurrence, and reoperation rates, risk ratios (RR) were used as summary statistics. A p-value of ≤0.05 was considered statistically significant.
Statistical heterogeneity was evaluated using the I2 inconsistency test. If the calculation results revealed an I2 value >50%, a random-effects model was applied to the quantitative synthesis; otherwise, a fixed-effect model was selected.
The meta-analysis and generation of graphical displays were undertaken using Review Manager 5.2 and Stata 15.1 software.
Results
Results of the literature search and characteristics of the study population
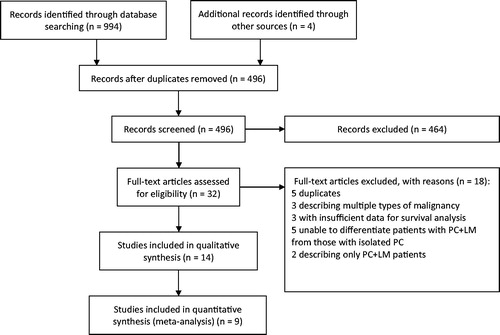
Our literature search identified 998 studies. Of these, 966 studies were excluded, based on title and abstract assessment. The full texts of the remaining 32 studies were then assessed, of which 14 were included in qualitative analysis for comparison of survival outcomes [Citation9,Citation10,Citation15–26]. Finally, 9 studies that provided sufficient data for a meta-analysis of both OS and DFS were included in a quantitative synthesis [Citation15–23]. A flow diagram of the literature search procedure according to the PRISMA guidelines is shown in .
Figure 1. Flowchart of the literature search. A literature search was undertaken according to the PRISMA guidelines. A total of 998 studies were identified, of which 9 studies were included in the meta-analysis after screening.
All 9 studies included in the meta-analysis were published between 2013 and 2019 and had been undertaken in 5 different countries. In total, 746 patients with CRC were included in the meta-analysis. Of these, 221 patients presented with synchronous PC + LM, and had been treated with liver resection (or alternative therapy such as RFA or cryoablation) in combination with CRS and intraperitoneal chemotherapy (HIPEC or EPIC). The remaining 525 patients presented with isolated PC and had been treated with CRS and intraperitoneal chemotherapy. No study included patients who had been left untreated. All of the included studies had compared clinical features between the two groups before statistical analysis. None of the studies had reported significant differences in terms of age, sex, performance status, or complete cytoreduction rates between the groups, apart from 1 study that recorded a higher proportion of male patients in the PC + LM group [Citation21], and 1 study that did not report the completeness of cytoreduction [Citation19]. Two studies reported a higher peritoneal carcinomatosis index (PCI) value and 1 study reported a lower PCI value with statistically significant differences in the PC + LM group [Citation17,Citation21,Citation23]. Three studies reported a higher percentage of patients receiving preoperative chemotherapy in the PC + LM group [Citation17,Citation19,Citation23], 2 studies reported a higher HIPEC therapy rate in the PC group [Citation16,Citation17], and 1 study reported a higher percentage of patients receiving postoperative chemotherapy in the PC group [Citation19]. The main characteristics of the included studies are summarized in .
Table 1. Characteristics of the selected studies.
Survival outcomes
The primary outcomes in this study were OS and DFS in the different groups of patients with CRC. The survival period was defined as the time from having undergone CRS and HIPEC procedures to the endpoint of observation. Most included studies showed a trend toward a shorter median survival time in the PC + LM group. Among 14 studies included in the qualitative analysis, 5 studies identified LM as a negative independent prognostic factor for OS [Citation10,Citation16,Citation17,Citation24,Citation26], whereas 9 studies reported no significant difference between patients with or without LM. After excluding studies with insufficient data to evaluate OS and DFS, 9 articles were included in the quantitative synthesis. Of these, 2 studies reported a negative effect of LM on OS () and 4 studies identified LM as a poor prognostic factor for DFS (). The median OS concerning patients in the PC + LM and PC groups reported in the studies included in our meta-analysis ranged from 13 to 36.1 months and 20.5 to 65 months, respectively. The median DFS ranged from 5.1 to 24 months and 7.6 to 24 months in the PC + LM and PC groups, respectively ().
Figure 2. Median OS for different patient groups in the 9 included studies. Each circle represents one study group, with the name of the first author labeled in the center. The red font indicates a statistically significant difference in OS between the 2 groups. Blue and yellow circles represent the PC + LM and PC groups, respectively. The size of the circle represents the number of patients in the corresponding group.
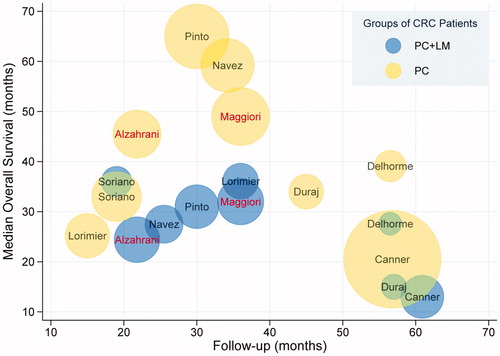
Figure 3. Median DFS in the different patient groups in the 9 included studies. Each circle represents one study group, with the name of the first author labeled in the center. The red font indicates a statistically significant difference in DFS between the 2 groups. Blue and yellow circles represent the PC + LM and PC groups, respectively. The size of the circle represents the number of patients in the corresponding group.
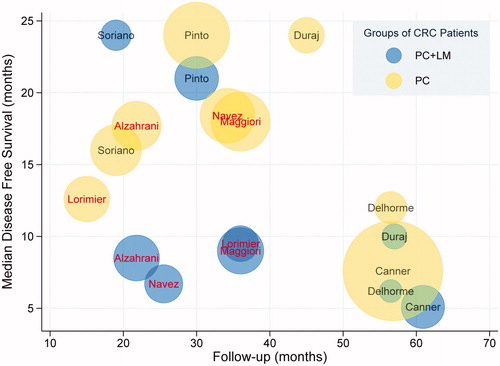
Table 2. Clinical outcomes of the selected studies.
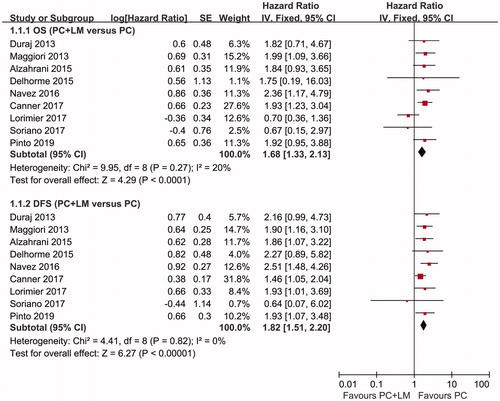
Our meta-analysis results showed that the pooled HR for OS was 1.68 (95% CI 1.33–2.13, p < 0.01) and the pooled HR for DFS was 1.82 (95% CI 1.51–2.20, p < 0.01). The survival analysis results indicated that patients with CRC with PC + LM had the poorer OS and DFS rates after complete resection of all tumors accompanied by intraperitoneal chemotherapy than patients with isolated PC. The results of the analysis are shown in .
Figure 4. Forest plots regarding OS and DFS. The pooled HR for OS was 1.68 (95% CI 1.33–2.13, p < 0.01) and the pooled HR for DFS was 1.82 (95% CI 1.51–2.20, p < 0.01). Both results indicated better survival in the PC group compared with the PC + LM group.
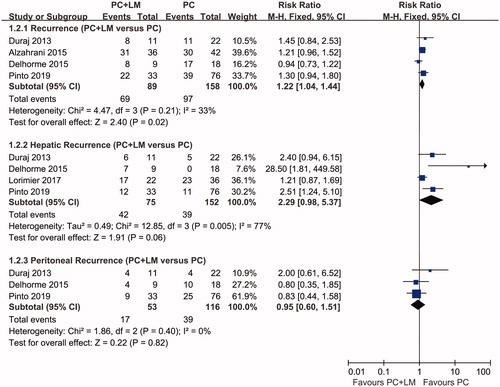
Four studies reported overall recurrence rates after curative surgery (). The recurrence rates for patients in the PC + LM group ranged from 67 to 73%, while those in the PC group ranged from approximately 50 to 95%. Three studies revealed a trend toward higher recurrence rates for patients in the PC + LM group, but the difference was not statistically significant. Pooled analyses indicated that patients in the PC + LM group were more likely to experience recurrence, with a calculated risk ratio (RR) of 1.22 (95% CI 1.04–1.44, p = 0.02). Some studies also evaluated recurrence rates in patients at different sites. According to the results of a pooled analysis, no significant difference was found between the PC + LM and PC groups in terms of the incidence of hepatic recurrence (4 studies, RR 2.29; 95% CI 0.98–5.37; p = 0.06) and peritoneal recurrence (3 studies, RR 0.95; 95% CI 0.60–1.51; p = 0.82).
Figure 5. Forest plots of recurrence. Pooled RRs concerning overall recurrence, hepatic recurrence, and peritoneal recurrence were 1.22 (95% CI 1.04–1.44, p = 0.02), 2.29 (95% CI 0.98–5.37, p = 0.06), and 0.95 (95% CI 0.60–1.51, p = 0.82), respectively. A fixed-effect model was used to calculate the RR of overall and peritoneal recurrence, whereas a random-effects model was used to calculate the RR of hepatic recurrence. The results of the analysis showed a higher risk of tumor recurrence in the PC + LM group.
Perioperative outcomes
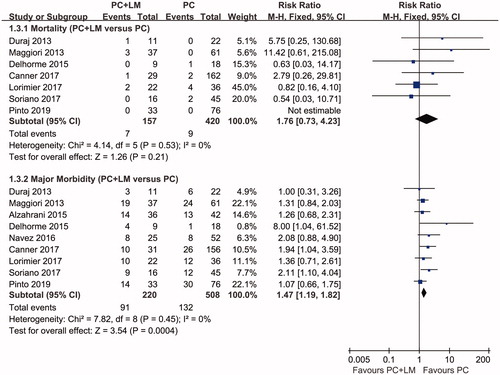
The incidence of postoperative mortality was reported in 7 studies included in the quantitative synthesis and ranged from 0 to 9.1% in the PC + LM group and from 0 to 11.1% in the PC group. No studies showed a significant difference in terms of postoperative mortality between the 2 groups, and this was confirmed in the meta-analysis results, with a pooled RR of 1.76 (95% CI 0.73–4.23, p = 0.21).
All 9 studies included in the quantitative synthesis reported the incidence of major morbidity, which ranged from 27.3 to 56.3% in the PC + LM group and from 11.1 to 39.5% in the PC group, respectively. Major postoperative morbidity was predominantly defined as the incidence of Dindo–Clavien grades III–IV complications postoperatively. Two studies identified higher morbidity with statistical significance in the PC + LM group [Citation18,Citation20]. The pooled RR obtained from our meta-analysis was 1.47 (95% CI 1.19–1.82, p < 0.01), indicating that complete resection of both PC and LM could be associated with a higher incidence of postoperative complications. Data concerning postoperative mortality, major morbidity, and the results of the meta-analysis are shown in and .
Figure 6. A Forest plot of postoperative mortality and major morbidity. The pooled RR of postoperative mortality was 1.76 (95% CI 0.73–4.23, p = 0.21) and the pooled RR of major postoperative morbidity was 1.47 (95% CI 1.19–1.82, p < 0.01). The results indicated a higher morbidity during the early period postoperatively in the PC + LM group, whereas there was no statistically significant difference in mortality between the 2 groups.
Operative time ranged from 366 min to 586 min in the PC + LM group and from 456 to 480 min in the PC group, as reported in 3 studies. A pooled analysis showed a mean difference of 22.58 min (PC + LM minus PC, 95% CI −106.48 to 151.65, p = 0.73). Hospitalization duration ranged from 16.0 to 23.1 days in the PC + LM group and from 14.4 to 23.7 days in the PC group, as reported in 3 studies. A pooled analysis showed a mean difference of 2.17 days (PC + LM minus PC, 95% CI −5.85 to 10.20, p = 0.60). The re-operation rate ranged from 4.5 to 25.0% in the PC + LM group and from 9.6 to 26.3% in the PC group, as reported in 5 studies. The pooled RR was 1.01 (95% CI 0.63–1.62, p = 0.97). An analysis of the aforementioned clinical factors indicated no statistically significant difference between the 2 groups.
Heterogeneity and risk of bias in the included studies
Statistical heterogeneity underlying the results of the included studies was examined using the I2 inconsistency test. No evidence of significant heterogeneity was observed in the assessment of OS, DFS, mortality, major morbidity, overall recurrence, peritoneal recurrence, and reoperation rates (I2 = 0%, p = 0.49). Lorimier et al. and Delhorme et al.’s studies contributed most of the heterogeneity in the assessment of OS and overall recurrence, respectively. Excluding Lorimier et al.’s study from the pooled analysis for OS resulted in a modified pooled HR of 1.84 (95% CI 1.43–2.36, p < 0.01), and excluding Delhorme et al.’s study from the overall recurrence analysis resulted in a modified RR of 1.27 (95% CI 1.06–1.53, p = 0.01). Adjustment of the included studies could further decrease the I2 value of each analysis to 0%, while both results remained statistically significant. However, unacceptably high heterogeneity was observed in the assessment of hepatic recurrence, operative time (I2 = 91%, p < 0.01), and duration of hospitalization (I2 = 92%, p < 0.01). Therefore, it is more difficult to reach reliable conclusions from pooled analyses of these factors.
To identify reporting bias in the studies, funnel plot asymmetry of each clinical factor included in the meta-analysis was measured numerically. Tests previously proposed by Egger et al. and Harbord et al. were used to evaluate continuous variables and dichotomous variables, respectively [Citation27,Citation28]. The test results indicated that none of the clinical factors presented a funnel plot with significant asymmetry (). While some of the test conclusions may have limitations due to the insufficient number of included studies, no evidence was found to confirm the presence of publication bias, based on the asymmetry examination.
Table 3. Results of tests for funnel plot asymmetry.
Discussion
Currently, the role of radical treatment combining liver resection with CRS and HIPEC in patients with CRC and PC + LM remains controversial. A comparison of the therapeutic effect between curative resection plus HIPEC and palliative treatment for patients with PC + LM provided a suitable approach to help resolve this controversy. Some studies have reported that CRS and HIPEC combined with liver resection could provide better survival outcomes than palliative systemic chemotherapy; however, most of the evidence has been obtained from imprecise comparisons involving previously published historical data rather than data from well-designed controlled studies [Citation8,Citation11,Citation29]. To date, very few studies have compared the effect of these 2 therapeutic strategies within the same PC + LM population. Razenberg et al. reported that in the presence of concomitant PC and LM, patients treated with palliative chemotherapy showed a significantly lower median OS (12.5 months, 95% CI 11.30–13.08) than patients treated with CRS and HIPEC (23.1 months, 95% CI 13.90–36.07) [Citation30]. However, there are significant limitations in interpreting this result as no data were available to evaluate heterogeneity in terms of the characteristics between the 2 groups, including general patient status, the extent of disease, and history of prior treatment. Given there was a greater chance that patients with less extensive peritoneal involvement or fewer hepatic lesions would have been treated with radical surgery and HIPEC, improvements in OS for patients receiving radical therapy could potentially have been overestimated due to selection bias.
Comparing the efficacy of curative resection and HIPEC between patients with PC + LM and those with isolated PC can also provide evidence to assess the value of this therapy in the PC + LM population, as CRS and HIPEC have previously been shown to improve the survival of patients with isolated PC. This is the most widely used method reported in studies that have evaluated this issue. Although most studies undertaken after 2008 restricted the study population inclusion criteria and usually enrolled patients with a limited number of LM (median, 1–2), conflicting results can still be found among these comparative studies and the subsequent meta-analyses.
Following the publication of more recent studies, this issue has been reexamined. All the articles included in our meta-analysis were published after 2013, after duplicated studies had been excluded. Results from our meta-analysis indicated that both OS and DFS in patients with PC + LM were significantly lower than in those with isolated PC when treatment involved radical surgery and HIPEC/EPIC. Most recent studies, including all 9 studies included in our meta-analysis, had attempted to restrict heterogeneity through matching or comparing clinical features between PC + LM and PC groups, which had not usually been ensured in earlier studies.
Restricting heterogeneity between groups has been challenging. Currently, there is no clear consensus concerning which characteristics in patients with CRC and PC independently affect prognosis. Incomplete cytoreduction and extensive PC (usually measured using the PCI score) were considered negative prognostic factors in a randomized controlled trial undertaken in the Netherlands [Citation31], which has been accepted in other studies [Citation13,Citation32]. Other clinical features including age, sex, general patient status, lymph node metastasis, tumor differentiation, primary tumor location, the presence of ascites, the number of LM, and preoperative/postoperative chemotherapy have also been considered prognostic variables affecting survival after CRS and HIPEC in some of the retrospective studies [Citation16,Citation17,Citation24,Citation25,Citation33–36]. In the 9 studies we included, there were a variety of experimental designs comparing different variables between the 2 groups. Along with differing trials or types of patients, treatment modalities also varied in terms of the extent of organ resection, the HIPEC technique, and the chemotherapy regimen. In each of these studies, not all the relevant variables were compared or well-matched between the PC + LM and PC groups. Three of the studies showed a significant difference in PCI between the 2 groups. If these studies were excluded, a pooled analysis for OS from the remaining 6 studies provided a lower HR of 1.47 (95% CI 1.05–2.07, p = 0.02), while the modified HR for OS and DFS remained statistically significant. The percentage of patients who received preoperative chemotherapy was reported to be higher in the PC + LM group in 3 of the studies. Similarly, if these 3 studies were excluded, the pooled HR for OS and DFS remained statistically significant. No significant difference in age, general status, lymph node metastasis, primary sites of the tumor, or completeness of cytoreduction was observed in the included studies.
A pooled analysis for OS in this study provided an I2 value of 20%, indicating an acceptable heterogeneity among the included studies, with Lorimier et al.’s study contributing the most heterogeneity. When we excluded this study from the meta-analysis, the I2 value decreased to an extremely low level (I2 = 0%, p = 0.94). Lorimier et al. also reported a trend in terms of a longer OS in the PC + LM group when compared to the isolated PC group [Citation22]. Identifying the probable cause of heterogeneity was challenging, as no significant difference could be found in most of the relevant clinical features between the PC + LM and PC groups in this study. Moreover, there was no significant difference between this study and the others enrolled in the meta-analysis concerning the extent of hepatic involvement, the treatment process for LM, the number of patients receiving perioperative chemotherapy, the compound used in HIPEC, and the median period of follow-up. It was unclear whether a lower proportion of incomplete resections in the PC + LM group would have been the cause of the longer OS, as Lorimer at al.’s findings showed that the proportion of complete cytoreduction appeared higher in the PC + LM group than in the PC group (86.4 versus 69.4%); although this difference was not statistically significant.
Our findings also showed significantly higher postoperative morbidity for patients in the PC + LM group. A pooled analysis for morbidity revealed a result unfavorable to patients with PC + LM, which was not unexpected, as the addition of liver resection to the process of CRS + HIPEC usually represents a more aggressive treatment strategy with a greater extent of surgical injury. However, insufficient data were available to clarify the specific risk factors related to differences in morbidity, as the treatment procedures had not been standardized among the included studies. There was variation in the recorded postoperative complications among the different studies, including complications at the surgical site such as anastomotic leakage, intestinal obstruction, hemorrhage, and abdominal abscesses, and systemic manifestations such as sepsis, pulmonary embolism, and renal failure. Complications related to liver resection such as biliary leakage and liver failure were not commonly reported, and these complications appeared to be associated more frequently with the performance of major hepatectomy [Citation16,Citation18].
Our results showed that the application of liver resection accompanied by CRS + HIPEC for concomitant PC and LM could be associated with poorer survival, earlier onset of recurrence, and a higher risk of postoperative morbidity when compared with patients with isolated PC. These adverse clinical outcomes should be carefully considered when selecting patients for this aggressive therapy as overtreatment should be avoided, given it may impair the quality of care and provide minimal benefit in terms of the prognosis. Nevertheless, the results of our meta-analysis were in accordance with the assumption that a better prognosis could be achieved in a well-selected group of patients with CRC and PC + LM. It is important to emphasize that more restricted patient inclusion criteria should be designed, except in relation to the number of liver lesions. The PCI score was most commonly used as an additional factor for selecting patients eligible to receive curative resection. Maggiori et al. reported a median OS of 40 months in patients with a PCI <12 and ≤2 LM, and a higher PCI and more LM were associated with a lower OS [Citation16]. Alzahrani et al. reported that the median survival for patients with a PCI ≤7 and ≤3 LM was significantly longer than those with a PCI >7 and >3 LM [Citation17]. Elias et al. presented a nomogram aiming to develop a tool to estimate the clinical outcomes for patients with LM and peritoneal metastases that selected the PCI and the number of LM as predictors of survival, as these 2 variables had shown a significant effect in their multivariate analysis [Citation37]. Further research is necessary to determine the prognostic effect of these 2 variables, as well as the relationship between survival and other factors such as performance status, lymph node metastasis, and tumor histology, to identify an optimal subgroup of patients who may benefit from radical surgery and intraperitoneal chemotherapy. Future studies to compare the prognostic difference between radical treatment and palliative chemotherapy in patients with PC + LM would also be helpful in developing appropriate treatment strategies for this group of patients.
Our study was limited due to a lack of relevant randomized trials, and heterogeneity among the study population and in treatment modality. Furthermore, poor descriptions of statistical outcomes in some prior publications restricted the number of included studies, which may have increased the risk of selection bias of our study. Further research involving homogenous patient populations using standardized surgical and HIPEC techniques is likely to provide higher-quality evidence concerning this topic.
In conclusion, our meta-analysis findings showed that the strategy of curative resection combined with HIPEC for CRC patients with synchronous hepatic and peritoneal metastases may be associated with worse survival and higher morbidity when compared with patients with isolated PC. More restricted inclusion criteria for patient selection should be established to facilitate an optimal prognosis for this patient group.
Ethical approval
This study was approved by the ethics committees of the Sixth Affiliated Hospital of Sun Yat-sen University. We have obtained consent to publish this paper from all the participants of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- van der Geest LG, Lam-Boer J, Koopman M, et al. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015;32(5):457–465.
- Koppe MJ, Boerman OC, Oyen WJ, et al. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243(2):212–222.
- Jones RP, Jackson R, Dunne DF, et al. Systematic review and meta-analysis of follow-up after hepatectomy for colorectal liver metastases. Br J Surg. 2012;99(4):477–486.
- Huang CQ, Min Y, Wang SY, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: a systematic review and meta-analysis of current evidence. Oncotarget. 2017;8(33):55657–55683.
- Thomassen I, van Gestel YR, Lemmens VE, et al. Incidence, prognosis, and treatment options for patients with synchronous peritoneal carcinomatosis and liver metastases from colorectal origins. Dis Colon Rectum. 2013;56(12):1373–1380.
- Franko J, Shi Q, Meyers JP, et al. Analysis and Research in Cancers of the Digestive System (ARCAD) Group. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–1719.
- Esquivel J, Elias D, Baratti D, et al. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol. 2008;98(4):263–267.
- Chua TC, Yan TD, Zhao J, et al. Peritoneal carcinomatosis and liver metastases from colorectal cancer treated with cytoreductive surgery perioperative intraperitoneal chemotherapy and liver resection. Eur J Surg Oncol. 2009;35(12):1299–1305.
- Varban O, Levine EA, Stewart JH, et al. Outcomes associated with cytoreductive surgery and intraperitoneal hyperthermic chemotherapy in colorectal cancer patients with peritoneal surface disease and hepatic metastases. Cancer. 2009;115(15):3427–3436.
- Baratti D, Kusamura S, Iusco D, et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum. 2014;57(7):858–868.
- de Cuba EM, Kwakman R, Knol DL, et al. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases: systematic review of all literature and meta-analysis of observational studies . Cancer Treat Rev. 2013;39(4):321–327.
- Kwakman R, Schrama AM, van Olmen JP, et al. Clinicopathological parameters in patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer metastases: a meta-analysis. Ann Surg. 2016;263(6):1102–1111.
- Hallam S, Tyler R, Price M, et al. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open. 2019;3(5):585–594.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
- Duraj FF, Cashin PH. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal and hepatic metastases: a case-control study. J Gastrointest Oncol. 2013;4(4):388–396.
- Maggiori L, Goéré D, Viana B, et al. Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent? A case-control study. Ann Surg. 2013;258(1):116–121.
- Alzahrani N, Ung L, Valle SJ, et al. Synchronous liver resection with cytoreductive surgery for the treatment of liver and peritoneal metastases from colon cancer: results from an Australian centre. ANZ J Surg. 2017;87(11):E167–E172.
- Delhorme JB, Dupont-Kazma L, Addeo P, et al. Peritoneal carcinomatosis with synchronous liver metastases from colorectal cancer: who will benefit from complete cytoreductive surgery? Int J Surg. 2016;25:98–105.
- Navez J, Remue C, Leonard D, et al. Surgical treatment of colorectal cancer with peritoneal and liver metastases using combined liver and cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: report from a single-centre experience. Ann Surg Oncol. 2016;23(5):666–673.
- Soriano RM, Canis JMM, Romero XM, et al. Influence of simultaneous liver and peritoneal resection on postoperative morbi-mortality and survival in patients with colon cancer treated with surgical cytoreduction and intraperitoneal hyperthermic chemotherapy. Cir Esp. 2017;95(4):214–221.
- Downs-Canner S, Shuai Y, Ramalingam L, et al. Safety and efficacy of combined resection of colorectal peritoneal and liver metastases. J Surg Res. 2017;219:194–201.
- Lorimier G, Linot B, Paillocher N, et al. Curative cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis and synchronous resectable liver metastases arising from colorectal cancer. Eur J Surg Oncol. 2017;43(1):150–158.
- Pinto A, Hobeika C, Philis A, et al. Synchronous liver metastases and peritoneal carcinomatosis from colorectal cancer: different strategies for curative treatment? Langenbecks Arch Surg. 2019;404(4):477–488.
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Cin Oncol. 2004;22(16):3284–3292.
- Shen P, Hawksworth J, Lovato J, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11(2):178–186.
- Cavaliere F, De Simone M, Virz S, et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol. 2011;37(2):148–154.
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–3457.
- Randle RW, Doud AN, Levine EA, et al. Peritoneal surface disease with synchronous hepatic involvement treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol. 2015;22(5):1634–1638.
- Razenberg LGEM, van Gestel YRBM, Creemers GJ, et al. Trends in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of synchronous peritoneal carcinomatosis of colorectal origin in the Netherlands. Eur J Surg Oncol. 2015;41(4):466–471.
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Cin Oncol. 2003;21(20):3737–3743.
- Goéré D, Souadka A, Faron M, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. 2015;22(9):2958–2964.
- Verwaal VJ, van Tinteren H, van Ruth S, et al. Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br J Surg. 2004;91(6):739–746.
- Elias D, Glehen O, Pocard M, et al. A comparative study of complete cytoreductive surgery plus intraperitoneal chemotherapy to treat peritoneal dissemination from colon, rectum, small bowel, and nonpseudomyxoma appendix. Ann Surg. 2010;251(5):896–901.
- Bruin SC, Verwaal VJ, Vincent A, et al. A clinicopathologic analysis of peritoneal metastases of colorectal and appendiceal origin. Ann Surg Oncol. 2010;17(9):2330–2340.
- Tonello M, Sommariva A, Pirozzolo G, et al. Colic and rectal tumors with peritoneal metastases treated with cytoreductive surgery and HIPEC: one homogeneous condition or two different diseases? A systematic review and meta-analysis. Eur J Surg Oncol. 2019;45(11):2003–2008.
- Elias D, Faron M, Goere D, et al. A simple tumor load-based nomogram for surgery in patients with colorectal liver and peritoneal metastases. Ann Surg Oncol. 2014;21(6):2052–2058.
