Abstract
Purpose
This study aimed to compare the efficacy and safety of ultrasound-guided percutaneous microwave ablation (MWA) for hepatocellular carcinoma (HCC) adjacent to large vessels with those far from large vessels.
Methods
The clinical data of patients who underwent ultrasound-guided percutaneous MWA for HCC were retrospectively analyzed between January 2011 and December 2018 in Shengjing Hospital. Patients with HCC adjacent to large vessels were included in the Vessel group, the remaining patients were included in the Control group. Propensity score matching analysis was used to reduce confounding bias. The rates of complete ablation, local recurrence, recurrence-free survival (RFS), overall survival (OS) and complications were compared between the two groups.
Results
A total of 134 patients with 157 nodules (size range, 0.6–3.8 cm) were enrolled in this study, 23 in the Vessel group and 111 in the Control group. A total of 21 patients in the Vessel group (91.3%) and 105 patients in the Control group (94.6%) achieved complete ablation (p = .902). Following 1:2 propensity score matching, 22 patients were included in the Vessel group and 40 patients were enrolled in the Control group. Local recurrence was observed in 2 (9.1%) patients in the Vessel group and 5 (12.5%) in the Control group (p = .86). No significant difference in local recurrence rate, RFS and OS were observed between the two groups.
Conclusions
Ultrasound-guided percutaneous MWA appears to be a safe procedure and can achieve comparable oncological efficacy for HCC abutting large vessels.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers in the Chinese population, and it ranks the second leading cause of cancer-related death for males and the fifth for females [Citation1]. Traditionally, liver resection is considered as the curative treatment for HCCs. However, in the past two decades, local thermal ablation including microwave ablation (MWA), radiofrequency ablation (RFA) and cryoablation, have emerged as other standard treatments for early stage HCCs [Citation2–5]. Previous studies have confirmed that thermal ablation can achieve long-term survival, similar to liver resection [Citation6]. However, the efficacy of ablation was affected by proximity with large vessels, tumor adjacent to large vessels was a risk factor for local tumor progression [Citation5]. The main reason is the cooling effect of blood in large vessels, which is also known as the ‘heat sink effect’ [Citation7]. Second, there exists a risk of surrounding vascular damage [Citation8]. Compared with RFA, MWA has advantages of generating higher coagulation temperatures in shorter ablation time, larger ablation zone and the ability to use multiple probes simultaneously [Citation9]. Furthermore, MWA is less affected by the ‘heat sink effect’ [Citation7,Citation10].
With further developments in thermal technology and the accumulation of clinical experience, more researchers have tried to treat tumors adjacent to large vessels with MWA or RFA, and the results were also comparable with those far away from large vessels [Citation11–13]. Considering the advantages of MWA, it may be a more suitable option. However, the previous studies lack strict inclusion criteria and the final results were affected by confounding bias. In this regard, propensity score matching (PSM) is an emerging statistical technique, which is widely used in processing data from observational studies. PSM can effectively reduce the confounding bias, eliminate the interference factors between groups, and make the results statistically more reliable. In this study, we used PSM analysis to retrospectively compare the outcomes of HCCs adjacent to large vessels, which were treated by ultrasound (US)-guided percutaneous MWA, with those far away from the large vessels. The risk factors affecting therapeutic efficacy were also analyzed.
Materials and methods
Patients
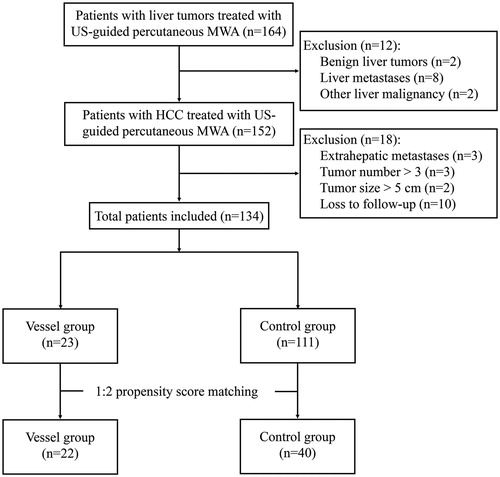
This study was approved by the Ethics Committee of Shengjing Hospital. Informed written consent was obtained from all patients included in the study before treatment. Treatment options for all patients with HCC were discussed by our multidisciplinary team including hepatobiliary surgeon, interventional radiologist, interventional sonographer, and oncologist, which included liver resection, thermal ablation, transarterial chemoembolization and systemic therapies. A total of 164 patients underwent US-guided percutaneous MWA for liver tumors in Shengjing Hospital of China Medical University (Shenyang, China) between January 2011 and December 2018. Patients underwent contrast enhanced US (CEUS), enhanced computed tomography (CT), and/or gadoxetic acid disodium-enhanced magnetic resonance imaging (MRI) scans before treatment. Liver functions were evaluated by blood tests and the alpha-fetoprotein (AFP) levels were detected. HCC was diagnosed based on the following criteria: (i) histologic evidence by liver biopsy; (ii) elevated AFP level and confirmed diagnoses by at least two imaging methods (CEUS/CT/MRI). Then, patients were enrolled using the following inclusion criteria: (i) Child-Pugh A or B; (ii) received MWA as a curative treatment and without concomitant treatment; (iii) single nodule with a diameter <5 cm; (iv) a maximum of three nodules with a diameter <3 cm; (v) absence of vascular invasion and extrahepatic metastases. The exclusion criteria were: (i) patients with benign liver tumors or other type liver malignancies; (ii) patients with severe concomitant diseases; (iii) patients with blood platelet level <30 × 10^9/L.
Large vessels were defined as vessels (inferior vena cava, hepatic vein, portal vein) of diameters ≥3 mm shown in CT and MRI [Citation12]. HCC lesions adjacent to large vessels were defined as tumors located ≤5 mm from large vessels [Citation11]. Patients who met the inclusion criteria were further divided into two groups according to tumor location: the Vessel group (tumor adjacent to large vessel) and the Control group (the remaining patients). Finally, a total of 134 patients were enrolled in our study, and then divided into Vessel group (n = 23) and Control group (n = 111) ().
MWA procedure
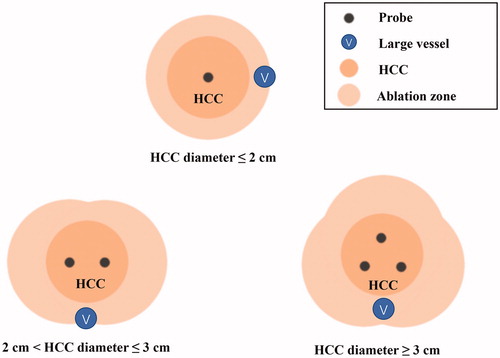
A cooled-shaft system (ECO-100AI10, ECO Microwave System Co, Nanjing, China) with max power of 80 W at 2450 MHz was used in our institution. This system was equipped with cooling circulation technology and provided real time temperature monitoring. The needle antenna (2.0 mm in diameter, 14 Gauge, 20 cm in length) was easily visualized under US. The procedure was performed by a hepatobiliary surgeon (5–10 years’ experience in MWA) with the assistance of a US physician (10 years’ experience in MWA), and modified according to the previous report [Citation14]. First, all patients underwent US (Aplio500 and PVT-375BT, Toshiba, Tokyo, Japan) examination to locate the tumor and to clarify the relationship with the surrounding anatomy. Based on the tumor location, patients need to constantly change their posture (supine or in the left lateral decubitus position) and cooperate with breathing during preoperative ultrasound examination in order to determine the best needle point and path to avoid vascular or organ injury. Then, midazolam (2 mg, intramuscular injection) and pethidine (100 mg, intramuscular injection) were given to all patients for sedation and analgesia. Local anesthesia of 2% lidocaine was given before 1 cm skin incision was made. After local anesthesia, insertion of the microwave antenna was assisted by needle guidance under real time US guidance (). For nodules with diameter ≤2cm, the antenna was placed at the center of the tumor. For nodules with a diameter between 2 and 3 cm, antenna was placed at the left and right side to the tumor center. Multiple overlapping ablations by repositioning the antenna was necessary for nodules with diameter >3cm (). In order to reduce the impact of the gas hyperechoic cloud, we preset the route of multiple punctures before ablation and waited for the bubble to subside for 5–10 min between the two punctures. An emission power of 60 W for 180 s was routinely used as one ablation cycle. Each puncture point was ablated for 2 cycles. If the heat-generated hyper-echoic bubbles did not completely encompass the tumor area, additional ablation cycle was applied. Complete ablation was considered when desired range was reached without any visible residual tumor. At the end of the procedure, the needle tract was coagulated with the continuation of microwave energy to prevent bleeding and seeding. Finally, US examination was performed to check for possible bleeding, pneumothorax or fluid accumulation.
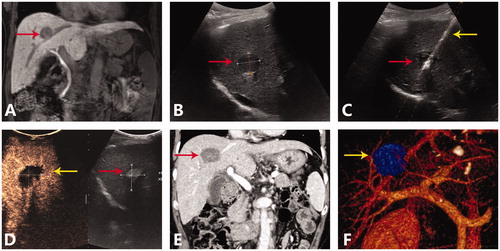
Figure 2. A representative case of ultrasound-guided percutaneous MWA for HCC abutting large vessel. (A) Preoperative MRI scan showed single nodule (red arrow) adjacent to the middle hepatic vein. (B) Preoperative ultrasound showed a hyperechoic nodule (red arrow) about 2.2 cm and 2.4 cm in size. (C) Insertion of antenna (yellow arrow) to the nodule (red arrow) under the US guidance. (D) Postoperative CEUS examination showed complete ablation of tumor. (E) On the venous phase of postoperative CT scan, no enhancement was seen in the ablation zone, the middle hepatic vein was unobstructed without thrombosis. (F) Postoperative three dimensional reconstruction showed that the ablation zone (yellow arrow) had exceeded the middle hepatic vein, and there was no related vein injury.
Assessment of therapeutic efficacy and follow-up
All patients underwent blood tests and liver function tests on postoperative days 1, 3, 5, and 7. Local therapeutic efficacy was evaluated by a combination of enhanced CT scans, MRI and/or CEUS 5–7 days after treatment [Citation15] (). The preoperative imaging data were used as a comparison, the complete ablation was defined as absence of peripheral enhanced portions and an ablation zone with at least a 5 mm margin that cover the initial tumor size [Citation15]. Any observed enhanced portions or the margin of the necrotic area close to that of the nodules were considered incomplete ablation and then an additional MWA cycle was conducted instantly in the same session. Any tumor residue found by postoperative CT, MRI and/or CEUS would be given additional MWA session ().
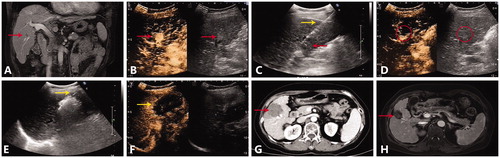
Figure 4. A representative case of multiple MWA sessions for HCC abutting large vessel. (A) Preoperative MRI scan showed one nodule (red arrow) adjacent to the right anterior branch of portal vein. (B) Contrast-enhanced ultrasound showed a hyperechoic nodule with enhancement in the arterial phase. (C) Insertion of antenna (yellow arrow) to the nodule (red arrow) under the guidance of ultrasound. (D) After the first MWA session, reexamination of contrast-enhanced ultrasound showed local enhancement of the focus (red circle). (E) Second MWA session was performed, the antenna (yellow arrow) was inserted into the residue focus. (F) After the second MWA session, contrast-enhanced ultrasound showed that the nonenhancing ablation zone (yellow arrow) covered the original focus, the nodule was completely ablated. (G) On the arterial phase of postoperative CT scan, no enhancement was observed in the ablation zone. (H) On the MRI scan one and a half year after MWA, the ablation zone (red arrow) shrank with no local recurrence.
Patients were regularly followed up with contrast-enhanced CT scans and/or MRI scans and serum AFP levels at 2 months postoperatively, then once every 3 months thereafter. Disease progression included local recurrence, intrahepatic recurrence and extrahepatic metastases. Tumor regrowth or new lesions that appeared at or adjacent to the successfully treated zone was defined as local recurrence. Early recurrence was defined as tumor relapse occurring ≤12 months and late recurrence as that occurring >12 months [Citation3]. Disease-free survival (DFS) was considered to be survival time from MWA to tumor recurrence or last follow up. Overall survival (OS) time was calculated as the interval from the initial MWA to death or last follow-up.
Statistical analyses
Pearson χ2 analysis or Fisher exact test was used to compare the categorical variables, while continuous variables were compared by independent t-test. The quantitative data were presented as mean ± standard deviation (SD). The OS and DFS were estimated with Kaplan–Meier survival analysis and was assessed using the log-rank test. Univariate and multivariate Cox regression analysis was performed to identify the independent survival prognostic factors. We further compared the characteristics between the early and late recurrence groups and binary logistic regression model was used to detect risk factors for early recurrence. Two tailed p < .05 was considered as statistically significant. All statistical analyses were performed using SPSS v22.0 software (SPSS Inc, Chicago, IL, USA).
To minimize selection bias, we conducted the PSM method to generate groups with similar baseline characteristics. Since the sample size varied greatly between the Vessel and Control groups, we performed one-to-two nearest-neighbour matching algorithm applied with a caliper of 0.2 (Propensity Score Matching in SPSSV R; SPSS Inc., Chicago, IL).
Results
Patients’ characteristics
A total of 134 HCC patients with 157 nodules were enrolled in the study. Demographic characteristics and clinical features of these patients are shown in . There were 103 males and 31 females, with a mean age of 57.6 ± 8.9 years (range, 32–80 years). 60 of 134 patients had initial HCC and underwent percutaneous MWA as first treatment, 74 of 134 patients had recurrent HCC. With respect to tumor number, 117 (87.3%) patients had a single nodule,11 (8.2%) patients had two nodules and 6 (4.5%) patients with three nodules. The mean size of tumor was 2.2 ± 0.6 cm (range, 0.6–3.8 cm), with 24 (15.3%) tumors adjacent to the larger vessels. Complete ablation was achieved in 126 (94.0%) patients and 149 (94.9%) tumors. The rest 8 patients showed incomplete ablation and all underwent a second MWA session, no patients underwent a third MWA session. Finally, a 100% technical success rate was reached with no MWA related death.
Table 1. Demographic characteristics for the enrolled patients before and after 1:2 propensity score matching.
Patients were divided into the Vessel group (n = 23) and the Control group (n = 111). The covariates including age, sex, ablation time, tumor number, tumor size, AFP level, liver cirrhosis and Child-Pugh grade were used to build the propensity score. After 1:2 PSM matching, there were 22 patients in the Vessel group and 40 patients in the Control group. As shown in , no matter before or after PSM matching, there were no significant differences between the two groups in gender, age, liver cirrhosis, portal hypertension, AFP level, albumin level, total bilirubin level, γ-glutamyltranspeptidase (GGT) level, alkaline phosphatase (ALP) level, Child–Pugh classification, and tumor number and size.
Complications
The severity of complications was reported using the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 of the National Cancer Institute. Minor complications including fever (56/134, 41.8%), ablation-related pain (53/134, 39.6%) and nausea (18/134, 13.4%) were observed. These complications were all CTCAE grade 1 or 2, the symptoms were all subsided within 2–3 days after symptomatic antipyretic and analgesic medication. Major complications were observed in two patients in the Control group. One patient experienced diaphragmatic injury and pleural effusion (Grade 3), the patient finally recovered by conservative therapies. Intestinal injury (Grade 4) occurred in one patient and developed to perforation of duodenum on postoperative day 2, the patient underwent open surgery and was discharged well.
Recurrence and survival rates
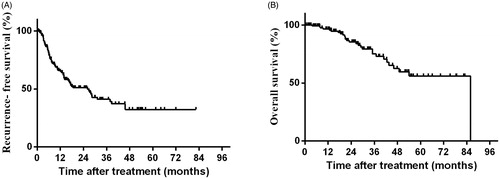
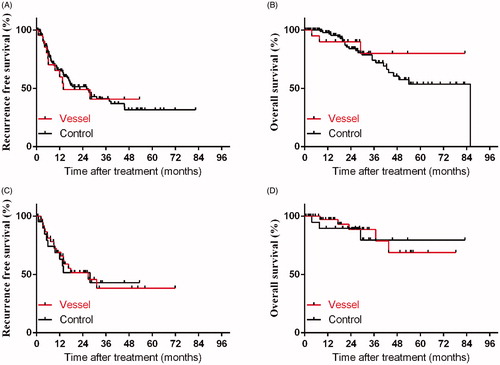
For all enrolled 134 patients, the 1-, 2-, and 3-year RFS rates were 65.1%, 51.1% and 41.2%. Meanwhile, the 1-, 2-, and 3-year OS rate were 96.6%, 85.3% and 75.2%, respectively (). During the follow-up period, there were 11 (11/23, 47.8%) patients in the Vessel group and 56 (56/111, 50.5%) patients in the Control group developed tumor recurrence. The cumulative 1-, 2- and 3-year local recurrence rates were 7.2%, 9.9% and 10.8% in the Vessel group and 4.3%, 8.7% and 8.7% in the Control group, respectively (p = .79). After PSM matching, 10 (10/22, 45.5%) patients in the Vessel group had tumor recurrence (including 2 local recurrence and 8 intrahepatic recurrence), 18 (18/40, 45.0%) patients in the Control group had tumor recurrence (including 5 local recurrence and 13 intrahepatic recurrence). Before PSM matching, the 1-, 2-, and 3-year RFS rates in the Vessel and Control groups were 59.9%, 49.0%, 40.8% and 66.2%, 51.4%, 41.2%, respectively (p = .87). Following PSM matching, the 1-, 2-, and 3-year RFS rates were 63.1%, 51.6% and 43.0% in the Vessel group, and 69.1%, 51.5%, and 38.2% in the Control group, respectively (p = .95) (). Before PSM, the 1-, and 3-year OS rates in the Vessel and Control groups were 90.0%, 80.0% and 97.9%, 74.2%, respectively (p = .59). Following PSM matching, the 1-, and 3-year OS rates were 89.5% and 79.5% in the Vessel group, 97.1% and 78.7% in the Control group, respectively (p = .82) ().
Prognostic factors associated with RFS and OS
In univariate analysis, recurrent type, male, multiple nodules, tumor size > 3 cm, high GGT level and AFP level were potential risk factors for RFS. In multivariate analysis, independent risk factors for RFS include recurrent type (HR = 2.1; 95% CI: 1.208, 3.663; p = .009), multiple nodules (HR = 2.32; 95% CI: 1.159, 4.645; p = .017), tumor size > 3 cm (HR = 3.29; 95% CI: 1.589, 6.804; p = .001) and high GGT level (HR = 1.74; 95% CI: 1.064, 2.965; p = .028). High AFP level, high GGT level and multiple nodules were significant risk factors for OS in univariate analysis. Whereas, the results of further multivariate analysis showed that high AFP level (HR = 4.03; 95% CI: 1.910, 11.277; p = .002), multiple nodules (HR = 3.61; 95% CI: 1.265, 10.293; p = .016), and tumor size (HR = 3.58; 95% CI: 1.342, 9.540; p = .011) were associated with OS (). Besides, tumor adjacent to large vessels was not an independent risk factor for either RFS or OS.
Table 2. The results of univariate and multivariate analysis using Cox proportional hazards mode.
Comparison between the early and late recurrence groups
Comparisons of the baseline characteristics between the early recurrence group (n = 41) and the late recurrence group (n = 26) are illustrated in . There were significant differences between the two groups in age, gender, ALP level and tumor number. The OS in the early recurrence group was significantly longer than late recurrence group (p = .003). The results of univariate and multivariate analyses revealed that patient age > 60 years old (HR = 2.25; 95% CI: 1.134, 4.464; p = .02) and multiple nodules (HR = 2.47; 95% CI: 1.215, 5.079; p = .014) were independent risk factors for early recurrence. However, tumor adjacent to large vessel was not the risk factor for early recurrence ().
Table 3. Characteristics of 67 patients with tumor recurrence.
Table 4. Univariate and multivariate analyses of factors associated with early recurrence.
Discussion
Percutaneous thermal ablation has been recognized worldwide as an effective and safe treatment for patients with early stage HCC. The reported complete ablation rate of MWA or RFA ranged from 92.6% to 97.2% [Citation3,Citation11,Citation16–19]. Compared with surgical resection, it is characterized by minimal invasive and shorter hospitalization, which allows for repeated sessions of ablation [Citation20]. However, there has been a longstanding debate on whether thermal ablation is suitable for tumors adjacent to large vessels. Some researchers consider it as a contraindication for thermal ablation, because the therapeutic effect can be easily affected by the ‘heat sink effect’ [Citation21]. The result of the study by Lu et al. showed that the rate of incompletely treated or local tumor recurrence was significantly lower in the non-perivascular group than that in the perivascular group (7% vs 48%, p < .01). A large peritumoral vessel was also an independent risk factor of oncologic outcome [Citation12]. Recently, with improvement in the design of the antenna, the internal cooling of MWA technology can decrease the heating of the shaft, therefore, deliver higher temperature to the target lesion and yield a larger ablation zone. Continuous and fast energy output also makes MWA less susceptible to ‘heat sink effect’ [Citation7,Citation22,Citation23]. Hence, MWA can become a more effective treatment and potentially be adopted in treating perivascular tumors.
In the present study, the complete ablation rate was 94.0% for patients and 94.9% for nodules. There was also no significant difference in complete ablation rate between the Vessel group and Control group before and after PSM. Similar with the results by Huang et al., the 1-, 2- and 3-year RFS and the 1-, 2- and 3-year OS in the two groups had no significant differences [Citation11]. An et al. assessed the efficacy of percutaneous MWA for patients with HCC in challenging locations. The results also demonstrated that OS and RFS were not influenced by tumors whether near large vessels or not [Citation24]. In multivariate analysis, recurrent type, multiple nodules, large tumor size and high GGT level were risk factors for poor RFS. In addition, multiple nodules, large tumor size and high AFP level were risk factors for poor OS. This result suggested that the tumor characteristics play a key role in the prognosis of patients, which was consistent with previous studies [Citation24,Citation25].
At present, imaging technologies like CEUS, CT and MRI are most commonly used to evaluate the technical effectiveness of MWA [Citation26]. However, it is difficult to detect a microscopic non-necrotic residual disease by these imaging, the tumor residual will lead to early recurrence or local progression. Besides, increased intra-tumoral pressure during thermal ablation might result in the spreading of tumor cells through the nearby large vessels, leading to diffuse intrahepatic recurrence [Citation27]. Therefore, we conducted subgroup analysis of recurrent patients, and the results were encouraging. Tumor abutting large vessel was not the risk factor of early recurrence. However, old patients and multiple nodules were associated with early recurrence.
There are also concerns about the safety and complications of MWA for HCC adjacent to large vessels. With larger zones of necrosis, MWA may increase the risk of potential collateral injury to adjacent non-target organs such as gastrointestinal tract, diaphragm, gallbladder, etc [Citation9,Citation24,Citation28]. Furthermore, the higher energy might cause injury to surrounding bile ducts and vessels, leading to cholangiectasis, bile leakage, thrombosis, occlusion of blood vessels and segmental liver infarction [Citation8,Citation11,Citation24,Citation29]. In this study, no related biliary tract damage and vascular injury were observed in the Vessel group. All of the two major complications occurred in the Control group. Theoretically, the vascular related complication mainly comes from the direct mechanical trauma by the puncture needle and the thermal injury of vascular wall. When ablating tumors near blood vessels, the circulating blood could take away part of heat and protect the vascular endothelium from severe damage during ablation. That might help to explain why the reported thermal injury to the vessels was minor and recoverable [Citation11,Citation24]. However, due to lack of circulating blood, the risk of collateral injury increases for tumors located in liver parenchyma and near non-target organs. Thus, we believe that by precise needle insertion and real-time temperature monitoring, MWA can be performed safely for tumors near large vessels.
As used in our center, US is still the most common guidance method in percutaneous thermal ablation for its low cost, no radiation and real time imaging. However, in selected cases such as nodules with poor conspicuity, US guidance is challenging due to decreased contrast resolution when compared with CT and MRI [Citation30]. Therefore, these patients have to receive more invasive treatments such as laparoscopic and open hepatectomy. Recently, new guiding modalities like CT, MRI, PET/CT and fusion imaging have been proved feasible, and expanded the use of ablation for tumors initially not well visible on US and tumors in challenging locations [Citation30–32]. Especially the fusion imaging system, no matter fusion of US, CT or MRI, can improve the detectability of HCC nodules and increase the treatment chance of HCC nodules by percutaneous thermal ablation [Citation30]. Non-contrast-enhanced US is generally used for monitoring of ablation. However, there exits difficulty in the judgment of tumor residues after initial ablation, the gas bubbles merged in ablation often mislead the evaluation of the ablation zone. We have also met not a small number of patients who underwent second MWA session due to insufficient ablative margin that was undiscovered by intraprocedural US. To solve the problem, Mauri et al. [Citation33] recommended the use of immediate post-procedural CEUS to evaluate the effectiveness. This strategy had comparable accuracy with CT or MRI scan at 24 h after ablation and can greatly reduce the re-treatments [Citation34]. Another solution is to employ fusion imaging systems in guidance of thermal ablation, these techniques can provide better contrast resolution and improve intra-procedural evaluation of ablation margin [Citation30,Citation31,Citation35].
Insertion of the antenna into the center of tumor is commonly used in clinical practice. For tumor with small size and away from large vessels, it can result in enough ablation zone. However, for tumors abutting large vessels and in consideration of heat sink effect, the overlapping ablation technique seems to be more suitable. In our study, we performed multiple overlapping ablations by switching the insertion point, it can produce larger and more homogeneous ablation zone. Recently, multi-applicator ablation devices have been proved safe and effective in facilitating MWA [Citation35]. It can result in simultaneous heat input into the target area and at the same time, shorten the total ablation time. Thus, multi-applicator MWA may be a better option for perivascular HCC because it allows more continuous and simultaneous heat generation without switching of electrodes. However, this technique needs parallel needles which are difficult to operate by US guidance. A combination of multi-applicator MWA and three-dimensional fusion imaging may be the future direction. This novel guiding technique can provide more accurate and objective pre-procedural planning and post-procedural evaluation, helping to achieve a higher complete ablation rate in the first MWA session [Citation14,Citation35].
This study has several limitations that should be resolved. Firstly, this was a retrospective study with unavoidable selection bias. Secondly, this is a single-center study. Thirdly, the sample size of our study is small. There were only 21 patients in the Vessel group, the results might be different with enlarged sample size. It is essential to conduct a multi-center controlled clinical trial with a larger cohort. Fourthly, we used a single antenna to ablate tumors larger than 3 cm by multi-point puncture, and the microbubbles generated by ablation may affect the accuracy of puncture and the completeness of ablation. Thus multiple antennas should be a better choice without considering the cost of treatment. Fifthly, this US-guided percutaneous MWA procedure was performed under conscious analgesic sedation. Several of patients required repeated pauses during the procedure due to intolerable pain. Therefore, the optimal ablation procedure should be performed under deep sedation or general anesthesia, even with the help of advanced guided tools such as CT, MRI and fusion imaging.
Conclusion
In conclusion, there are no significant differences in complete ablation rate, RFS and OS between the Vessel group and Control group. US-guided percutaneous MWA is a safe and effective way in treating HCC adjacent to large vessels with an acceptable incidence of complications.
Acknowledgements
The authors are very grateful to Dr. Samiksha Wasnik, from Loma Linda University, for her careful editing of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Feng RM, Zong YN, Cao SM, et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics?. Cancer Commun (Lond). 2019;39(1):22.
- Kikuchi L, Menezes M, Chagas AL, et al. Percutaneous radiofrequency ablation for early hepatocellular carcinoma: risk factors for survival. World J Gastroenterol. 2014;20(6):1585–1593.
- Waki K, Aikata H, Katamura Y, et al. Percutaneous radiofrequency ablation as first-line treatment for small hepatocellular carcinoma: results and prognostic factors on long-term follow up. J Gastroenterol Hepatol. 2010;25(3):597–604.
- Hakime A, Yevich S, Tselikas L, et al. Percutaneous thermal ablation with ultrasound guidance. Fusion imaging guidance to improve conspicuity of liver metastasis. Cardiovasc Intervent Radiol. 2017;40(5):721–727.
- Shyn PB, Mauri G, Alencar RO, et al. Percutaneous imaging-guided cryoablation of liver tumors: predicting local progression on 24-hour MRI. AJR Am J Roentgenol. 2014;203(2):W181–W191.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802.
- Pillai K, Akhter J, Chua TC, et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore). 2015;94(9):e580.
- Livraghi T, Meloni F, Solbiati L, et al. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35(4):868–874.
- Izzo F, Granata V, Grassi R, et al. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 2019;24(10):e990–e1005.
- Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236(1):132–139.
- Huang S, Yu J, Liang P, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol. 2014;83(3):552–558.
- Lu DSK, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14(10):1267–1274.
- Teratani T, Yoshida H, Shiina S, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43(5):1101–1108.
- Ren H, An C, Liang P, et al. Ultrasound-guided percutaneous microwave ablation assisted by athree-dimensional visualization treatment platform combined with transcatheter arterial chemoembolization for a single large hepatocellular carcinoma 5 cm or larger: a preliminary clinical application. Int J Hyperthermia. 2019;36(1):44–54.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Xu Y, Shen Q, Liu P, et al. Microwave ablation for the treatment of hepatocellular carcinoma that met up-to-seven criteria: feasibility, local efficacy and long-term outcomes. Eur Radiol. 2017;27(9):3877–3887.
- Yin XY, Xie XY, Lu MD, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115(9):1914–1923.
- Hoffmann R, Rempp H, Keßler DE, et al. MR-guided microwave ablation in hepatic tumours: initial results in clinical routine. Eur Radiol. 2017;27(4):1467–1476.
- Sun AX, Cheng ZL, Wu PP, et al. Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J Gastroenterol. 2015;21(10):2997–3004.
- Li D, Kang J, Golas BJ, et al. Minimally invasive local therapies for liver cancer. Cancer Biol Med. 2014;11(4):217–236.
- Liang P, Yu J, Lu MD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol. 2013;19(33):5430–5438.
- Huang S, Yu J, Liang P. Percutaneous Microwave ablation for liver tumors adjacent to large vessels. In: Microwave Ablation Treatment of Solid Tumors. Dordrecht: Springer; 2015. p. 79–87.
- Dou JP, Yu J, Yang XH, et al. Outcomes of microwave ablation for hepatocellular carcinoma adjacent to large vessels: a propensity score analysis. Oncotarget. 2017;8(17):28758–28768.
- An C, Cheng Z, Yu X, et al. Ultrasound-guided percutaneous microwave ablation of hepatocellular carcinoma in challenging locations: oncologic outcomes and advanced assistive technology. Int J Hyperthermia. 2020;37(1):89–100.
- Wang T, Lu XJ, Chi JC, et al. Microwave ablation of hepatocellular carcinoma as first-line treatment: long term outcomes and prognostic factors in 221 patients. Sci Rep. 2016;6:32728.
- Bouda D, Lagadec M, Alba CG, et al. Imaging review of hepatocellular carcinoma after thermal ablation: the good, the bad, and the ugly. J Magn Reson Imaging. 2016;44(5):1070–1090.
- Mori Y, Tamai H, Shingaki N, et al. Diffuse intrahepatic recurrence after percutaneous radiofrequency ablation for solitary and small hepatocellular carcinoma. Hepatol Int. 2009;3(3):509–515.
- Ding J, Jing X, Liu J, et al. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol. 2013;68(6):608–615.
- Zhi-Yu H, Ping L, Xiao-Ling Y, et al. A clinical study of thermal monitoring techniques of ultrasound-guided microwave ablation for hepatocellular carcinoma in high-risk locations. Sci Rep. 2017;7:41246.
- Calandri M, Mauri G, Yevich S, et al. Fusion imaging and virtual navigation to guide percutaneous thermal ablation of hepatocellular carcinoma: a review of the literature. Cardiovasc Intervent Radiol. 2019;42(5):639–647.
- Mauri G, Gennaro N, De Beni S, et al. Real-time US-18FDG-PET/CT image fusion for guidance of thermal ablation of 18FDG-PET-positive liver metastases: the added value of contrast enhancement. Cardiovasc Intervent Radiol. 2019;42(1):60–68.
- Filippiadis DK, Spiliopoulos S, Konstantos C, et al. Computed tomography-guided percutaneous microwave ablation of hepatocellular carcinoma in challenging locations: safety and efficacy of high-power microwave platforms. Int J Hyperthermia. 2018;34(6):863–869.
- Mauri G, Porazzi E, Cova L, et al. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. 2014;5(2):209–216.
- Meloni MF, Andreano A, Zimbaro F, et al. Contrast enhanced ultrasound: roles in immediate post-procedural and 24-h evaluation of the effectiveness of thermal ablation of liver tumors. J Ultrasound. 2012;15(4):207–214.
- Zhang D, Liang W, Zhang M, et al. Multiple antenna placement in microwave ablation assisted by a three-dimensional fusion image navigation system for hepatocellular carcinoma. Int J Hyperthermia. 2019;35(1):122–132.