Abstract
Introduction
Aim of this randomized controlled trial was to evaluate the effects of serial locally applied water-filtered infrared A radiation (sl-wIRAR) in patients with axial spondyloarthritis (axSpA).
Methods
axSpA patients with active disease undergoing a 7-day multimodal rheumatologic complex treatment under non-steroidal anti-inflammatory drug (NSAID) therapy were eligible. Patients were randomly assigned in a 1:1 ratio. The intervention group (IG) received additional sl-wIRAR treatment of the back (2 treatments for 30 min per day for 6 days) to assess whether locally applied hyperthermia can i) reduce pain levels, ii) reduce disease activity and improve functionality and iii) whether an effect on tumor necrosis factor α (TNFα) levels is detectable. Additionally, it was examined whether a reduction in NSAID therapy could be achieved after trial completion.
Results
71 patients completed the trial (IG: 36 patients, control group (CG) 35 patients). sl-wIRAR led to a significant pain reduction measured by a numeric rating scale (p < .0005) and in comparison, to the CG (p = .006). sl-wIRAR treatment resulted in a significant reduction in the Bath Anyklosing Spondylitis Disease Activity Index (BASDAI) (p = .004) and Bath Ankylosing Spondylitis Functional Index (p = .004) with no significant difference to the CG. TNF-α levels were significantly decreased (p = .001) only in the IG with a significant difference to the CG (p = .01). 26 (76%) of patients in the IC reduced their NSAID therapy after trial completion.
Conclusion
sl-wIRAR treatment in axSpA leads to a rapid reduction in pain allowing NSAID dosage reduction. A reason for these desirable effects could be a change in TNFα levels.
Introduction
Axial spondyloarthritis (axSpA) is a chronic rheumatic inflammatory disease that mainly affects the axial skeleton [Citation1] with a prevalence of 9 to 30 per 10000 in the general population and an incidence of about 3.1 per 100,000/year [Citation2,Citation3]. Usually patients with axSpA present with chronic back pain and stiffness of the pelvis and lower back, but any part of the spine and even the peripheral joints can be affected [Citation1]. AxSpA leads to an increased morbidity and mortality and affected patients often experience a loss of function as most patients develop structural changes of the axial skeleton at some point in their lives [Citation1,Citation2]. Over the last two decades relevant innovation and advances in disease modifying anti-rheumatic drugs (DMARDs) led to the so called ‘biologic era’ with effective pharmacological therapy for rheumatic diseases and especially for axSpA [Citation4]. Nonetheless, current data from the German Collaborative Arthritis Centers shows that a significant proportion of patients with axSpA still have moderate to high disease activity despite pharmacological treatment [Citation5]. Concerning trends in outcome in axSpA a two-sided view can be applied. For one, the portion of patients with good functional status increased from 36% in 2000 to 49% in 2012 in Germany. On the other side, more than 50% of patients with axSpA continue to experience at least a limited functional status [Citation6]. Assessment of SpondyloArthritis International Society (ASAS)/European League Against Rheumatism (EULAR) recommendations for the management of axSpA therefore clearly recommend a combination of non-pharmacological and pharmacological treatment for optimal management [Citation7]. In non-pharmacological treatment physical therapy interventions play a central role in the treatment algorithm of axSpA [Citation8,Citation9].
The use of hyperthermia in physical medicine and in particular its use in axSpA in form of whole-body hyperthermia applications such as mud baths, low-dose radon exposure in combination with whole-body hyperthermia, overheating baths and mild water-filtered infrared A radiation (wIRAR) have shown to reduce pain and disease activity [Citation10–14]. Additionally, the use of whole-body hyperthermia in treating axSpA has an effect on pro- and anti-inflammatory cytokines [Citation13–15]. Of all application forms of hyperthermia wIRAR is particularly noteworthy. It is a form of infrared heat radiation in the range of 780-1400 nm with high tissue penetration and low thermal load on the skin surface, which is easy to apply and contact-free [Citation16]. The water filtering reduces the radiation components in the undesired infrared B and C range (<5%). wIRAR has both, temperature-dependent and non-dependent effects, which are without relevant thermal energy transfer and/or relevant temperature changes [Citation16]. It is therefore not only used in acute and chronic wound healing as it promotes perfusion, alleviates pain and has anti-infectious effects [Citation17], but is also used in oncology [Citation18], dermatology [Citation16] and rheumatology [Citation12]. Until now, wIRAR has only been use in rheumatology and especially in treating axSpA as a whole-body treatment.
Based on the positive effects of whole-body hyperthermia due to whole-body wIRAR in axSpA [Citation12], we hypothesized that the use of serial locally applied wIRAR (sl-wIRAR) of the back in treating axSpA could also achieve positive effects. Therefore, we evaluated sl-wIRAR effects on (i) pain levels (ii) disease activity and functionality (iii) levels of molecular markers of disease activity (tumor necrosis factor (TNF) -α) and (iv) dosage of non-steroidal anti-inflammatory drug (NSAID) in this exploratory trial.
Methods
To evaluate the effects of sl-wIRAR of the back in patients with axSpA we conducted a prospective monocentric randomized controlled trial with an assessor-blinded parallel group design. Participants were randomly assigned in a 1:1 ratio to one of two treatment groups using simple randomization procedures (computerized random numbers). All patients aged 18 to 80 with axSpA fulfilling the ASAS classification criteria [Citation19] receiving stable NSAID therapy or stable non-pharmacological therapy for at least 4 weeks prior to treatment with moderate disease activity defined by a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) of 4 to 7 receiving an inpatient 7-day multimodal rheumatologic complex treatment (MRCT) were eligible. The ASAS classification criteria can be applied in patients with back pain over 3 months and who are younger than 45 years at symptom onset. Eligible patients fulfill the criteria if sacroiliitis is seen on imaging (MRI or radiographic) and they have more than one SpA feature (e.g. elevated CRP, HLA-B27 positivity or arthritis) or if they are HLA-B27 positiv and have more than two SpA features (e.g. elevated CRP, arthritis, uveitis, psoriasis, good response to NSAIDs) [Citation19]. BASDAI is a validated tool for assessing disease activity of axSpA and contains 6 questions determining fatigue, back and joint pain, pain at the tendons and morning stiffness (cf. ) [Citation20]. Patients were excluded if they received glucocorticoids (systemic or local) and/or conventional synthetic (cs)- and/or biologic (b-) DMARDs treatment or if they needed one of these treatments now due to disease manifestation. Patients with contraindications to hyperthermia (e.g. sensitivity disorders, heat intolerance, therapy with photosensitizing drugs, heat urticaria, new manifested hematomas, severe (chronic) kidney injury or severe (chronic) heart failure or acute infections were also excluded. The study was performed at Campus Kerckhoff of Justus Liebig University of Giessen, Dept. of Rheumatology, Immunology, Osteology and Physical Medicine, Germany. This study was conducted according to the declaration of Helsinki and after receiving approval by the local ethics committee of the Faculty of Medicine of the Justus-Liebig-University Gießen (vote no. 17/16).
Table 3. Change in functionality and disease activity between baseline and after trial completion.
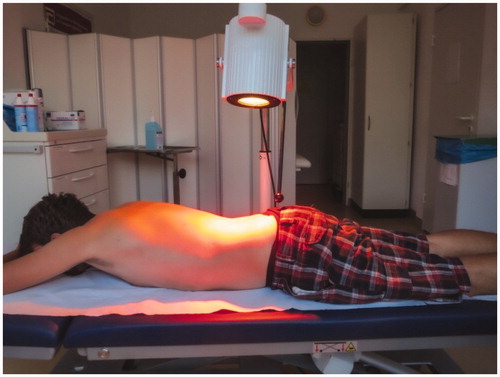
Both the intervention group (IG) as well as the control group (CG) received a 7-day MRCT in an inpatient setting. MRCT is a specific concept of German inpatient care focusing on physical therapy in addition to occupational therapy, behavioral therapy and patient education for patients with rheumatic diseases suffering from exacerbated pain and functional impairment [Citation21,Citation22]. The 7-day MRCT delivered 11 h of different MRCT modalities to each patient with every modality being applied for a duration of 30 min. In total, 22 applications (7x physiotherapy, 3x pain processing strategies, 7x classic massage, 3x electrotherapy, 2x patient disease training program) were delivered to each patient. In addition, the IG received standardized sl-wIRAR treatment of the back (2 applications daily (morning/afternoon) for 30 min for 6 days totaling in 12 applications). sl-wIRAR was performed using Hydrosun®750 (Hydrosun Medizintechnik GmbH, Müllheim, Germany). Radiation was applied vertically in a radiation field of 25 cm encompassing the lower thoracic and lumbar area (see ). Radiation strength was 160 mW/cm2 with a radiation distance of 35 cm.
Primary outcome was a change in pain levels measured on a numeric rating scale (NRS) (0 = no pain, 10 = outmost pain) before and after trial completion. Secondary outcome parameters were a change to baseline after the last intervention in disease activity measured by BASDAI (0-10), in disease-specific functionality measured by the Bath Ankylosing Spondylitis Functionality Index (BASFI), in TNFα levels as a pro-inflammatory cytokine and molecular marker of inflammatory activity and lastly in NSAID dosage. BASFI is a validated and recommended tool to determine functional restrictions due to axSpA using 10 questions related to everyday activities (0 = no restriction, 10 = poor functionality) [Citation20]. The first 8 questions evaluate activities related to functional anatomical limitations due to the course of this inflammatory disease. The final 2 questions evaluate the patients’ ability to cope with everyday activities. Laboratory analysis of TNFα was performed using Human TNF-α ELISA Kit, Company RayBiotech, Inc.Ca#: ELH-TNFalpha-001 (measuring range: 3.5 − 6000 pg/ml). Additionally, this exploratory study has drawn particular attention to adverse and severe adverse events.
As there was no data on the effects of sl-wIRAR in axSpA, power calculations of this exploratory trial were challenging. Using the reported effects of whole-body-hyperthermia utilizing whole body wIRAR in axSpA [Citation12] we aimed to recruit 35 patients per sequence group with a dropout rate of approximately 20%.
In statistical analysis the data was first listed descriptively (mean ± SD). Data was reviewed for normality by Q-Q plots and the Shapiro–Wilk test, which resulted in a contradiction to the hypothesis of a normal distribution. Several comparisons between the two groups were therefore conducted with the non-parametric Mann–Whitney U test. To assess the changes over time between the beginning and the end of the serial therapy, each parameter of the corresponding differences was calculated. The Mann–Whitney U test was used to test for these differences as a standard for the change in variations among the group members. The nonparametric Wilcoxon test was used to measure comparisons between two points in time within one group. Although this is an exploratory trial, we corrected the significance level due to multiple comparisons the depending on the amount of measurement times of a parameter in accordance with Bonferroni’s method. The multiple alpha level of the study lies at p < .05.
Results
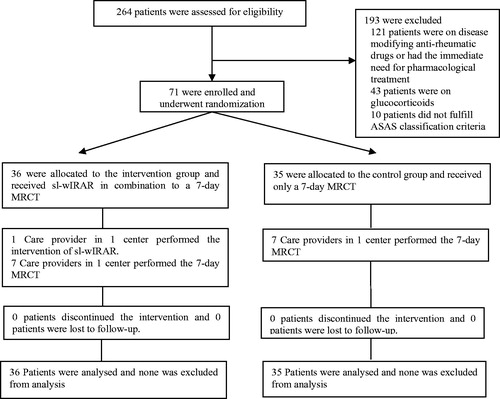
71 patients were recruited and completed the trial. All patients could be analyzed with 36 patients in the IC and 35 patients in the CG ().
Mean age was 51 years with a mean disease duration of 5.9 years. For further baseline characteristics of trial participants see .
Table 1. Patient characteristics.
Concerning the primary outcome parameter patients treated with sl-wIRAR had a significant pain reduction after 6 days of treatment compared to baseline (p < .0005). The CG receiving a 7-day MRCT alone did not achieve a significant reduction in pain (p = .088). Comparing the IG and CG revealed a significant difference in reduction of pain for the IG (p = .006) ()
Table 2. Change in pain levels between baseline and after trial completion.
In analysis of the secondary outcome parameters, both the IG and the CG experienced a significant reduction of disease activity measured by BASDAI. Only the IG experienced a significant improvement in functionality measured by BASFI. There was no significant difference between the IG and the CG in BASFI nor BASDAI after trial completion (). TNFα levels were only significantly reduced in the IG between baseline and after trial completion. There was a significant difference between IG and CG concerning the response to the trial measured in TNFα levels ().
Table 4. Change in TNFα levels [pg/ml] between baseline and after trial completion.
26 (76%) of patients in the IG decreased their NSAID therapy after trial completion (at day 7). Of these 26, 10 (29.4%) opted for a complete stop of their NSAID therapy at day 7. In contrast, only 1 patient of the CG decreased NSAID therapy.
No adverse or severe adverse events were recorded in both groups.
Discussion
This was the first prospective randomized controlled trial to evaluate the effects of sl-wIRAR in patients with a rheumatoid disorder (here in axSpA).
Here, sl-wIRAR in the background of a 7-day MRCT significantly reduced pain levels and disease activity and improved functionality while significantly decreasing TNFα levels. Compared to the control group auxiliary sl-wIRAR led to significant benefits in terms of pain reduction (p = .006). While the control group showed a pain reduction of −0.3 (mean) and therefore no significant change, the intervention group experienced a change of −1.6 (mean) (p < .0005) in pain levels in this 6-day long trial. Hence, sl-wIRAR is a rapid acting and effective method to significantly reduce pain in axSpA. In addition, sl-wIRAR can be dosed very well by varying i) the radiation distance and thus the radiance strength and radiation field as well as ii) the duration of the radiation. Pain reduction leads to improved physiotherapeutic mobilization ultimately resulting in an improvement in quality of life. In addition, 76% of patients in the IG decreased their NSAID dosage after trial completion in various extend, some even stopping NSAID therapy. Although it cannot be stated how long patients continued with the reduced dosage, potential side effects of NSAID could be minimized for at least one day.
Of importance was the significant reduction (p = .001) of TNFα in the intervention group from (mean) 8.8 pg/ml (baseline) to 5.8 pg/ml (after trial completion) with a significant difference between the IG and the CG (p = .01). Interestingly, the control group had no significant alteration of TNFα levels (p = .635). The change in TNFα levels in the intervention group could be a possible explanation for the profound effect of the intervention in this study on pain. In a model of knee inflammation for example, a reduction of TNFα had pronounced antinociceptive effects [Citation23]. These effects resulted from a neuronal site of action rather than from a reduction of inflammation. As the effects on TNFα appeared only in the intervention group they are probably related to sl-wIRAR treatment. In line, wIRAR is a special form of heat radiation with high tissue penetration and a low thermal load to the surface of the skin [Citation16]. wIRAR has both, temperature-dependent and non-dependent effects leading to various changes in the treated area, e.g. an increased perfusion [Citation16]. In context of this study, we consider the temperature-dependent effects of wiRAR to be particularly important. In addition, previous studies on whole-body hyperthermia [Citation10–13] and whole-body wIRAR [Citation12,Citation15] have shown to influence pro- and anti-inflammatory cytokines, while whole-body wIRAR led to a change in TNFα levels [Citation12,Citation15], too.
In this study both the IG as well as the CG showed a significant reduction in disease activity measured by BASDAI, while only the IG showed a significant increase in functionality capacity measured by BASFI. However, there were no significant differences between both groups. Since only axSpA patients on NSAID therapy with a BASDAI between 4 and 7 correlating to active disease (moderate to high disease activity) were eligible to participate, we considered it unethical to test against placebo without any additional active treatment in addition to baseline NSAID therapy. Therefore all patients received a 7-day MRCT, which provided each patient with a high volume (22 units) of physical therapy modalities and were shown to be effective in treating axSpA in a 14-day program [Citation22]. While this choice was pro-patient and may have led to no dropouts in this trial, it may have influenced outcomes in these secondary parameters of disease activity (BASDAI) and functionality (BASFI). The more so as the trial period only lasted 7 days and BASDAI and BASFI were designed as disease-specific outcome measures to assess differences over a longer period of time and are normally assessed in routine care once every 12 weeks. If BASDAI and BASFI should be primary outcome parameters in another trial evaluating sl-wIRAR in axSpA patients we would therefore recommend a longer trial period with a follow-up or a placebo treated CG without any other active disease-modifying treatment to show a significant change. A limitation of this exploratory trial is the missing follow-up period as the aim of this trial was to assess whether sl-wIRAR has effects in treating axSpA. We cannot quantify how long effects of the intervention lasted after trial completion and how long the effects of the intervention on NSAID therapy lasted (76% of patients in the intervention group decreased their NSAID therapy after trial completion). Based on studies of whole-body hyperthermia in axSpA and our clinical expertise we estimate that effects last up to 3 months. Nevertheless, these aspects should be addressed in a follow-up trial comparing sl-wIRAR as a form of locally applied hyperthermia to whole-body hyperthermia.
Regarding non-pharmacological treatment in axSpA physical therapy interventions play a central role [Citation7], although its potential is often not exploited in everyday practice [Citation5]. In this study, similar eligibility criteria as BASDAI > 4 under NSAID therapy and DMARD naivete were used as in randomized controlled trials for pharmacological treatments [Citation24]. It could be shown that an enhanced additive physical therapy reduced pain and disease activity and improved physical function. Therefore, additive and or enhanced physical therapy should be considered for every axSpA patients with insufficient response to pharmacological treatment and can in some cases be preferred to pharmacological therapy escalation.
In summary, this trial shows for the first time that in axSpA patients locoregional applied hyperthermia in the form of sl-wIRAR leads to pain reduction with consecutively reduced pain medication, beneficial effects on inflammation, disease activity and functional capacity.
Trial registration
DRKS00021257; German Clinical Trial Register (www.drks.de)
Author contributions
All authors approved the submitted manuscript and contributed actively to the study and the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73–84.
- Sieper J, Braun J, Dougados M, et al. Axial spondyloarthritis. Nat Rev Dis Prim. 2015;1:15013.
- Wang R, Ward MM. Epidemiology of axial spondyloarthritis: an update. Curr Opin Rheumatol. 2018;30(2):137–143.
- Godfrin-Valnet M, Prati C, Puyraveau M, et al. Evaluation of spondylarthritis activity by patients and physicians: ASDAS, BASDAI, PASS, and flares in 200 patients. Joint Bone Spine . 2013;80(4):393–398.
- Albrecht K, Huscher D. Verordnen wir ausreichend Physikalische Medizin? Aktuelle Daten aus der Kerndokumentation der Arbeitsgemeinschaft Regionaler Kooperativer Rheumazentren. Akt Rheumatol. 2017;42(02):118–121.
- Huscher D, Thiele K, Rudwaleit M, et al. Trends in treatment and outcomes of ankylosing spondylitis in outpatient rheumatological care in Germany between 2000 and 2012. RMD Open. 2015;1(1):e000033.
- van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76(6):978–991.
- Dagfinrud H, Hagen KB, Kvien TK. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2008. DOI:10.1002/14651858.CD002822.pub3
- Perrotta FM, Musto A, Lubrano E. New insights in physical therapy and rehabilitation in axial spondyloarthritis: a review. Rheumatol Ther. 2019;6(4):479–486.
- Dischereit G, Goronzy JE, Müller-Ladner U, et al. Effects of serial mud baths on inflammatory rheumatic and degenerative diseases. Z Rheumatol. 2019;78(2):143–154.
- Dischereit G, Neumann N, Müller-Ladner U, et al. Einfluss einer seriellen niedrig-dosierten Radonstollen-Hyperthermie auf Schmerz, Krankheitsaktivität und zentrale Zytokine des Knochenmetabolismus bei ankylosierender Spondylitis – eine Prospektivstudie. Akt Rheumatol. 2014;39(05):304–309.
- Lange U, Müller-Ladner U, Dischereit G. Wirkung iterativer Ganzkörperhyperthermie mit wassergefilterter Infrarot-A-Strahlung bei ankylosierender Spondylitis – eine kontrollierte, randomisierte, prospektive Studie. Akt Rheumatol. 2017;42(02):122–128.
- Moder A, Hufnagl C, Lind-Albrecht G, et al. Effect of combined low-dose radon-and hyperthermia treatment (LDRnHT) of patients with ankylosing spondylitis on serum levels of cytokines and bone metabolism markers: a pilot study. IJLR. 2010;7(6):423–435.
- Lange U, Dischereit G. Effects of different iterative whole-body hyperthermia on pain and cytokines in rheumatic diseases: a current review. Aktuelle Rheumatol. 2018;43:479–483.
- Tarner IH, Müller-Ladner U, Uhlemann C, et al. The effect of mild whole-body hyperthermia on systemic levels of TNF-alpha, IL-1beta, and IL-6 in patients with ankylosing spondylitis. Clin Rheumatol. 2009;28(4):397–402.
- Hoffmann G. Clinical applications of water-filtered infrared-A (wIRA) – a review. Phys Med Rehab Kuror. 2017;27(05):265–274.
- Hoffmann G, Harte M, Mercer JB. Heat for wounds – water-filtered infrared-a (wIRA) for wound healing – a review. GMS Ger Med Sci. 2016;14:Doc08.
- Notter M, Thomsen AR, Nitsche M, et al. Combined wIRA-hyperthermia and hypofractionated re-irradiation in the treatment of locally recurrent breast cancer: evaluation of therapeutic outcome based on a novel size classification. Cancers (Basel). 2020;12(3):606.
- Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–783.
- Zochling J. Measures of symptoms and disease status in ankylosing spondylitis: Ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Sp. Arthritis Care Res. 2011;63(S11):S47–S58.
- Klemm P, Hudowenz O, Asendorf T, et al. Multimodale rheumatologische Komplexbehandlung bei rheumatoider Arthritis – eine monozentrische Retrospektivanalyse. Z Rheumatol. 2019;78(2):136–142.
- Klemm P, Hudowenz O, Asendorf T, et al. Evaluation of a special concept of physical therapy in spondyloarthritis: German multimodal rheumatologic complex treatment for spondyloarthritis. Clin Rheumatol. 2020;39(5):1513–1520.
- Boettger MK, Hensellek S, Richter F, et al. Antinociceptive effects of tumor necrosis factor alpha neutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis Rheum. 2008;58(8):2368–2378.
- Sieper J, van der Heijde D, Dougados M, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis. 2013;72(6):815–822.