Abstract
Purpose
Our purpose is to assess the efficacy and safety of percutaneous US-guided microwave ablation (MWA) for cervical metastatic lymph nodes from papillary thyroid carcinomas (PTC).
Methods
In total, 37 patients with 98 cervical metastatic lymph nodes from PTC were enrolled in this retrospective study. Among them, 8 had subtotal thyroidectomy, 4 lobectomy, 2 no operation, and the rest total thyroidectomy. A multipoint and multiplane fixed ablation method was used. Monitoring of ablation process and clinical follow-up consisted of US or CEUS.
Results
All 98 metastatic lymph nodes successfully treated in a single session with 100% complete ablation. The average longest and shortest diameter of the tumors were reduced from 13.21 ± 5.86 mm to 6.74 ± 5.66 mm (p =.00) and from 9.29 ± 4.09 mm to 4.31 ± 3.56 mm (p =.00) at the final follow-up. There were no evidence of recurrence at ablated sites. The common intraoperative complications were a burning sensation and pain. Only 3 patients had vagal reflex.
Conclusions
US-guided MWA can effectively control cervical metastatic lymph nodes from PTC. MWA may become an alternative therapy in selected PTC patients with cervical lymph node metastasis.
Introduction
Thyroid cancer, the most prevalent endocrine malignancy, accounts for 1% of all malignant neoplasms and has become an increasingly common diagnosis for Americans over the past 25 years [Citation1–3]. The situation is as well as in China [Citation4]. Papillary thyroid carcinoma (PTC) is the most common histological subtype of thyroid cancer, comprising 80–85% of all thyroid malignancies [Citation1,Citation2]. Despite this dramatic rise in incidence, mortality due to thyroid cancer has remained stable, with a 10-year survival >91% and 15-year survival >87% [Citation2,Citation3]. Central lymph node metastasis is observed in 20–90% of those patients [Citation5,Citation6]. So, prophylactic central lymph node dissection is frequently performed in patients with clinically negative central lymph nodes (CN0), although there remains controversial [Citation7]. When cervical lymph node metastasis detected in postoperative patients, surgery, followed by radioactive iodine therapy and/or thyroid hormone therapy is still the mainstream standard treatment. However, repeated operations are sometimes challenged to perform due to severe fibrosis and distortion of the normal tissue planes by scar formation and a higher rate of complications [Citation7].
Ultrasonography-guided percutaneous thermal ablations, such as radiofrequency ablation (RFA), microwave ablation (MWA), and laser ablation (LA), are a family of minimally invasive local therapy that have been shown to provide promising results for local tumor control in a variety of organs and recommended in the corresponding guidelines [Citation8–11]. These methods have also been applied to benign thyroid tumors [Citation12–17]. Iodine 131 is a routine therapy for metastasis lymph node of PTC. For some lesions that do not absorb iodine, ablation can be used and has been recommended by the guidelines of ATA [Citation7]. However, there have only several published references which reported the application to the treatment on the postoperative patients with cervical metastatic lymph nodes [Citation18–23]. Compared with other thermal ablation techniques, MWA has obvious advantages of higher intratumoral temperatures, larger ablation volumes, faster ablation times and usefulness of multiple applicators. Therefore, a larger complete ablation volume can be achieved within a shorter time [Citation24,Citation25]. In this paper, we share our own experience of MWA for the treatment of cervical metastatic lymph nodes in the patients of PTC.
Materials and methods
Study population
The study was performed at our medical institution (Chinese PLA General Hospital, Beijing, China). Written informed consent was obtained from the patients and/or relatives, when appropriate, before treatment. Ethical approval was obtained from the Ethical Committees of Chinese PLA General Hospital.
Inclusion criteria were: (1) patients received total or subtotal thyroidectomy for PTC, and at least one subsequent neck dissection; (2) patients who had contraindications for surgery or refused surgery, or refused to undergo further surgical resection and absent radioiodine uptake at post-therapeutic 131I whole-body scan; (3) patients with at least one metastatic lymph node (which were ready for treatment) confirmed by US-guided biopsy, or confirmed by US and CEUS ( and ) [Citation26]; (4) no evidence of distant metastases. The flowchart of patient selection process was shown in .
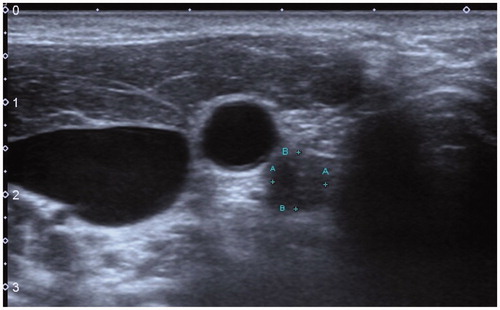
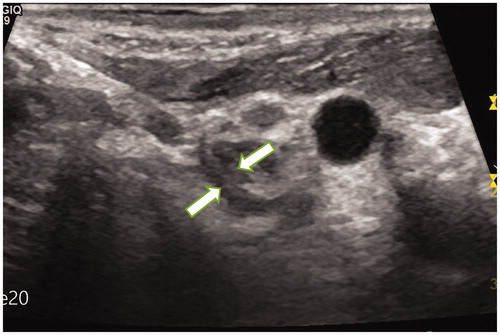
Figure 1. A 22-year-old male patient with PTC was found to have metastatic lymph nodes in area VI of the right neck (Ultrasound image obtained from head side of patients). The lymph node was located between the common carotid artery and the trachea. The size of lymph nodes was about 1.2cmx1.2 cm.
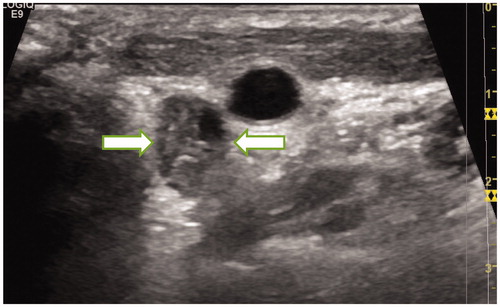
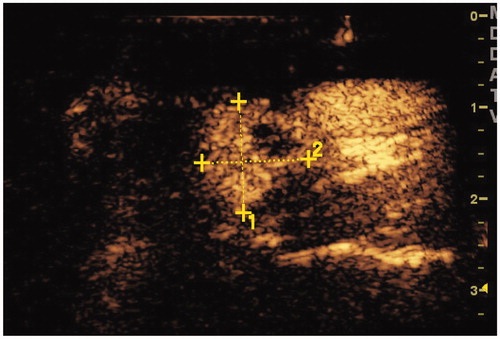
Figure 2. The CEUS of the metastatic lymph nodes in the above patients showed a high enhancement mode.
All enrolled patients were reviewed without risk of bleeding, with a normal platelet count and normal coagulation parameters. Laboratory assessment of the thyroid function included serum TSH, free and total T4, free and total T3, and thyroglobulin (Tg) in need.
Between June 2015 and January 2020, thirty-seven patients (male 17, female 20; mean age 43.58 ± 13.77 years, range 14 − 74 years) were enrolled in this study. Among the 37 patients, 8 (21.6%, 8/37) had subtotal thyroidectomy, 4 (10.8%, 4/37) had lobectomy, 2 (5.4%, 2/37) had no operation, and the rest (62.2%, 23/37) had total thyroidectomy. Of the 2 patients with lymph node metastases who failed to receive surgical resection, one was severe primary pulmonary hypertension, the other was after mitral and aortic valve replacement. They all received MWA as a palliative treatment for their disease.
Equipment and methods
The microwave ablation system (KY2000, Kangyou Medical Instruments, Nanjing, China) consisted of two independent MW generators, two flexible coaxial cables and two cooled shaft antennae. The generator was capable of producing 1 to 100 W of power at 2,450MHz, which could drive up to 1 antenna. The cooled shaft antenna had a 16-gauge shaft coated with polytetrafluoroethylene to prevent adhesion. The distance from the aperture of the MW emission to the needle tip was 3 mm and the emission aperture was 1 mm. Inside of the antenna shaft, there were dual channels through which distilled water was circulated by a peristaltic pump, continuously cooling the shaft to prevent shaft overheating. The MWA procedure was performed under real-time ultrasound guidance (model: LOGIQ E9, GE Health Care) with 10-MHz linear probe.
In order to achieve complete ablation, a multipoint and multiplane moving shot ablation method was used. Before ablation, the targeted lymph node should be divided into multiple conceptual planes, and every plane was divided into multiple conceptual points. The ablation was performed point by point and plane by plane through moving the antenna. Initially, the antenna tip was positioned in the deepest of the targeted lymph nodes to enable easy monitoring of the antenna tip abating the disturbance of microbubbles. The ablation power was 20 W, lasting for 5–10 s in each point. In order to achieve complete ablation, each point usually needs 2–3 cycles. During the ablation, all the patients were under the local anesthesia of 1% Lidocaine (Shuanghe; Beijing Pharmaceuticals, Beijing, China) 5–10 ml. If a patient could not tolerate pain during ablation, shorten each ablation time until the desired goal is achieved. The symptoms and complications of all patients were monitored during and immediately after ablation.
Hydro-dissection technique was applied to all cases in this study. A 21-gauge needle was introduced percutaneously between the targeted lymph node and its surrounding structures. Then the NS was carefully injected around the targeted lymph nodes, especially between those lymph nodes and the vital structures (). NS was injected discontinuous during the ablation to keep effective hydro-dissection.
Figure 4. Normal saline was injected between the targeted lymph nodes and the trachea to isolate them.
Continuous real-time US monitoring with gray scale imaging was performed to identify proper antenna position and assess microbubble formation during the ablation (). CEUS were performed before and immediately after the procedure to evaluate the therapeutic effect (). The targeted lymph nodes were completely ablated through the multipoint and multiplane moving shot ablation method. The complete ablation was defined that both the capsule and the parenchyma of the targeted lymph nodes were no enhanced in CEUS immediately after the procedure and confirmed by the follow-up results of 1 month. The assessments were achieved by consensus of both operators and another radiologist who did not perform any of the procedures.
Figure 5. The patient received MWA ablation of metastatic lymph nodes in July 2016. Ultrasound monitoring showed that some lymph nodes were covered by microbubbles.
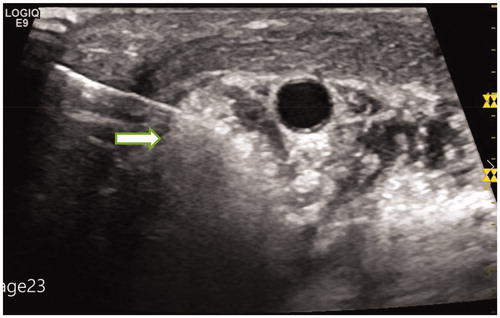
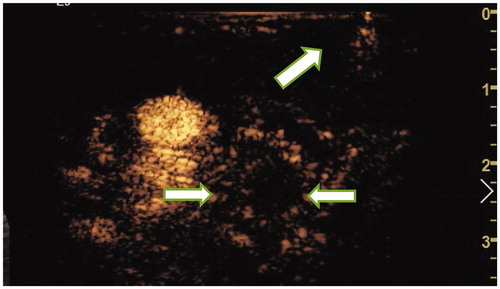
Figure 6. On the second day after ablation, CEUS showed that the ablated lymph nodes had no enhancement (ultrasound image obtained from foot side of patients).
Clinical follow-up consisted of US or CEUS. Follow-up examinations were performed 1, 3, 6, and 12 months after treatment and then every 6 months thereafter. The changes of size and intratumoral vascularity of the ablated lymph nodes were evaluated (). The follow up of US or CEUS was also performed under real-time US guidance (model: LOGIQ E9, GE Health Care) with 10-MHz linear probe. Standard terminology of thermal ablation was used in this study [Citation27,Citation28].
Statistical analysis
Descriptive data were expressed as mean ± SD. Statistical analysis was performed using SPSS statistical software (Version 19.0; SPSS, Chicago, IL). The paired t-test was used to compare the longest and shortest diameter before MWA with the last follow-up. A difference with p < .05 was considered as statistically significant.
Results
Of the 37 patients, 3 received two times of ablation and 1 received three times of ablation for different recurrent lymph nodes found during follow-up period. The CEUS showed that the arterial phase of the targeted lymph nodes was significantly enhanced, and then quickly washout. A total of 98 targeted lymph nodes was ablated in 37 patients. On average, 2.5 (range, 1–10) lymph nodes were ablated in each patient. These lymph nodes were mainly located in level III (26/98), IV(43/98), VI(19/98) and a few in levelI(2/98),II(7/98), V(1/98). The targeted lymph node characteristics before and after MWA were summarized in .
Table 1. Characteristics of the targeted lymph nodes before and after MWA.
The procedures were well tolerated in all 37 patients to completion without obvious bleeding or/and serious complications. Nearly all patients suffered a burning sensation, pain, or both, the symptoms were relieved when stopping the ablation. Three patients who the targeted lymph nodes couldn’t separate well with Vagal nerve showed vagal reflex with decreased heart rate and blood pressure after ablation for about 5 s. The measure was to stop ablation immediately. After the heart rate and blood pressure returned to normal, the ablation duration was shortened to 2–3 s. The ablation was stopped immediately when the vagal reflexes appeared again. Repeated like this until the whole lesion was ablated. The average ablation time of each targeted lymph node was 206.55 s ± 193.59 s.
The average longest and shortest diameter of the tumors were reduced from 13.21 ± 5.86 mm (range, 5–35 mm) to 6.74 ± 5.66 mm (range, 0–26 mm) and 9.29 ± 4.09 mm (range, 4–24 mm) to 4.31 ± 3.56 mm (range, 0–15 mm) at the final follow-up. The results of paired t-test showed that there were significant differences in the longest and shortest diameter before and after operation (p = .00). After ablation both the capsule and the parenchyma of all targeted lymph nodes were no enhanced in CEUS. After and during the follow-up period, the treated lymph nodes demonstrated increased echogenicity in the tumor and surrounding soft tissue, and the absence of intratumoral color Doppler signals in comparison with the previous treatment. There was no evidence of recurrence at ablated sites.
In addition to the vagal reflex in 3 patients during ablation (all lymph nodes in level lV), there were also two patients with skin scald in the ablation area, manifesting as small blisters.
Discussion
Although the amount of literature published now is very small, US-guided thermal energy ablation has been reported as an effective form of thermal ablation technique for the treatment of nodal metastases in PTC [Citation16,Citation18–23]. Our initial results showed that MWA under US-guidance for the treatment of cervical metastatic lymph nodes in the patients of PTC also had the same results.
Compared with RFA and LA, MWA has obvious advantages of higher intratumoral temperatures, larger ablation volumes, faster ablation times and usefulness of multiple applicators. Therefore, a larger complete ablation volume can be achieved within a shorter time [Citation24,Citation25]. However, these advantages are not all necessarily beneficial to the ablation of superficial cervical lymph nodes. Therefore, the power and time of MWA must be adjusted properly. So the ablation power of our study was 20 W and the time was lasting for 5–10 s in each point. The main purpose was to reduce the thermal damage of surrounding tissues. In addition, compared with RF electrode and laser fiber, microwave antenna is relatively less sharp. In order to facilitate the accurate placement of the antenna, we isolated the surrounding liquid after the antenna was accurately inserted into the target position. However, the most outstanding point of MWA is that it can obtain intratumoral temperatures and was not affected by carbonization [Citation24,Citation25]. Microwave power can be continually applied to produce extremely high temperatures (>150 °C), which can improve ablation efficacy by increasing thermal conduction into the surrounding tissue [Citation29]. In order to achieve complete ablation, we usually ablated 2–3 cycles at the same point and didn't worry about carbonization. Previous studies have illustrated that both RFA and laser ablation for cervical lymph node metastasis of thyroid malignancy is a feasible, effective, and safe therapy [Citation16,Citation18–23], while no comparative studies were available to show the difference and beneficial outcomes of different techniques. Laser for example provides effective results by using very small applicators (21 G) and lower energy deposition, but it might increase the times of adjustment of laser fiber. Comparative studies are needed to illustrate advantages and disadvantages of different technique in ablating lymph node.
Despite this dramatic rise in incidence, mortality due to thyroid cancer has remained stable. Moreover, total thyroidectomy carries a risk of injury to either recurrent laryngeal nerve (or, rarely, both of them) and a risk of hypoparathyroidism due to damage to all four parathyroid glands; it also necessitates lifelong thyroid hormone replacement. Therefore, less extensive lobectomy is often the best choice because this surgery carries a lower risk of those of total thyroidectomy [Citation3]. However, in case of lymph node metastasis, adjuvant therapy with radioactive iodine (RAI) must be preceded by total thyroidectomy [Citation3,Citation7]. That is why about 80% of patients who have surgery for localized papillary thyroid cancer (≤2 cm in diameter) undergo a total thyroidectomy [Citation3]. In our study, 4 patients (10.8%, 4/37) had lobectomy. Moreover, 2 patients (5.4%, 2/37) with lymph node metastases failed to receive surgical resection because the one was with severe primary pulmonary hypertension and the other was after mitral and aortic valve replacement. They all received MWA as a palliative treatment for their thyroid disease. MWA was good at controlling their lymph node metastasis, although some patients needed more than one course of treatment.
There are some limitations in our study which should be taken into account. First, the sample size of this study was small with a relatively short follow-up period. Secondly, the diagnosis of biopsy was limited to individual lymph nodes. The diagnosis of other lymph nodes depended on the US and CEUS. So there might be bias. Thirdly, in addition to the low malignancy of PTC itself, partial ablation of lymph nodes was smaller, which might be one of the reasons for good results.
In conclusion, our initial results show that MWA under US guidance is effective, and safe for the treatment of cervical lymph nodal metastases from PTC and may become an alternative therapy in selected PTC patients with cervical lymph node metastasis, who were ineligible or refused to undergo repeated operation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
- Lundgren CI, Hall P, Dickman PW, et al. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106(3):524–531.
- Welch GH, Doherty GM. Saving thyroids – overtreatment of small papillary cancers. N Engl J Med. 2018;379(4):310–312.
- Wang J, Yu F, Shang Y, et al. Thyroid cancer: incidence and mortality trends in China, 2005-2015. Endocrine. 2020;68(1):163–173.
- Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5(1):43–63.
- Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134(6):946–955.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid Cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
- Kouri BE, Abrams RA, Al-Refaie WB, et al. ACR appropriateness criteria radiologic management of hepatic malignancy. J Am Coll Radiol. 2016;13(3):265–273.
- Liang P, Yu J, Lu MD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol. 2013;19(33):5430–5438.
- Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA Guideline. J Urol. 2017;198(3):520–529.
- Ye X, Fan W, Wang H, et al. Expert consensus workshop report: guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition). J Cancer Res Ther. 2018;14(4):730–744.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Cesareo R, Palermo A, Benvenuto D, et al. Efficacy of radiofrequency ablation in autonomous functioning thyroid nodules. A systematic review and meta-analysis. Rev Endocr Metab Disord. 2019;20(1):37–44.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperthermia. 2019;36(2):13–20.
- Kim JH, Baek JH, Lim HK. Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology, et al. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632–655.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: A systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Mauri G, Cova L, Tondolo T, et al. Percutaneous laser ablation of metastatic lymph nodes in the neck from papillary thyroidcarcinoma: preliminary results. J Clin Endocrinol Metab. 2013;98(7):E1203–7.
- Papini E, Bizzarri G, Bianchini A, et al. Percutaneous ultrasound-guided laser ablation is effective for treating selected nodal metastasesin papillary thyroid cancer. J Clin Endocrinol Metab. 2013; 98(1):E92–7.
- Wang L, Ge M, Xu D, et al. Ultrasonography-guided percutaneous radiofrequency ablation for cervical lymph node metastasis from thyroid carcinoma. J Can Res Ther. 2014;10(7):144–149.
- Guang Y, Luo Y, Zhang Y, et al. Efficacy and safety of percutaneous ultrasound guided radiofrequency ablation for treating cervical metastatic lymph nodes from papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2017;143(8):1555–1562.
- Teng D, Ding L, Wang Y, et al. Safety and efficiency of ultrasound-guided low power microwave ablation in the treatment of cervical metastatic lymph node from papillary thyroid carcinoma: a mean of 32 months follow-up study. Endocrine. 2018;62(3):648–654.
- Zhou W, Chen Y, Zhang L, et al. Percutaneous microwave ablation of metastatic lymph nodes from papillary thyroid carcinoma: preliminary results. World J Surg. 2019;43(4):1029–1037.
- Skinner MG, Iizuka MN, Kolios MC, et al. A theoretical comparison of energy sources-microwave, ultrasound and laser-for interstitial thermal therapy. Phys Med Biol. 1998;43(12):3535–3547.
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and vivo porcine livers. Eur J Radiol. 2011;79(1):124–130.
- Hong YR, Luo ZY, Mo GQ, et al. Role of contrast-enhanced ultrasound in the pre-operative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma. Ultrasound Med Biol. 2017;43(11):2567–2575.
- Ahmed M, Solbiati L, Brace CL. Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Yang D, Converse MC, Mahvi DM, et al. Measurement and analysis of tissue temperature during microwave liver ablation. IEEE Trans Biomed Eng. 2007;54(1):150–155.