Abstract
Purpose
Quantitative dynamic contrast-enhanced ultrasonography (CEUS) reflects tumor blood perfusion. There are very few studies on the relationship between intrahepatic recurrence of hepatocellular carcinoma (HCC) and tumor perfusion. We investigated the correlation of dynamic CEUS parameters with intrahepatic recurrence after radiofrequency ablation (RFA).
Methods
This retrospective study enrolled 125 native HCC patients who underwent RFA between September 2017 and January 2019 with curative intent. Pre-ablation quantitative dynamic CEUS was performed. CEUS parameters were extracted from time–intensity curves. The correlation of CEUS parameters with intrahepatic recurrence was investigated.
Results
The mean follow-up time was 21.6 ± 7.9 months. The recurrence rate was 33.6%. Univariate and multivariate analyses revealed that tumor peak intensity (PI) was a significant independent risk factor for intrahepatic recurrence after RFA (hazard ratio (HR), 0.3; 95% CI, 0.1–0.9). A PI of 58.8% (area under curve, 0.72; 95% CI, 0.63–0.81) was considered as the optimal cutoff level to predict the intrahepatic recurrence of HCC in patients after RFA. The recurrence-free survival rate in patients with a PI > 58.8% was 94.4% at 1 year and 77.8% at 2 years. Subgroup analysis showed that the HR of time to peak (TTP) in intrahepatic recurrence was 1.1417 (95% CI, 0.9748–1.1436; p = 0.1973) in the patient group with tumor diameter > 31 mm.
Conclusion
CEUS is commonly used in HCC patients who undergo RFA. The CEUS parameters PI and TTP are associated with intrahepatic recurrence after RFA, and can thus be used to identify patients at risk for intrahepatic recurrence.
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related deaths worldwide [Citation1]. Although surgical therapy can achieve long-term survival in early-stage HCC, many patients are not appropriate candidates for this procedure. Radiofrequency ablation (RFA) is recommended as one of the locoregional curative treatment methods [Citation2–5]. The 1-year, 2-year, and 3-year recurrence-free survival rates are 60.4%, 46.2%, and 30.3%, respectively [Citation6]. HCC intrahepatic recurrence is an important prognostic factor for overall survival after RFA [Citation7]. Therefore, early prediction of HCC intrahepatic recurrence is important to apply suitable and individualized management of early-stage HCC.
Previous studies have detected a few risk factors for HCC intrahepatic recurrence after RFA, including tumor size, tumor number, serum tumor marker, proximity to a large vessel, insufficient safety margin, subcapsular location, and poor pathologic differentiation of tumor cells [Citation6,Citation8–11]. The perfusion of the tumor has been identified as an important factor influencing the energy distribution of thermal ablation [Citation12]. Thus, it is closely related to intrahepatic recurrence after RFA.
Contrast-enhanced ultrasonography (CEUS) is applied in the diagnosis of HCC [Citation13]. CEUS involves an intravenous injection of microbubble contrast agents, which are purely intravascular. CEUS has the ability to show the blood perfusion characteristics of liver lesions after the bolus injection of ultrasound contrast agents. Currently, many ultrasound instruments are equipped with contrast-enhanced ultrasound quantitative analysis software. The analysis software enables quantitative assessment of tumor perfusion through time–intensity curves [Citation14]. Time–intensity curves are analyzed to evaluate tumor response after antiangiogenic therapies [Citation15–17]. However, there are few studies on the association between CEUS-assessed tumor perfusion and intrahepatic recurrence of HCC after RFA, and their results are controversial [Citation18–20]. Hence, in this study, we used CEUS to evaluate the blood perfusion characteristics of HCC and analyzed perfusion parameters influencing HCC intrahepatic recurrence after RAF.
2. Materials and methods
2.1. Patients
This retrospective study received approval from the Institutional Review Board of the Harbin Medical University Cancer Hospital and followed the principles of the Declaration of Helsinki. HCC patients who were treated with RFA in our hospital from September 2017 to January 2019 were enrolled in this cohort study. Of 275 HCC patients, 150 were excluded and 125 were included in this study (). The diagnosis of HCC was based on pathology or diagnostic imaging criteria based on the guidelines of the American Association for the Study of Liver Diseases. HCC shows arterial hyperenhancement and washout during the portal venous and/or delayed phases on both computed tomography (CT) scans and magnetic resonance imaging (MRI) results. The inclusion criteria were as follows: (1) a single lesion with the largest diameter of 50 mm or two or three lesions with the largest diameter of ≤30 mm; (2) an absence of portal vein thrombosis or extrahepatic metastases; (3) a platelet count number less than 5 × 109/mm3 and prothrombin activity no less than 50%; (4) child class A or B; and (5) no severe heart or lung disease. In addition to the above criteria, the target lesions met the following requirements for the quantitative dynamic CEUS analyses: hypervascularization, size >10 mm, necrosis rate <50%, and sites with the best acoustic window that lasts for 2 min without disappearance of the tumor. The exclusion criteria were as follows: (1) previous treatment history for HCC; (2) other previous or concomitant malignancies; (3) tumor inconspicuous on conventional ultrasound; (4) ascites; (5) contraindications to the administration of SonoVue; and (6) extrahepatic metastases after RFA. Each patient was informed of the technique and signed an informed consent.
2.2. CEUS imaging
All patients underwent CEUS imaging one week before RFA. First, conventional ultrasound was performed to determine the lesion location, quantity, size, blood supply, and relationship to peripheral structures. Patients with lesions that could not be seen clearly were excluded from the study. Subsequently, CEUS was performed on the section of the lesion with the largest blood flow using a Siemens Acuson S3000 color Doppler ultrasound system (Siemens, Germany) with a transducer frequency of 2.0–5.0 Hz and a mechanical index of 0.06. The contrast agent was sulfur hexafluoride microbubbles (SonoVue, Bracco, Italy). It was prepared by dissolving 24.98 mg dry powder in 5 ml saline (0.9% NaCl) to a final concentration of 8 ml/mL. Microbubbles (2.4 ml) were injected through an antecubital vein in the left arm with a 20-gauge syringe. A microbubble bolus injection was followed by a 5 ml saline flush. The timer on the control panel was started at the beginning of the saline flush. The entire vascular phase of liver CEUS consists of the arterial phase, portal venous phase, and late phase, according to the CEUS Liver Imaging Reporting and Data System criteria [Citation21]. The enhancement pattern of each tumor was observed, and a dynamic sequence of 2 min or more was recorded. SonoVue uptake and washout and echo intensity within the tumor were recorded for later analysis. In cases of multiple hepatic lesions, the largest lesion was analyzed using CEUS. The built-in auto contrast software was used to analyze the region of interest (ROI) quantitatively. The ROIs of the tumors were defined in the most enhanced area of the HCCs. The sizes of all the ROIs were 1 cm. Time–intensity curves were generated from the ROIs. Quantitative parameters were peak intensity (PI), time to peak (TTP), mean transit time (MTT), and area under curve (AUC). The operators of the ultrasound examination were experts in hepatology and ultrasonography, with more than 5 years of experience in ultrasound examination at the time of study initiation.
2.3. Percutaneous ultrasound-guided RFA
Before RFA, some enrolled patients underwent liver biopsies if required. RFA processes were performed percutaneously under ultrasound guidance. The patients were placed in the supine position, and two grounding pads were placed on their thighs. Each patient’s blood pressure and heart rate were monitored throughout the procedure. After ultrasound puncture tract positioning, the patient was sedated with midazolam (0.075 mg/Kg) and fentanyl (0.1 µg/Kg). The radiofrequency (RF) needle (Cool-tip; Radionics, Burlington, MA, USA) was inserted into the lesion. The energy application was conducted for periods of up to 12 min. The RF needle is a 17-gauge internally cooled electrode with an exposed tip of 2–3 cm. In tumors with the longest diameter of 30 mm, one needle was placed at the center of the lesion; however, in larger tumors, two needles were applied for superposed ablation. At the end of treatment, the puncture pathway was solidified during needle withdrawal to avoid bleeding. After ablation, all the tumors were imaged using CEUS to detect any abnormal enhancement, which in the ablation zone was defined as a tumor residual. If a tumor residual was observed, the lesion was ablated again.
2.4. Follow-up
RFA-related complications and side effects were recorded according to the standardization of terminology and reporting criteria for image-guided tumor ablation proposed by Ahmed et al. An enhanced MRI was conducted to evaluate the effectiveness of ablation at 1 month after RFA, and complete ablation was defined as no enhancements in any area of the tumor. Thereafter, ultrasonography and contrast-enhanced CT/MRI were performed at follow-up, every 3 months during the first year and every 4–6 months for the next years. Contrast-enhanced CT and MRI were performed during alternate visits. Blood routine, liver function, and tumor markers were examined during each visit. The follow-up period was defined as the time from RFA to recurrence or May 2020. The primary outcome was assessed as recurrence-free survival (RFS), which was defined as the time from RFA to intrahepatic recurrence. Intrahepatic recurrences included intrahepatic local recurrence and intrahepatic distant recurrence. Local recurrence was diagnosed as a viable tumor inside or abutting the ablation zone during follow-up at least 1 month after RFA. Intrahepatic distant recurrence was defined as a viable tumor in the liver parenchyma outside the original ablation area on any post-procedural imaging during the follow-up period.
2.5. Statistical analysis
Empower(R) (www.empowerstats.com; X&Y solutions, Inc., Boston MA) and R software, version 3.6.1 (http://www.r-project.org), were used for all statistical analyses. Data are presented as means ± standard deviation for continuous variables and as frequencies (percentages) for categorical variables according to the characteristics of the patients and HCC lesions. The Cox regression model was used to assess the hazard ratio (HR) and conduct multivariate analysis. The outcome measure was intrahepatic recurrence during the follow-up period, and the optimal cutoff values for PI predicting intrahepatic recurrence were determined using receiver operating characteristic (ROC) curve analysis. The recurrence-free survival curve was studied in the Kaplan–Meier analyses using the log-rank test. A two-piecewise linear regression model was used to examine the threshold effect of tumor size on intrahepatic recurrence using a smooth function. The HRs and 95% CIs for the risk of intrahepatic recurrence that was associated with TTP were estimated with the use of the Cox proportional hazards models with adjustment for sex, age, etiology, number of lesions, tumor location, proximity to important structures, and serum α-fetoprotein level. Similarly, the HRs and 95% CIs of intrahepatic recurrence in response to tumor size were estimated, and their interactions were tested. p < 0.05 was considered to indicate statistical significance in all analyses.
3. Results
3.1. Patient characteristics and therapeutic results
Between September 2017 and January 2019, 275 consecutive patients underwent RFA for HCC at our institution. With only 125 patients out of 275 included in this study, none of them received antiviral therapy. All the patients were undergoing initial treatments. The characteristics of the patients and HCC lesions are listed in .
Table 1. Characteristics of patients and HCC lesions.
One RFA procedure was performed in 123 patients and two in two patients. All the patients experienced technical success according to contrast-enhanced MRI performed 1 month after the initial RFA. No RFA-related mortality or major complications were detected. Supportive care was provided to eight patients who reported abdominal pain (n = 5) and transient fever (n = 3).
Mean follow-up time was 21.6 ± 7.9 months with a range of 5–33 months. The analysis of intrahepatic recurrence in patients with HCC after RFA treatment revealed incidences of 16.8% and 32% at 1 and 2 years, respectively. The number of intrahepatic recurrence was 42.
3.2. Risk factor of PI for intrahepatic recurrence of HCC following RFA
Univariate analysis revealed that tumor peak intensity (PI) (HR, 0.9; 95% CI, 0.9–1.0; p < 0.001), proximity to a large vessel (HR, 0.3; 95% CI, 0.1–0.9; p = 0.04), tumor isoenhancement in the CEUS portal phase (HR, 0.5; 95% CI, 0.3–1.0; p = 0.048), and AUC (HR, 1.0; 95% CI, 1.0–1.0; p = 0.006) were significant risk factors for intrahepatic recurrence (). Mean tumor PI was 54.1 ± 10.7% in patients with intrahepatic recurrence and 63.4 ± 10.8% in patients without intrahepatic recurrence. Mean AUC was 3897.9 ± 1738.1% in patients with intrahepatic recurrence and 4908.9 ± 1608.2% in patients without intrahepatic recurrence.
Table 2. Potential risk factors affecting HCC recurrence after RFA.
Multivariate analysis revealed that tumor PI was a significant independent risk factor for intrahepatic recurrence (). However, proximity to a large vessel, tumor isoenhancement in the CEUS portal phase, and AUC were not significant factors for intrahepatic recurrence (p = 0.181, p = 0.747, and p = 0.335, respectively).
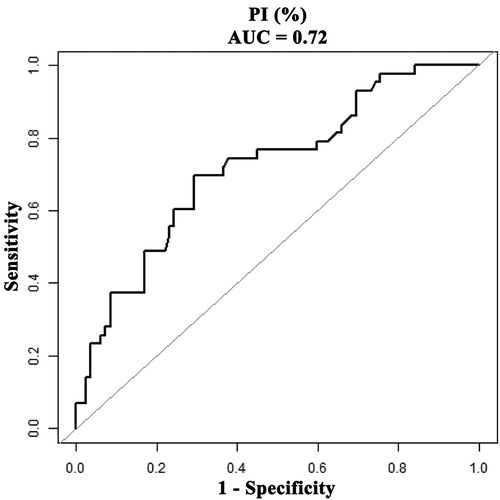
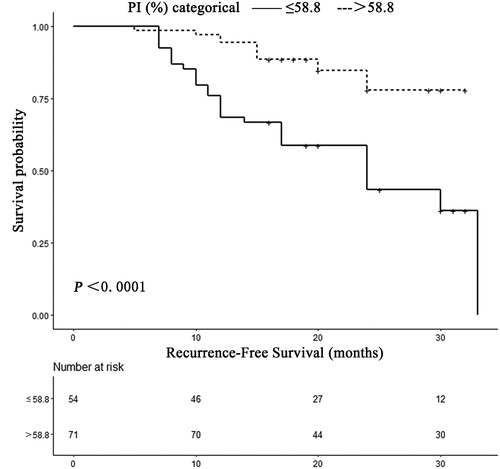
According to the results of ROC curve analysis, a PI of 58.8% (AUC, 0.72; 95% CI, 0.63–0.81) was considered as the optimal cutoff level to predict intrahepatic recurrence in HCC patients undergoing RFA with a sensitivity of 69.8% (95% CI, 63.9%–82.8%) and a specificity of 70.7% (69.7–80.3%), respectively (). The recurrence-free survival rate for patients with a PI of greater than 58.8% was 94.4% at 1 year and 77.8% at 2 years. This reduced to 68.5% at 1 year and 43.3% at 2 years in patients with PI less than or equal to 58.8% (p < 0.0001; ).
3.3. Risk factor of TTP for intrahepatic recurrence of HCC following RFA
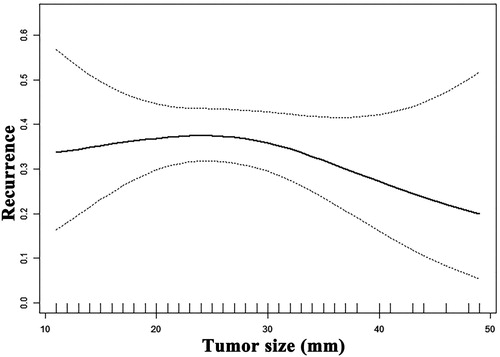
The correlation between the tumor size and risk of intrahepatic recurrence was not linear according to the smooth function (). The threshold value of tumor size was 31 mm. There were no significant differences in the risk of intrahepatic recurrence between tumor sizes of 10 mm to 31 mm. The HRs of tumor diameter in intrahepatic recurrence were 0.8 (95% CI, 0.7–1.0; p = 0.038) for larger than 31-mm tumors and 1.0 (95% CI, 1.0–1.1; p = 0.124) for smaller than 31-mm tumors. The logarithmic likelihood ratio was 0.01.
Interaction tests revealed an interaction between TTP and tumor size (p = 0.0195, ). We performed a subgroup analysis with the tumor diameter of 31 mm as the cutoff point between two groups. As shown in , we first observed a differential association between TTP and the risk of intrahepatic recurrence between the two subgroups. The HR of TTP in intrahepatic recurrence was 1.1430 (95% CI, 0.9865–1.1670; p = 0.1003) in the larger than or equal to 31 mm and 0.9827 (95% CI, 0.9333–1.0037; p = 0.0741) in the smaller than 31 mm groups. We also performed a multiple regression analysis. Model 1 was a Cox proportional hazards model that was adjusted for sex and age. Model 2 was a Cox proportional hazards model that was adjusted for sex, age, etiology, number of lesions, tumor location, proximity to important structures, and serum α-fetoprotein level. In model 2, the HR of TTP in intrahepatic recurrence was 1.1417 in the larger than or equal to 31 mm and 0.9726 in the smaller than 31 mm groups. The effect values were not significantly different in the different models. p values of interaction were all less than 0.05.
Table 3. Relationship between TTP and HCC recurrence after RFA in subgroup of tumor size.
4. Discussion
The overall survival of patients with HCC treated with RFA varies between 53.2 months and 66 months [Citation22]. It has a comparable overall survival outcome to liver resection for early-stage HCC (5- and 10-year overall survival rates, 66.4% and 41.8% vs. 66.5% and 47.6%, respectively; p = 0.531) [Citation23]. However, the rate of distant tumor recurrence after RFA at 3, 5, and 10 years is 49–63%, 58–81%, and 80–88%, respectively [Citation24]. The occurrence of intrahepatic recurrence has an adverse effect on the overall survival rate [Citation25]. We observed the relationship between preoperative CEUS parameters and intrahepatic recurrence after RFA to identify possible risk factors. According to the imaging indications, patients with a high risk of recurrence were followed up closely and treated accordingly to prolong the survival of the patients.
In this study, PI is an independent risk factor for HCC recurrence after RFA (HR, 0.9; 95% CI: 0.9, 1.0;p = 0.009). A tumor with a smaller PI is more likely to recur. A previous study also identified PI as a significant factor for intrahepatic recurrence [Citation26]. Maruyama et al. showed that intensity differences during the early arterial phase were risk factors for distant recurrence [Citation18]. One explanation for the low intensity is the rapid outflow of the microbubble from a drainage route, such as the portal vein, into the adjacent hepatic sinusoids [Citation19]. One of the most important factors limiting the coagulation zone size is blood perfusion through the capillaries, which causes a thermal sink effect [Citation25]. We hypothesized that the rapid outflow of blood from the tumor led to incomplete ablation. Some preclinical studies have suggested that incomplete ablation might promote tumor aggressiveness through epithelial-mesenchymal transition [Citation27–29]. Maruyama et al. used contrast-enhanced ultrasound with perflubutane microbubble agent (sonazoid). A characteristic feature of sonazoid is that it accumulates in the reticuloendothelial system, such as the liver and spleen [Citation30]. In contrast, this study used SonoVue for CEUS. SonoVue produces microbubbles with a size similar to that of red blood cells. Because of their size distribution, these microbubble contrast agents are purely intravascular and do not extravasate into the interstitial fluid. Therefore, SonoVue might more objectively reflect the hemodynamic changes of the tumor. Our study further confirmed that PI is an independent protective factor for intrahepatic recurrence after HCC ablation. This supports the theory that there is a relationship between PI and blood flow velocity.
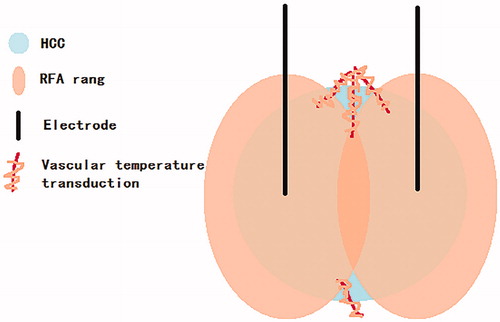
Previous studies have found that TTP is a risk factor for HCC recurrence after ablation [Citation26]. In our study, a relationship between TTP and HCC recurrence was not observed in univariate or multivariate analysis. Therefore, we applied the method of interaction test to analyze whether the effect of TTP on HCC recurrence had an interaction with other factors. The interaction test found an interaction between tumor size and TTP. The relationship between tumor size and recurrence was nonlinear based on smooth curve fitting. The threshold of tumor size was 31 mm. Therefore, all patients were divided into two groups, with the tumor diameter of 31 mm as the cutoff point. The results of the subgroup analysis showed that TTP was a risk factor for HCC recurrence after ablation in the subgroup with tumor diameters greater than or equal to 31 mm. After adjusting for sex, age, etiology, number of lesions, tumor location, proximity to important structures, and serum α-fetoprotein level, multiple regression analyses showed that the interaction and the effect value was stable in different models. In model 2, the HR of the <31mm group was 0.9726, indicating that TTP was a protective factor for intrahepatic recurrence in this subgroup. The incidence of intrahepatic recurrence was reduced by 2.74% for each additional 1 s of TTP. Meanwhile, the HR of the ≥31mm group was 1.1417, indicating that TTP was a risk factor for intrahepatic recurrence in this subgroup. The incidence of intrahepatic recurrence was increased by 14.17% for each additional 1 s of TTP. Thus, tumors with a larger TTP are more likely to recur. Contrary to the previous study [Citation26], our study has found an interaction between tumor diameter and TTP owing to the correlation between TTP and recurrence. Single-electrode overlapping RFA is applied for tumors with a diameter greater than 30 mm [Citation31]. However, there is a risk of incomplete ablation in single-electrode overlapping ablation of the irregularly shaped HCC [Citation31]. In this study, two electrodes were used for RFA when the tumor diameter was greater than 30 mm. The ablation zones were extended along a vessel [Citation32], and the resulting vascular temperature transduction eventually led to complete coagulative necrosis of the tumor.
We hypothesized that the temperature of an incomplete ablation zone with more vessels was higher than that of a zone with fewer vessels. Furthermore, sublethal heat treatment of HCC promotes intrahepatic metastasis and stemness [Citation33]. Thus, a tumor with fewer microvessels is more likely to recur after a sublethal heat treatment. Furthermore, microvessel density was shown to be negatively correlated with TTP [Citation20]; the larger the TTP, the smaller the microvessel density. A tumor with smaller TTP and larger microvessel density is less likely to recur (). Randomized controlled trials showed better recurrence-free survival, and overall survival for RFA + transarterial chemoembolization compared to RFA alone [Citation34–36]. However, the validity of this treatment modality in a western cirrhotic population with HCCs between 3 and 5 cm compared to new ablation methods, remains controversial [Citation24]. However, our hypothesis can explain this result.
Figure 5. The hypothesis of overlapping ablation temperature conduction along the vessel to the residual tumor.
Although CT and MRI can be used for the surveillance of HCC, patients undergoing RFA can easily undergo an ultrasound, which has the highest temporal and spatial resolution among all the current imaging tools. CEUS with a second-generation contrast agent is a simple, noninvasive, and reproducible imaging technique that provides a new method to assess changes in tumor vascularity. Owing to its high sensitivity in detecting weak intratumoral blood flow signals, CEUS has been employed for several years in the differential diagnosis of focal hepatic lesions as well as in the follow-up of intralesional therapy to identify the residual areas of intratumoral enhancement that could reflect incomplete necrosis or disease recurrence [Citation37]. Therefore, CEUS parameters can be easily obtained and used to predict intrahepatic recurrence after RFA.
One limitation of our study is the absence of histologic diagnosis for all the lesions. This is partly because needle biopsies present a potential risk for seeding of tumors, and thus, international consensus recommends the diagnosis of HCC with definite imaging criteria [Citation38]. Therefore, the relationship between recurrence patterns and cellular differentiation was not examined. The cutoff value shown here is dependent on the study population, and thus not generally applicable. Another limitation is the retrospective nature of the study. In subsequent studies, we will investigate the risk factors for recurrence after HCC ablation in vital signs.
In this study, it was confirmed that PI and TTP could reflect the characteristics of the blood supply of HCC. Thus, CEUS parameters are associated with intrahepatic recurrence after RFA. These findings can be used to developan individualized treatment plan to prolong patient survival.
Geolocation information
This study was conducted in Harbin, Heilongjiang Province, China.
Acknowledgments
The authors thank all the participants of the Department of Ultrasound, Harbin medical university cancer Hospital, for their valuable contributions. The authors thank Editage (www.editage.cn) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Global Burden of Disease Cancer C; Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749–1768.
- Bureau of Medical Administration. National Health Commission of the People’s Republic of China. Standardization for diagnosis and treatment of primary hepatic carcinom. Chin J Digest Surg. 2019;2020:1–20.
- Korean Liver Cancer Association; National Cancer Center. Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver. 2019;13:227–299.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma [published correction appears in J Hepatol. 2019 Apr;70(4):817]. J Hepatol. 2018;69:182–236.
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750.
- Hermida M, Cassinotto C, Piron L, et al. Multimodal percutaneous thermal ablation of small hepatocellular carcinoma: predictive factors of recurrence and survival in Western patients. Cancers (Basel). 2020;12:313.
- Rossi S, Ravetta V, Rosa L, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53:136–147.
- Yoo J, Lee MW, Lee DH, et al. Evaluation of a serum tumour marker-based recurrence prediction model after radiofrequency ablation for hepatocellular carcinoma. Liver Int. 2020;40:1189–1200.
- Lee MW, Kang D, Lim HK, et al. Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma < 3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol. 2020;30:2391–2400.
- Ryu T, Takami Y, Wada Y, et al. Actual 10-year survival after surgical microwave ablation for hepatocellular carcinoma: a single-center experience in Japan. Ann Surg Oncol. 2019;26:4126–4133.
- Canale M, Ulivi P, Foschi FG, et al. Clinical and circulating biomarkers of survival and recurrence after radiofrequency ablation in patients with hepatocellular carcinoma. Crit Rev Oncol Hematol. 2018;129:44–53.
- Liu Z, Ahmed M, Sabir A, et al. Computer modeling of the effect of perfusion on heating patterns in radiofrequency tumor ablation. Int J Hyperthermia. 2007;23:49–58.
- Quaia E. State of the Art: LI-RADS for Contrast-enhanced US. Radiology. 2019;293:4–14.
- Fröhlich E, Muller R, Cui XW, et al. Dynamic contrast-enhanced ultrasound for quantification of tissue perfusion. J Ultrasound Med. 2015;34:179–196.
- Zocco MA, Garcovich M, Lupascu A, et al. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol. 2013;59:1014–1021.
- Lo GM, Al Zahrani H, Jang HJ, et al. Detection of early tumor response to axitinib in advanced hepatocellular carcinoma by dynamic contrast enhanced ultrasound. Ultrasound Med Biol. 2016;42:1303–1311.
- Zhang P, Chen Y, Liu J, et al. Quantitative evaluation of combretastatin A4 phosphate early efficacy in a tumor model with dynamic contrast-enhanced ultrasound. Ultrasound Med Biol. 2018;44:840–852.
- Maruyama H, Takahashi M, Shimada T, et al. Pretreatment microbubble-induced enhancement in hepatocellular carcinoma predicts intrahepatic distant recurrence after radiofrequency ablation. AJR Am J Roentgenol. 2013;200:570–577.
- Kitao A, Zen Y, Matsui O, et al. Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography–radiologic-pathologic correlation. Radiology. 2009;252:605–614.
- Tan L, Chen S, Wei G, et al. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett. 2019;460:29–40.
- Ahmed M, Solbiati L, Brace CL, et al.; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273:241–260.
- Izzo F, Granata V, Grassi R, et al. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 2019;24:e990–e1005.
- Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104:1775–1784.
- Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2018;68:783–797.
- Kang TW, Lim HK, Lee MW, et al. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology. 2015;276:274–285.
- Gao Y, Zheng DY, Cui Z, Ma Y, et al. Predictive value of quantitative contrast-enhanced ultrasound in hepatocellular carcinoma recurrence after ablation. WJG. 2015;21:10418–10426.
- Yoshida S, Kornek M, Ikenaga N, et al. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2013;58:1667–1680.
- Zhang N, Wang L, Chai ZT, et al. Incomplete radiofrequency ablation enhances invasiveness and metastasis of residual cancer of hepatocellular carcinoma cell HCCLM3 via activating β-catenin signaling [published correction appears in PLoS One. 2015;10(3):e0120472]. PLoS One. 2014;9:e115949.
- Dong S, Kong J, Kong F, et al. Insufficient radiofrequency ablation promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through Akt and ERK signaling pathways. J Transl Med. 2013;11:273.
- Maruyama H, Sekimoto T, Yokosuka O. Role of contrast-enhanced ultrasonography with Sonazoid for hepatocellular carcinoma: evidence from a 10-year experience. J Gastroenterol. 2016;51:421–433.
- Lee J, Lee JM, Yoon JH, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012;13:34–43.
- Singh S, Siriwardana PN, Johnston EW, et al. Perivascular extension of microwave ablation zone: demonstrated using an ex vivo porcine perfusion liver model. Int J Hyperthermia. 2018;34:1114–1120.
- Zhan Y, Zhou F, Yu X, et al. Quantitative dynamic contrast-enhanced ultrasound may help predict the outcome of hepatocellular carcinoma after microwave ablation. Int J Hyperthermia. 2019;35:105–111.
- Wang W, Shi J, Xie WF. Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: a meta-analysis. Liver Int. 2010;30:741–749.
- Peng ZW, Zhang YJ, Liang HH, et al. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689–700.
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–432.
- Cao J, Dong Y, Mao F, et al. Dynamic three-dimensional contrast-enhanced ultrasound to predict therapeutic response of radiofrequency ablation in hepatocellular carcinoma: preliminary findings. Biomed Res Int. 2018;2018:6469703.
- Silva MA, Hegab B, Hyde C, et al. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596.