Abstract
Introduction
Mucinous appendiceal carcinoma is a rare malignancy that commonly spreads to the peritoneum leading to peritoneal metastases. Complete cytoreduction with perioperative intraperitoneal chemotherapy (PIC) is the mainstay of treatment, administered as either hyperthermic intra peritoneal chemotherapy (HIPEC) or early post-operative intraperitoneal chemotherapy (EPIC). Our goal was to assess the perioperative and long term survival outcomes associated with these two PIC methods.
Materials and methods
Patients with mucinous appendiceal carcinoma were identified in the US HIPEC Collaborative database from 12 academic institutions. Patient demographics, clinical characteristics, and survival outcomes were compared among patients who underwent HIPEC vs. EPIC with inverse probability weighting (IPW) used for adjustment.
Results
Among 921 patients with mucinous appendiceal carcinoma, 9% underwent EPIC while 91% underwent HIPEC. There was no difference in Grade III–V complications between the two groups (18.5% for HIPEC vs. 15.0% for EPIC, p=.43) though patients who underwent HIPEC had higher rates of readmissions (21.2% vs. 8.8%, p<.01). Additionally, PIC method was not an independent predictor for overall survival (OS) or recurrence-free survival (RFS) after adjustment on multivariable analysis.
Conclusions
Among patients with mucinous appendiceal carcinoma, both EPIC and HIPEC appear to be associated with similar perioperative and long-term outcomes.
Introduction
Mucinous appendiceal carcinoma is a rare malignancy that has a predilection for peritoneal dissemination leading to peritoneal metastases [Citation1]. Given the indolent nature, typical confinement to the peritoneal cavity and superficial mucinous nature, surgical resection with perioperative intraperitoneal chemotherapy (PIC) has been the cornerstone of treatment for patients with peritoneal metastases [Citation2–4].
There are two methods by which PIC can be administered: as hyperthermic intraperitoneal chemotherapy (HIPEC) or as early post-operative intraperitoneal chemotherapy (EPIC). While definitions and techniques have been clearly standardized for cytoreductive surgery [Citation5], there remains no standardization of PIC treatment and the method of PIC used has generally been based on surgeon preference and training practices. Important pharmacological variables to be considered in defining the optimal PIC include; chemotherapeutic agent, temperature, dose, duration and timing of application.
The early use of PIC began with EPIC using chemotherapy and/or radioisotopes [Citation6]. PIC has the advantages of allowing repeated administration and longer dwell times of chemotherapeutic agents including anti-metabolites. However, this administration technique is limited by patient discomfort and post-operative adhesions limiting peritoneal exposure. Additionally, EPIC does not permit the application of hyperthermia.
In contrast, HIPEC has the perceived benefits of increased patient comfort, improved cytotoxicity and local concentration, as well as the ability to achieve hyperthermia [Citation7,Citation8]. The addition of hyperthermia may augment the penetration and effectiveness of some chemotherapeutic agents [Citation9]. However, it implies a single application over a short duration and therefore non cell-cycle specific chemotherapeutic agent is necessary.
Currently, HIPEC is more commonly used than EPIC, however, both are still used today without a clear superiority of one technique over the other [Citation10]. Additionally, techniques and chemotherapy regimens continue to differ considerably across the world without standardization.
While numerous studies have reported acceptable safety and efficacy with either PIC method, there has not been a direct comparison in regards to their impact on post-operative or long-term outcomes. Two small retrospective series demonstrated no difference in the recurrence-free or overall survival (OS) between patients with peritoneal carcinomatosis from appendiceal malignancies [Citation11,Citation12], and there are conflicting results regarding HIPEC and/or EPIC in peritoneal carcinomatosis from colon cancer [Citation10,Citation13]. In order to advance the field, more investigations identifying the optimal chemotherapeutic agent(s), dose, duration, temperature and delivery method are necessary in order to standardize approaches for patient care throughout the world. Our goal was to assess the perioperative and survival outcomes associated with the two PIC methods to determine if there is a benefit to one method over the other for patients with peritoneal metastases from mucinous appendiceal carcinoma.
Materials and methods
Patients were identified in the US HIPEC Collaborative database as those who underwent curative intent surgery for mucinous appendiceal carcinoma from 2000 to 2017. The US HIPEC Collaborative includes the following 12 academic institutions: Mayo Clinic, The Ohio State University, MD Anderson Cancer Center, Moffitt Cancer Center, University of California San Diego, Medical College of Wisconsin, Emory University, University of Cincinnati, University of Massachusetts, University of Wisconsin, City of Hope National Medical Center, and Johns Hopkins University [Citation14,Citation15]. This study was approved by the Institutional Review Board at all 12 institutions. Clinical, pathologic, post-operative and survival outcomes were collected. Patients were divided into two groups: CRS with HIPEC and CRS with EPIC.
Only one institution (Mayo Clinic) performed EPIC and this was standardized with 5-fluorouracil (5-FU) and radioactive phosphorus. This was the only form of PIC used at this institution. When patients were clinically stable following CRS, they received 3 d of intraperitoneal 5-FU infusion at a dose of 1000 mg/d. On the 4th day, patients were given 10 mCi of intraperitoneal chromic phosphate (P32) and peritoneal drain was then removed. HIPEC regimens were performed by the other 11 institutions with nearly 99% using mitomycin C (MMC).
There were no PCI values available for the EPIC group so the number of resections for each patient was calculated and used to estimate extent of disease. See Supplementary Table 1 for greater detail on how number of resections was determined. The highest number of resections was 25 with an IQR of 3–10. This was dichotomized into extensive disease (more than 10 resections) and less extensive disease (less than or equal to 10) given that 10 was the 75th percentile.
Statistical analysis
Differences in patient demographics, clinical characteristics, and early complications were assessed using Chi-square for categorical factors and Wilcoxon rank-sum tests for continuous factors. Categorical data are reported as number and percent while continuous data are reported as median and IQR. Univariate and multivariable Cox proportional hazards regression was utilized to assess factors associated with recurrence and post 90-d survival. All clinically relevant variables were included in the multivariable models. Of note, for the multivariable analysis, 46% of patients had missing clinical variables and were excluded to obtain an accurate analysis. Baseline characteristics were compared between the included and excluded group and there were minimal significant differences noted (Supplementary Table 2). Inverse probability weighting (IPW) was utilized for adjustment with the weight developed from a logistic regression model predicting EPIC vs. HIPEC (C-statistic = 0.87) where the sole predictor was surgery year. Analysis was performed using SAS version 9.4 (SAS Inc., Cary, NC) and p-values <.05 were considered statistically significant.
Results
Clinical and pathological characteristics
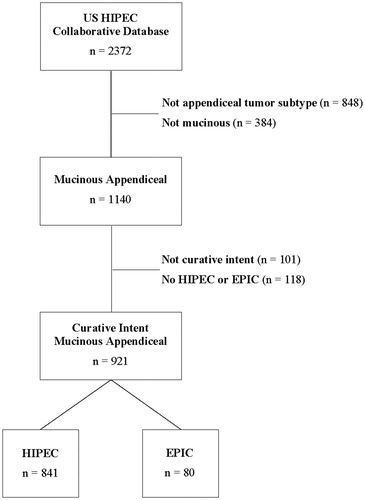
Out of the 2372 patients in the US HIPEC Collaborative database, 1140 patients had a diagnosis of mucinous appendiceal carcinoma. Of these, 921 underwent a curative intent resection with HIPEC or EPIC (). Eighty patients underwent EPIC (8.7%) and 841 underwent HIPEC (91.3%). No patient underwent both EPIC and HIPEC. Mean age across the entire cohort was 55 years old and 60.6% (n = 558) were male.
Figure 1. Flowchart for patients included in the analysis. HIPEC: hyperthermic intraperitoneal chemotherapy; EPIC: early post-operative intraperitoneal chemotherapy.
There were some statistically significant clinical and pathologic differences between the two groups (). Patients in the EPIC group were older (58.3 years old vs. 54.8, p=.02) and more racially homogenous (96.3% white vs. 82.4%, p=.02) while patients in the HIPEC group had higher ASA grades (78.2% Grade III–IV vs. 36.7%, p<.01). More patients in the EPIC group had a previous CRS (41.3% with one previous CRS vs. 21.7%, p<.01) and moderately or poorly differentiated tumors (42.3% vs. 26.6%, p<.01) while neoadjuvant (21.3% vs. 5.0%, p<.01) and adjuvant (15.5% vs. 2.5%, p<.01) chemotherapy were used more often in the HIPEC group. The marker for extent of disease, number of resections, was not statistically different between the two groups with a median of seven resections each. Patients with moderately or poorly differentiated tumors were more likely to receive neoadjuvant chemotherapy (34% vs. 12%, p<.01) and adjuvant chemotherapy (27% vs. 7%, p<.01) than patients with well-differentiated tumors.
Table 1. Demographics and clinical characteristics.
There was no difference in rates of Grade III–V complications between the two groups (18.5% for HIPEC vs. 15.0% for EPIC, p=.43) (). HIPEC operations took longer to complete (7.9 (IQR: 6.1, 9.9) hours vs. 5.4 (IQR: 3.3, 7.2), p<.01) but had fewer CC2/3 resections than the EPIC group (7.1% vs. 13.8%, p=.03). Patients undergoing HIPEC had higher rates of readmissions (21.2% vs. 8.8%, p<.01) when compared to those receiving EPIC (). There was no difference in 90-d mortality between the two groups (0.0% in EPIC and 1.4% in HIPEC, p=.29).
Table 2. Complications.
Survival
Median follow-up in the overall cohort was 35 months (IQR: 17.6, 59.5) and was not statistically different between the two PIC cohorts. PIC method was not found to be an independent predictor for OS (HR 1.25, 95% CI 0.56–2.81, p=.59) or RFS (HR 1.24, 95% CI 0.81–1.90, p=.31) after adjustment on multivariable analysis (). Factors that were independent predictors of worse OS include ASA 3–4 (HR 1.93, 95% CI 1.01–3.70, p=.046), moderate or poorly differentiated histology (HR 2.64, 95% CI 1.51–4.63, p<.01), CC score (HR 2.12, 95% CI 1.02–4.39, p=.04), neoadjuvant chemotherapy (HR 2.98, 95% CI 1.59–5.57, p=.01) and adjuvant chemotherapy (HR = 2.05, 95% CI 1.10–3.84, p=.03). Variables that were independent predictors of worse RFS included older age (HR 1.47, 95% CI 1.05–2.06, p=.03), moderate or poorly differentiated histology (HR 1.40, 95% CI 1.01–1.94, p=.045), higher CC score (HR 1.69, 95% CI 1.05–2.72, p=.03), neoadjuvant chemotherapy (HR 3.08, 95% CI 2.08–4.57, p<.01), and adjuvant chemotherapy (HR 1.75, 95% CI 1.18–2.59, p<.01).
Table 3. Post 90 d multivariable Cox proportional Hazards models for death.
Given the significant role that tumor differentiation has on survival and recurrence, the patients were divided into well-differentiated and moderate- or poorly-differentiated and analyzed separately. In the well-differentiated cohort, PIC method was not an independent risk factor for survival or recurrence (). Similarly, PIC method was not significant for survival or recurrence in the moderate- and poorly-differentiated cohort ().
Table 4. Post 90 d multivariable Cox proportional hazards models for death – well differentiated.
Table 5. Post 90 d multivariable Cox proportional Hazards models for death – moderate/poor differentiated.
Discussion
CRS and PIC have become the standard of care for the treatment of peritoneal metastases from mucinous appendiceal neoplasms with multiple retrospective series supporting its safety and efficacy [Citation7,Citation16,Citation17]. Similar to our study, two previous small single institutional studies have failed to demonstrate any difference in survival outcomes between HIPEC and EPIC for mucinous appendiceal neoplasms [Citation11,Citation12]. No study to our knowledge has compared postoperative morbidity, hospital length of stay and readmissions between the two techniques. The randomized phase II ICARuS (NCT01815359) trial is currently enrolling patients with peritoneal carcinomatosis from the appendix, colon or rectum to EPIC with floxuridine (FUdR) and leucovorin or HIPEC with Mitomycin-C after complete (CC-0 and CC-1) cytoreduction. The primary outcome of disease-free survival and the secondary outcomes of surgical and chemotherapy-related grade III–V toxicity is anticipated to provide further data regarding the optimal method of PIC.
The question of whether the combination of HIPEC and EPIC could potentially provide additional survival benefit for patients with peritoneal metastases from mucinous appendiceal neoplasms compared to HIPEC alone remains unanswered and is an area with a conflicting conclusion in the literature [Citation18]. A previous study from Australia of 185 patients with high grade appendiceal peritoneal metastasis found that the combination of HIPEC and EPIC resulted in improved OS (hazard ratio [HR] = 0.42) and disease-free survival (HR = 0.66), without increasing postoperative morbidity and mortality [Citation19].
In contrast, a Canadian study of 198 patients with peritoneal metastases from a multitude of tumor types reported a higher rate of grade III/IV complications in patients who received HIPEC and EPIC when compared to patients who received HIPEC alone (44.7% vs. 31.0%, p=.05) [Citation20]. A second study from the same group looked at 93 patients with peritoneal metastasis from high grade appendiceal or colorectal cancer and found no difference in OS and RFS between patients treated with CRS and HIPEC + EPIC vs. HIPEC alone but also found a higher incidence of grade III/IV complications [Citation13]. A second group also found higher rates of grade III and above complications in 111 patients receiving EPIC after CRS and HIPEC compared to CRS and HIPEC alone (58% vs. 25%, p=.05) [Citation21]. Further investigation is warranted to determine what if any benefit EPIC may have in addition to HIPEC for patients with peritoneal metastasis from mucinous appendiceal neoplasms.
There are several limitations in this study. First, it was a retrospective study so there is significant risk for confounding and selection bias. Missing data resulted in exclusion of a large subset of patients from the multivariable analysis. Though there were some differences between the patients who remained in the analysis and those that were excluded (Supplementary Table 2), it is unlikely that this has resulted in significant changes to the data or conclusions generated from them. Chemotherapy agent, dose, and duration were not standardized for the HIPEC arm but were standardized for the EPIC arm which can introduce bias. There were significant differences in some of the baseline characteristics between patients treated with HIPEC and EPIC, which were controlled for in the multivariate model. However, other unmeasured differences could also have biased the results. Additionally, prospective PCI information was not available for the EPIC group and was therefore unable to be used in the analysis. We attempted to account for this as described with number of resections but this is of course not identical. EPIC was used at a single institution and as such had a significantly smaller sample size than the HIPEC group which can impact these results as well. Despite these limitations, our study reflects the largest multi-institutional US experience comparing two methods of PIC. We conclude that there is no difference in neither perioperative nor survival outcomes between HIPEC and EPIC for patients with peritoneal dissemination of mucinous appendiceal adenocarcinoma.
Conclusions
For patients with mucinous appendiceal carcinoma, both EPIC and HIPEC when combined with high-quality cytoreductive surgery are associated with excellent short- and long-term outcomes. Until prospective trials clarify the optimal regimen, either method of PIC can be used.
Supplemental Material
Download PDF (274.1 KB)Supplemental Material
Download PDF (241.3 KB)Acknowledgments
This work was presented at the 2019 International Symposium on Regional Cancer Therapies in Phoenix, AZ.
Disclosure statement
The authors have no financial disclosures or conflict of interest. This work has not been previously or concurrently been submitted for publication.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Smeenk RM, van Velthuysen ML, Verwaal VJ, et al. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol. 2008;34(2):196–201.
- Polanco PM, Ding Y, Knox JM, et al. Outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion in patients with high-grade, high-volume disseminated mucinous appendiceal neoplasms. Ann Surg Oncol. 2016;23(2):382–390.
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Cin Oncol. 2012;30(20):2449–2456.
- Levine EA, Stewart JH, IV Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204(5):943–953.
- Sugarbaker PH. Cytoreductive surgery & perioperative chemotherapy for peritoneal surface malignancy. Woodbury, NY: Cine-Med Publishing; 2012.
- Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach . Ann Surg. 1994;219(2):112–119.
- Sideris L, Mitchell A, Drolet P, et al. Surgical cytoreduction and intraperitoneal chemotherapy for peritoneal carcinomatosis arising from the appendix. Can J Surg. 2004;52(2):19–43.
- Elias D, Matsuhisa T, Sideris L, et al. Heated intra-operative intraperitoneal oxaliplatin plus irinotecan after complete resection of peritoneal carcinomatosis: pharmacokinetics, tissue distribution and tolerance. Ann Oncol. 2004;15(10):1558–1565.
- Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia. 2007;23(5):431–442.
- Elias D, Blot F, El Otmany A, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92(1):71–76.
- SØrensen O, Flatmark K, Reed W, et al. Evaluation of complete cytoreductive surgery and two intraperitoneal chemotherapy techniques in pseudomyxoma peritonei. Eur J Surg Oncol. 2012;38(10):969–976.
- Chua TC, Liauw W, Zhao J, et al. Comparative analysis of perioperative intraperitoneal chemotherapy regimen in appendiceal and colorectal peritoneal carcinomatosis. Int J Clin Oncol. 2013;18(3):439–446.
- Lam J, McConnell Y, Rivard J, et al. Hyperthermic intraperitoneal chemotherapy + early postoperative intraperitoneal chemotherapy versus hyperthermic intraperitoneal chemotherapy alone: assessment of survival outcomes for colorectal and high-grade appendiceal peritoneal carcinomatosis. Am J Surg. 2015;210(3):424–430.
- Beal EW, Ahmed A, Grotz T, et al. Trends in the indications for and short-term outcomes of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Am J Surg. 2020;219(3):478–783.
- Zaidi MY, Lee RM, Gamboa AC, et al. Preoperative risk score for predicting incomplete cytoreduction: a 12-institution study from the US HIPEC collaborative. Ann Surg Oncol. 2020;27(1):156–164.
- Baratti D, Kusamura S, Cabras AD, et al. Diffuse malignant peritoneal mesothelioma: Long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Cancer. 2013;49(15):3140–3148.
- Marcotte E, Sideris L, Drolet P, et al. Hyperthermic intraperitoneal chemotherapy with oxaliplatin for peritoneal carcinomatosis arising from appendix: preliminary results of a survival analysis. Ann Surg Oncol. 2008;15(10):2701–2708.
- Soucisse ML, Liauw W, Hicks G, et al. Early postoperative intraperitoneal chemotherapy for lower gastrointestinal neoplasms with peritoneal metastasis: a systematic review and critical analysis. Pleura Peritoneum. 2019;4(3):20190007.
- Huang Y, Alzahrani NA, Liauw W, et al. Early postoperative intraperitoneal chemotherapy for low-grade appendiceal mucinous neoplasms with pseudomyxoma peritonei: is it beneficial? Ann Surg Oncol. 2017;24(1):176–183.
- McConnell YJ, Mack LA, Francis WP, et al. HIPEC + EPIC versus HIPEC-alone: differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol. 2013;107(6):591–596.
- Tan GH, Ong WS, Chia CS, et al. Does early post-operative intraperitoneal chemotherapy (EPIC) for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) make a difference? Int J Hyperthermia. 2016;32(3):281–288.
