Abstract
Background and aims
Surgical resection is currently the cornerstone of liver tumor treatment in children. In adults radiofrequency ablation (RFA) is an established minimally invasive treatment option for small focal liver tumors. Multiprobe stereotactic RFA (SRFA) with intraoperative image fusion to confirm ablation margins allows treatment for large lesions. We describe our experience with SRFA in children with liver masses.
Methods
SRFA was performed in 10 patients with a median age of 14 years (range 0.5–17.0 years) suffering from liver adenoma (n = 3), hepatocellular carcinoma (n = 1), hepatoblastoma (n = 2), myofibroblastic tumor (n = 1), hepatic metastases of extrahepatic tumors (n = 2) and infiltrative hepatic cysts associated with alveolar echinococcosis (n = 1). Overall, 15 lesions with a mean lesion size of 2.6 cm (range 0.7–9.5 cm) were treated in 11 sessions.
Results
The technical success rate was 100%, as was the survival rate. No transient adverse effects higher than grade II (Clavien and Dindo) were encountered after interventions. The median hospital stay was 5 d (range 2–33 d). In two patients who subsequently underwent transplant hepatectomy complete ablation was histologically confirmed. Follow-up imaging studies (median 55 months, range 18–129 months) revealed no local or distant recurrence of disease in any patient.
Conclusions
SRFA is an effective minimal-invasive treatment option in pediatric patients with liver tumors of different etiologies.
Introduction
Liver masses in childhood may be benign (as with cyst or hemangioma), ambiguous in potential (adenoma), or malignant (hepatoblastoma [HB], hepatocellular carcinoma [HCC], metastases). Pain, compromise of liver function, risk of rupture with hemorrhage and malignancy (definite, suspected or prospective) may warrant intervention. Surgical extirpation of the mass lesion is the cornerstone of treatment for most entities. For malignant tumors, chemotherapy in combination with surgery, whether mass excision or liver transplantation (LTx), is the gold standard [Citation1–3].
Radiofrequency ablation (RFA) is a minimally invasive and potentially curative treatment option for liver lesions. Electrodes are percutaneously precisely placed into the tumor under image guidance; dispersive electrodes are placed on the skin. Application of high-frequency alternating current between tumor electrodes and skin electrodes causes targeted heat of 60 °C and controlled tumor destruction [Citation4,Citation5]. RFA is applied in adult patients with primary and secondary liver tumors who are not candidates for liver resection [Citation6,Citation7]. For small lesions the complete response rate is comparable with that achieved by liver resection, with lower morbidity and mortality [Citation6,Citation8–11]. Therefore, conventional RFA is an accepted alternative to liver resection in small HCC (<3 cm) [Citation12]. RFA also serves in bridging therapy before LTx [Citation13]. An advance beyond conventional RFA is stereotactic RFA (SRFA) in which sophisticated planning and precise electrode placement generate overlapping ablations for effective treatment of large tumors [Citation14–16].
In children reported experience with (S)RFA in hepatic tumors is scant [Citation8,Citation17]. Based on longstanding local expertise in SRFA in adults [Citation10,Citation15,Citation18,Citation19], we offered SRFA to selected pediatric patients as an alternative to radical primary resection or as a bridging strategy while awaiting LTx.
Here we present the first report of use of SRFA in a cohort of children with liver mass lesions. We safely deployed SRFA (11 sessions, 15 lesions) in 10 pediatric patients with liver adenoma, HCC, HB, multifocal inflammatory myofibroblastic tumor (IMT), hepatic metastases of primary non-liver tumors and infiltrative hepatic cysts of alveolar echinococcosis, both in bridging (two patients) and as primary treatment (eight patients). We describe our approach and our uniformly favorable results.
Materials and methods
Study
Over a period of 11 years (2007–2018) 10 patients (8 female, 2 male; median age 14 years, range 0.5–17.0 years) with 15 liver masses of different pathology underwent SRFA in 11 sessions. Written informed consent for intervention was obtained from either patients (>14 years) or legal guardians. SRFA was conducted only when chosen explicitly by patients and parents who had received detailed information on treatment options. Patients were treated only if an experienced interventional radiologist judged all visible hepatic masses technically treatable. Due to the possibility to treat large and multiple tumors within one session the size and number of masses were not limited. Exclusion criteria were platelet counts <50,000/μL and coagulopathy. All SRFA treatments were performed with curative intent. Detailed demographic data are shown in and .
Table 1. Patients, underlying diseases and ablation histories.
Table 2. Statistical summary of patients and interventions included in this study.
Retrospective analysis was performed in accordance with the ethics council of the Medical University of Innsbruck (Number of approval: AN4357, 300/4.17, 393/5.23 (4369a).
Indications for SRFA treatment
SRFA treatment was indicated for the following reasons: in patient 1 () SRFA was performed for local disease control of recurrent chemo-resistant hepatoblastoma after left hemihepatectomy, with increasing alpha fetoprotein levels while awaiting LTx (). Indication for SRFA in patient 2 with Beckwith–Wiedemann syndrome was suspected hepatoblastoma as described previously [Citation17]. In patient 3, SRFA was intended as ‘bridging’ therapy to LTx as the definite diagnosis and exclusion of a potential neuromuscular impairment were not yet determined (). In both patients with Tyrosinemia type I (patients 4 and 5, ), SRFA was indicated to prevent imminent risk of malignancy with rising alpha-fetoprotein values. Patient 6 suffered from glycogen storage disease. Liver tumor biopsy showed beta catenin alterations. SRFA was performed due to the adenoma’s size and the associated risk of bleeding. An extended mesohepatic liver resection was not applicable to patient 7, suffering from Echinococcus multilocularis infection. Therefore, minimal invasive percutaneous SRFA was offered as an alternative treatment (). In patient 8, four liver metastases of Stage IV neuroblastoma were treated by SRFA in a palliative setting. Patient 9 suffered from multifocal IMTs with liver lesions. The patient underwent SRFA after tumor extirpation and crizotinib therapy [Citation20]. In patient 10, SRFA was opted for a single liver metastasis of a solid pancreatic pseudopapillary tumor.
Figure 1. A 9-year-old patient with (A) recurrent HB (margin, arrowhead). Planning-CT immediately before SRFA showing hypervascular lesion with 6 cm in diameter. (B) CT image immediately after SRFA showing complete necrosis. A 5-month-old patient with (C) a highly differentiated HCC 3 cm in diameter (CT image, black arrows). Adjacent organs are highlighted (D duodenum, P pancreas). (D) Skin fiducials are attached to the patient and prepared for the intervention. (E) Multiple probe trajectories (colored lines) are defined by using multiplanar and 3D reconstructed images before the ablation procedure. (F) CT image of the tumor after ablation.
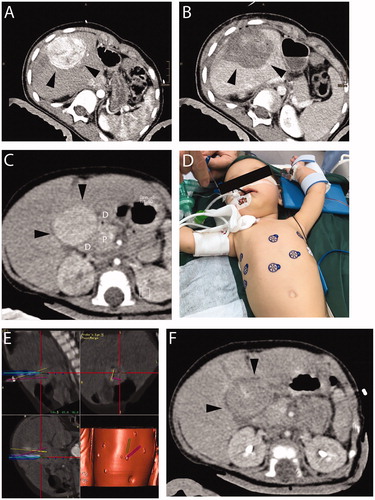
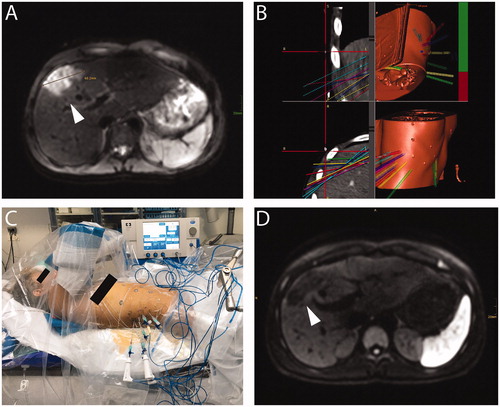
Figure 2. A 15-year-old patient with hepatic subcapsular cyst (Echinococcus multilocularis). (A) A 5.2 × 4.4 cm E. multilocularis lesion (white arrow). (B) Multiple probe trajectories (colored lines) are defined by using multiplanar and 3D reconstructed images before the ablation procedure. (C) SRFA electrodes are sequentially introduced into the coaxial needles. (D) MRI image 3 months after SRFA (white arrowhead) depicts scar tissue without evidence of cystic lesions.
SRFA
A rapid-switching multiple-electrode RFA system (Medtronic, Dublin and Ireland) was applied, consisting of a 200-W generator, three internally cooled monopolar applicators with an active tip length of 3 cm, and a rapid switching multiple-electrode impedance control. As described elsewhere in detail, the anesthetized patient is fixed in a vacuum cushion at the computerized tomography (CT) intervention table [Citation10,Citation15,Citation18,Citation21]. Skin fiducials are attached to the patient (). A single dose of antibiotic (cephalosporin) is administered intravenous 30 min before needle placement. A contrast-enhanced planning CT is obtained during temporary tracheal tube disconnection for respiratory motion control. The stereotactic navigation system is used to define multiple trajectories employing multiplane 3D reconstructed images (). Subsequently, a dynamic reference frame is mounted to the patient table, and skin fiducials are registered using a navigation probe correlated with virtual markers on the CT data set. After skin disinfection the entire interventional field is draped with translucent plastic film. A sterilized aiming device consisting of an adjustable mechanical arm and two pivot joints for holding the probe of the navigation system is used for navigated trajectory alignment. The aiming device is fixed when calculated trajectory alignment is correct, and depth from aiming device to target is automatically calculated by navigation software. Coaxial needles (15/16 gauge), rigidly guided by the aiming device, are positioned during temporary tracheal tube disconnection. After placement of all coaxial needles, a native control CT, performed during temporary tracheal tube disconnection, is fused with the planning CT using the navigation system’s image 3D registration algorithm to verify correct needle placement (). Via the coaxial needles, after a biopsy sample is obtained radiofrequency probes are introduced for tumor ablation. In difficult locations (patient 3) temperature monitoring probes are introduced through the coaxial needles to prevent injury to surrounding organs. After all positions are ablated, contrast-enhanced CT images, again obtained during temporary tracheal tube disconnection, are fused with the planning CT to verify ablated-region size and to assess for complications (). To avoid spreading of infectious or malignant material, every probe tract is coagulated as the probe is retracted.
Follow-up
Follow-up contrast-enhanced magnetic resonance imaging (MRI) was performed at 3-month intervals in the first year and annually thereafter. Complete ablation was defined as demonstration of a circumscribed non-enhancing region with a well-defined margin that lay within or beyond the borders of the mass. Local recurrence was defined as demonstration of masses within or immediately adjacent to the ablation zone. Distant recurrence was defined as demonstration of masses remote from the site of ablation. Technical effectiveness rate was assessed for every lesion in terms of presence or absence of residual mass on first follow-up MRI. As surgical excision was the alternative to SRFA, complications were classified accordingly (Clavien and Dindo [Citation22]. Patient survival was calculated from date of first SRFA to date of death attributable to malignancy or other causes (i.e., event) or to most recent follow-up visit (i.e., censoring). Overall survival was evaluated per patient, whereas local recurrence-free time was evaluated per lesion.
Histopathologic evaluation
Routine sections (formalin, paraffin and hematoxylin-eosin) of hepatectomy specimens from patients 1 and 3 were assessed by light microscopy. As after SRFA necrotic tissue may resemble viable carcinoma, parallel sections were subjected to terminal deoxynucleotidyl transferase (TdT)-mediated uridine triphosphate nick-end labeling (TUNEL) assay (ApopTag, Merck Millipore, Darmstadt, Germany), according to manufacturer’s instructions, to detect fragmented DNA and thereby to identify nuclei of cells in apoptosis. As described, TdT was allowed to incorporate digoxigenin-conjugated nucleotides at DNA breakpoints and a chromogen-conjugated anti-digoxigenin antibody was used to identify newly added nucleotides, with hematoxylin counterstaining to point up histologic detail. Labeled nuclei were assessed by light microscopy [Citation19].
Results
Patient characteristics
Patient details, with types and sites of liver masses, are summarized in and . In total 10 patients (8 female, 2 male) underwent SRFA with a median age of 14 years (range 0.5–17 years) suffering from liver adenoma [Citation8] (n = 3), HCC [Citation23] (n = 1), HB [Citation17] (n = 2), IMT (n = 1), hepatic metastases of extrahepatic tumors (n = 2), and infiltrative hepatic cysts associated with alveolar echinococcosis (n = 1). Fifteen lesions were ablated in 11 sessions. Tumor localization, the SRFA procedure and follow-up imaging results for patients 1, 2 and 7, as examples, are depicted in and .
SRFA procedures (probes, ablation times and histologic findings in ablated regions)
The mean size of the treated liver mass was 2.6 cm (SD ± 2.6 cm). On average 5.7 (SD ± 2.6) coaxial needles and 9.5 (SD ± 9.0) probe positions were required per tumor with an ablation time of 30 min (range 21–102 min, ).
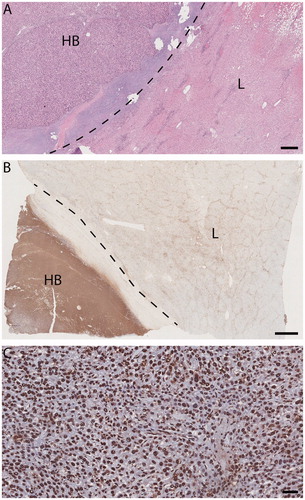
In two patients SRFA was performed as bridging therapy to LTx and ablated regions could be examined histopathologically in the explanted livers. In both, the ablated regions were free of viable tumor cells, including tumor pseudocapsules, as demonstrated with TUNEL staining in one explant ().
Figure 3. Light micrographs, explanted liver (patient 1) with SRFA-treated HB. (A) and (B) HB (HB hepatoblastoma, L adjacent liver). Dotted line, tumor pseudocapsule. A viable-appearing tumor and background liver. Hematoxylin/eosin, size bar, 500 µm. (B) Immunohistochemical evidence of response to injury much more pronounced in tumor than in background liver. TUNEL staining size bar, 2 mm. (C) HB: tumor-cell, endothelial and fibrocyte nuclei alike are marked TUNEL positive, indicating apoptosis in both tumor and stroma. TUNEL staining size bar, 50 µm.
Adverse effects and hospitalization time
After one ablation (9%) mild analgesics were required. Grade II complications were observed for two ablations (18%), with pain requiring morphine in both and temporary portal vein thrombosis in one. After one ablation (9%) temporary paresis of the diaphragm (grade I complication) was observed ( and ).
The median hospitalization lasted 5 d (range 2–33 d, ). However, hospitalization time after SRFA was not always clearly associated with SRFA, as other diagnostic or therapeutic procedures were performed during the same hospital stays.
Follow-up
Median post interventional follow-up is 55 months (range 18–129 months). The overall survival rate with SRFA is 100%. In all patients, shrinkage of the initial ablation zone was observed during follow-up ( and ). So far, no recurrence of disease has been detected by imaging studies in any patient.
Discussion
As to our knowledge, this report is the first pediatric series of SRFA, performed in 11 sessions for 15 liver masses in 10 patients. We know of only three previous reports of SRFA or even RFA in pediatric liver lesions [Citation8,Citation9,Citation24]. Patient selection for SRFA weighed the burdens and potential complications of primary surgery. Our multidisciplinary tumor board proposed all 10 patients for primary liver resection or LTx, with SRFA as a better alternative. For two patients (HB, HCC) SRFA was applied as bridging therapy for disease control while awaiting LTx; in both histopathologic examination found a complete response (), in keeping with a reported complete histopathologic response in 97.3% of nodules (183 of 188) in HCC patients [Citation19]. While RFA appears effective as reiterative treatment in children with metastatic HB [Citation24], no comparable SRFA studies have been performed. For patients with hepatic metastasis of neuroblastoma, pseudopapillary tumor of the pancreas, or IMT, SRFA was chosen because R0 resection by conventional hepatectomy could not be achieved without causing hepatic insufficiency.
SRFA was chosen to treat hepatocellular adenoma, which poses risks of rupture with life-threatening hemorrhage [Citation25] and of malignant transformation [Citation26], in three patients with underlying metabolic disease ().
Although LTx would have dealt with both metabolic disease and tumor, we considered it too drastic an intervention, as the children were relatively well. Resection also carried risk that we thought substantial (hemorrhagic complications requiring transfusion, 13% of cases) [Citation27]. Although RFA [Citation28] or transarterial embolization [Citation29] might have been employed, we thought that SRFA offered better prospects of extirpating the tumor entirely. SRFA in these three patients yielded complete radiological response with one grade I complication.
E. multilocularis infections in children are few, with overt clinical symptoms mostly in advanced disease [Citation30,Citation31]. Death rates are high because of destructive parenchymal infiltration in liver and lungs. For hepatic cysts of alveolar echinococcosis, early and complete radical resection remains the clinical standard [Citation32,Citation33]. The survival rate of patients undergoing R0 resection with clear margins was 100% after a 300-month follow-up [Citation34]. Nevertheless, the risk of mortality and postoperative complications including hemorrhage or sepsis is substantial [Citation34,Citation35]. As would have been the case in our patient, radical resection is generally difficult due to infiltrative disease or multiplicity of cysts involving different liver segments [Citation36]. SRFA was performed without bile leakage, abdominal cavity infection and pleural or abdominal effusion, the principal complications of palliative resection [Citation34,Citation35]. Long-term follow up for at least 10 years and continuous administration of albendazole for at least 2 years are planned [Citation37,Citation38].
In all patients, image fusion intraoperatively confirmed adequacy of ablation. While histological confirmation that ablation equaled R0 resection cannot be provided in eight of our patients, the explanted livers of the two patients with HCC and HB were of note for complete tumor apoptosis (). This finding strengthens our confidence that SRFA is an effective primary treatment in malignancy. Furthermore, all patients were free from disease recurrence on follow-up (median 55 months, range 18–129 months), as confirmed on contrast-enhanced MRI.
In adults, thermal ablation is associated with lower morbidity and mortality, less blood loss, greater preservation of functional liver parenchyma, less pain, shorter hospital stays and lower costs than surgical resection [Citation11]. The median hospital stay for our patients was 5 d, in our experience shorter than that for resection and in line with adult experience [Citation15,Citation39,Citation40]. Adverse events as defined by Clavien and Dindo [Citation22] occurred in four patients with four ablation sessions (36%) ( and ), including two grade I and two grade II complications (18% each). No patient required blood transfusions, which are often needed with surgery [Citation41,Citation42]. No adverse effect required treatment after discharge from hospital.
The limitations to our study include the lack of direct comparison with other treatment modalities, especially liver resection. Histopathologic response to SRFA could be assessed in only the two patients who underwent LTx. Other limitations of our case series include its retrospective nature, the small number of patients and the short follow-up.
In summary, we describe 10 pediatric patients with various liver masses in whom SRFA was a feasible and safe alternative to primary resection. The masses included adenoma, HCC, HB, metastatic myofibroblastic tumor, neuroblastoma, pancreatic pseudopapillary neoplasm and cysts of alveolar echinococcosis. SRFA is a treatment option in selected pediatric patients with liver masses. Since results of randomized studies are unavailable, we recommend an individual decision-making process with every patient including careful consideration of the benefits and risks of SRFA.
Acknowledgment
We thank A.S. Knisely, Institute of Pathology, Medical University of Graz, Graz, Austria, for histopathologic and editorial consultation. This study was presented in part at the 50th ESPGHAN Annual Meeting in Prague, 15 March 2017.
What is already known on this topic
RFA is a minimally invasive and potentially curative treatment option for liver lesions. SRFA generates overlapping ablations for effective and safe treatment of large tumors in adults.
What this study adds
SRFA seems to be effective minimal invasive treatment option in pediatric patients with liver tumors of different etiologies.
Author contributions
H.B., V.G.F., H.M., M.T. and B.R. designed the study, analyzed the data and wrote the manuscript. B.R. developed stereotactic radiofrequency ablation (SRFA). B.R., S.P. and P.D. performed the described interventions. O.G. performed histopathological analysis and TUNEL assays. K.G., M.B., C.R., K.D., M.T., S.C., E.A., S.S., N.C., C.G., J.A., F.K., M.K., S.G., R.O., S.S. and R.B. were involved in patient care, contributed to the manuscript and contributed to editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Emre S, Umman V, Rodriguez-Davalos M. Current concepts in pediatric liver tumors. Pediatr Transplant. 2012;16(6):549–563.
- Schnater JM, Aronson DC, Plaschkes J, et al. Surgical view of the treatment of patients with hepatoblastoma: results from the first prospective trial of the International society of pediatric oncology liver tumor study group. Cancer. 2002;94(4):1111–1120.
- von Schweinitz D. Management of liver tumors in childhood. Semin Pediatr Surg. 2006;15(1):17–24.
- Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249(1):20–25.
- Kim YS, Lee WJ, Rhim H, et al. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195(3):758–765.
- Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma </=3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59(1):89–97.
- Nishikawa H, Kimura T, Kita R, et al. Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia. 2013;29(6):558–568.
- Karall D, Scholl-Burgi S, Widmann G, et al. Stereotactic radiofrequency ablation for liver tumors in inherited metabolic disorders. Cardiovasc Intervent Radiol. 2014;37(4):1027–1033.
- Liu B, Zhou L, Huang G, et al. First experience of ultrasound-guided percutaneous ablation for recurrent hepatoblastoma after liver resection in children. Sci Rep. 2015;5:16805.
- Widmann G, Schullian P, Haidu M, et al. Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc Intervent Radiol. 2012;35(3):570–580.
- Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–307.
- EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Fontana RJ, Hamidullah H, Nghiem H, et al. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8(12):1165–1174.
- Laeseke PF, Sampson LA, Haemmerich D, et al. Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: in vivo porcine liver results. Radiology. 2006;241(1):116–124.
- Bale R, Widmann G, Stoffner DI. Stereotaxy: breaking the limits of current radiofrequency ablation techniques. Eur J Radiol. 2010;75(1):32–36.
- Widmann G, Schullian P, Haidu M, et al. Targeting accuracy of CT-guided stereotaxy for radiofrequency ablation of liver tumours. Minim Invasive Ther Allied Technol. 2011;20(4):218–225.
- Bachmann N, Crazzolara R, Bohne F, et al. Novel deletion in 11p15.5 imprinting center region 1 in a patient with Beckwith-Wiedemann syndrome provides insight into distal enhancer regulation and tumorigenesis. Pediatr Blood Cancer. 2017;64(3):e26241.
- Bale R, Widmann G, Haidu M. Stereotactic radiofrequency ablation. Cardiovasc Intervent Radiol. 2011;34(4):852–856.
- Bale R, Schullian P, Eberle G, et al. Stereotactic radiofrequency ablation of hepatocellular carcinoma - a histopathological study in explanted livers. Hepatology. 2019;70(3):840–850.
- Theilen TM, Soerensen J, Bochennek K, et al. Crizotinib in ALK(+) inflammatory myofibroblastic tumors-current experience and future perspectives. Pediatr Blood Cancer. 2018;65(4):e26920.
- Schullian P, Widmann G, Lang TB, et al. Accuracy and diagnostic yield of CT-guided stereotactic liver biopsy of primary and secondary liver tumors. Comput Aided Surg. 2011;16(4):181–187.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery. 2004;240(2):205–213.
- Waich S, Roscher A, Brunner-Krainz M, et al. Severe DGUOK deficiency in Austria: a six-patient series. J Pediatr Gastroenterol Nutr. 2018;68(1):e1–e6.
- Yevich S, Calandri M, Gravel G, et al. Reiterative radiofrequency ablation in the management of pediatric patients with hepatoblastoma metastases to the lung, liver, or bone. Cardiovasc Intervent Radiol. 2019;42(1):41–47.
- van Aalten SM, de Man RA, Ij JN, et al. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99(7):911–916.
- Deneve JL, Pawlik TM, Cunningham S, et al. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol. 2009;16(3):640–648.
- Dokmak S, Paradis V, Vilgrain V, et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137(5):1698–1705.
- Mironov O, Jaberi A, Beecroft R, et al. Retrospective single-arm cohort study of patients with hepatocellular adenomas treated with percutaneous thermal ablation. Cardiovasc Intervent Radiol. 2018;41(6):935–941.
- van Rosmalen BV, Coelen RJS, Bieze M, et al. Systematic review of transarterial embolization for hepatocellular adenomas. Br J Surg. 2017;104(7):823–835.
- Craig P. Echinococcus multilocularis. Curr Opin Infect Dis. 2003;16(5):437–444.
- McManus DP, Zhang W, Li J, et al. Echinococcosis. Lancet. 2003;362(9392):1295–1304.
- Kern P. Clinical features and treatment of alveolar echinococcosis. Curr Opin Infect Dis. 2010;23(5):505–512.
- Ayifuhan A, Tuerganaili A, Jun C, et al. Surgical treatment for hepatic alveolar echinococcosis: report of 50 cases. Hepatogastroenterology. 2012;59(115):790–793.
- Joliat GR, Melloul E, Petermann D, et al. Outcomes after liver resection for hepatic alveolar echinococcosis: a single-center cohort study. World J Surg. 2015;39(10):2529–2534.
- Qu B, Guo L, Sheng G, et al. Management of advanced hepatic alveolar echinococcosis: report of 42 cases. Am J Trop Med Hyg. 2017;96(3):680–685.
- Buttenschoen K, Carli Buttenschoen D, Gruener B, et al. Long-term experience on surgical treatment of alveolar echinococcosis. Langenbecks Arch Surg. 2009;394(4):689–698.
- Vuitton DA, Azizi A, Richou C, et al. Current interventional strategy for the treatment of hepatic alveolar echinococcosis. Expert Rev anti Infect Ther. 2016;14(12):1179–1194.
- Brunetti E, Kern P, Vuitton DA, et al. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114(1):1–16.
- Bale R, Richter M, Dunser M, et al. Stereotactic radiofrequency ablation for breast cancer liver metastases. J Vasc Interv Radiol. 2018;29(2):262–267.
- Bale R, Schullian P, Schmuth M, et al. Stereotactic radiofrequency ablation for metastatic melanoma to the liver. Cardiovasc Intervent Radiol. 2016;39(8):1128–1135.
- Zwintscher NP, Azarow KS, Horton JD. Morbidity and mortality associated with liver resections for primary malignancies in children. Pediatr Surg Int. 2014;30(5):493–497.
- Veenstra MA, Koffron AJ. Minimally-invasive liver resection in pediatric patients: initial experience and outcomes. HPB (Oxford). 2016;18(6):518–522.
