Abstract
Purpose
To characterize temperature fields and tissue damage profiles of large-volume hyperthermia (HT) induced by magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) in deep and superficial targets in vivo in a porcine model.
Methods
Nineteen HT sessions were performed in vivo with a commercial MRgHIFU system (Sonalleve® V2, Profound Medical Inc., Mississauga, ON, Canada) in hind leg muscles of eight pigs with temperature fields of cross-sectional diameter of 58-mm. Temperature statistics evaluated in the target region-of-interest (tROI) included accuracy, temporal variation, and uniformity. The impact of the number and location of imaging planes for feedback-based temperature control were investigated. Temperature fields were characterized by time-in-range (TIR, the duration each voxel stays within 40–45 °C) maps. Tissue damage was characterized by contrast-enhanced MRI, and macroscopic and histopathological analysis. The performance of the Sonalleve® system was benchmarked against a commercial phantom.
Results
Across all HT sessions, the mean difference between the average temperature (Tavg) and the desired temperature was −0.4 ± 0.5 °C; the standard deviation of temperature 1.2 ± 0.2 °C; the temporal variation of Tavg for 30-min HT was 0.6 ± 0.2 °C, and the temperature uniformity was 1.5 ± 0.2 °C. A difference of 2.2-cm (in pig) and 1.5-cm (in phantom) in TIR dimensions was observed when applying feedback-based plane(s) at different locations. Histopathology showed 62.5% of examined HT sessions presenting myofiber degeneration/necrosis within the target volume.
Conclusion
Large-volume MRgHIFU-mediated HT was successfully implemented and characterized in a porcine model in deep and superficial targets in vivo with heating distributions modifiable by user-definable parameters.
Introduction
Hyperthermia (HT) refers to increasing tissue temperature to 40–45 °C for an extended duration [Citation1]. It is one of the most potent treatment modalities for enhancing the efficacy of radiation therapy (RT) and chemotherapy [Citation2]. For skin cancer or sarcoma treatments, the combination of RT and HT (RT + HT) resulted in a significantly increased response rate compared with RT alone [Citation3–16]. A randomized trial [Citation8] in skin cancer reported a statistically significant (p = 0.008) 2-year local control rate in patients treated by RT + HT (46%, N = 34) compared to patients receiving RT only (28%, N = 34).
Several modalities have been introduced to induce HT for cancer therapy [Citation17,Citation18], including radiofrequency [Citation19–23], microwaves [Citation24,Citation25], lasers [Citation26,Citation27], magnetic fluids [Citation28–31], radiant heat (visible and infrared) [Citation32], and hot water immersion [Citation30,Citation31,Citation33]. Most of these techniques have limitations including superficial penetration or non-localized heating [Citation33,Citation34]. Temperature monitoring in almost all clinical HT devices is achieved by invasive thermocouples, which only determines the temperature in several isolated points, providing limited volumetric information [Citation35,Citation36].
Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) has recently been introduced for inducing HT using real-time temperature monitoring by MR thermometry [Citation37,Citation38]. HIFU can penetrate deep into the body and achieve high spatial targeting precision (in mm scale). MR is utilized for pretreatment planning and real-time monitoring during HT due to its superiority in soft tissue contrast, high image resolution, and the ability to provide volumetric thermometry during HT administration. A commercial MRgHIFU system (Sonalleve® V2, Profound Medical Inc., Mississauga, ON, Canada) has shown an acceptable safety profile in the muscles and uterus of pig models in vivo when employing heating fields of cross-sectional diameter (target-region-of interest (tROI) diameter, or cell diameter) up to 44 mm [Citation39,Citation40].
Target dimensions for RT + HT can be larger than 44-mm and are located at both deep and superficial locations. Due to extended heating durations, using multiple separate heating events to cover a large tumor is both inefficient and undesirable from a clinical-treatment perspective. Previous work [Citation39,Citation40] showed successful implementations of in vivo MRgHIFU-HT where depths of the tROI ranged from 35 mm to 80 mm when using a tROI Ø ≤ 44-mm. No successful implementation of large-volume HT (here, defined by tROI Ø = 58-mm) or for superficial targets (i.e., <25-mm from the skin) in vivo with satisfactory temperature statistics has been reported. Therefore, there is a pressing need to characterize large-volume HT for deep and superficial targets in vivo to facilitate clinical translation.
We aim to characterize the temperature distribution and tissue profile of large-volume HT in both deep and superficial locations by applying a series of large-volume HT treatment cells to both superficial and deep targets in vivo with different user-definable parameters. Temperature statistics within the tROI and temperature distributions were characterized. Three-dimensional measurements of the thermal dose distributions were benchmarked against a commercial phantom. Finally, contrast-enhanced MR images, macroscopic examination, and histopathological analysis were performed for tissue damage characterization.
Materials and methods
Animal overview
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee at Washington University in St. Louis following the National Institute of Health Guidelines for animal research. This study used eight female pigs (107.6 ± 4.3 day-of-treatment-age, 119.6 ± 5.9 lbs.). Pigs either received one (N = 1), two (N = 4), three (N = 2), or four (N = 1) HT sessions on a given day. For pigs that received multiple HT sessions, we applied each HT session to different areas of the hind leg muscles to avoid reapplying HT to the same area of the muscle.
Description of MRgHIFU system
The Sonalleve® V2 system [Citation41–43] comprises of a patient tabletop that can be locked into an MRI scanner bore (Ingenia 1.5 T, Philips, Best, the Netherlands). The tabletop accommodates a 2-channel RF receive coil and a 256-channel phased-array HIFU transducer. Another 3-channel pelvic RF receive coil were embedded in the pelvic surface-coil. The transducer is immersed in an oil bath inside the tabletop, and access to the target is provided through an acoustically transparent Mylar membrane. The transducer can operate within a frequency range of 0.85–1.45 MHz.
Temperature feedback control
A temperature feedback control algorithm introduced by Tillander et al. [Citation40] was employed to control the HT sonication by integrating electronic beam steering and mechanical movement of the transducer [Citation40,Citation44]. In short, HT was primarily regulated by the tROI temperature in the target plane. The aim is to raise the temperature to a pre-defined target temperature and to homogenously maintain the temperature in each voxel within the tROI [Citation43]. The 58-mm diameter circular tROI is divided into seven ‘subcells’ (each with Ø = 16-mm) arranged on a hexagonal grid [Citation40]. For heating within each subcell, only electronic steering was employed. The method of electronic beam steering, number of points within a subcell, and sonication point order have been described by Köhler [Citation44] and Tillander et al. [Citation40]. The transducer is mechanically translated between different subcells. The HT algorithm first increases the average temperature of the middle subcell to the target temperature, and then moves to one of the ‘coldest’ surrounding subcells. The sonication temporarily pauses if none of the subcell had an average temperature lower than the target temperature. The HT feedback algorithm also contains a maximum-threshold-temperature for preventing temperature overshoot in one or more user-defined and positioned plane(s), which are here collectively labeled as ‘threshold-plane’. If any voxel in the beampath cross-section within the threshold-plane exceeds a user-defined maximum temperature threshold, the sonication power would be set to zero until all voxels of interest cool to temperatures under the maximum threshold.
Experimental procedure
Animal preparation: The pig was anesthetized with an intramuscular injection of ketamine (2 mg/kg), xylazine (2 mg/kg), and telazol (4 mg/kg) in the cervical muscles, intubated and ventilated. General anesthesia was maintained using isoflurane at a concentration of 1–5%, and a tidal volume of 10 mL/kg. The blood oxygen saturation and pulse rate were monitored by a fiber-optic pulse oximeter (Nonin 7500FO, Plymouth, MN). The pigs’ body temperature was monitored rectally.
Animal placement: To acoustically couple the animals’ skin with the Mylar membrane and adjust the position of the targets into an accessible range, a 1.5-cm or 4.0-cm gel pad (Profound Medical Inc., Mississauga, ON, Canada) and modified mixture of ultrasound gel (Aquasonic, Parker Laboratories Inc., Fairfield, NJ, USA) and degassed water in a 3–4:1 ratio were utilized. Clippers were used to remove the hair from the hind leg, rump, and adjacent regions and any remaining hair was removed using depilatory cream (Nair, Church & Dwight Co., Princeton, NJ, USA). After laying the animal on the tabletop in a lateral position, the pelvic coil was secured over the target anatomy. Sandbags were used to further stabilize and support the animal on the HIFU table ().
Figure 1. (A&C) Schematic illustration of the experimental setup in an example superficial (A) and deep (C) HT session viewed in the transverse plane, with the animal placed on the HIFU tabletop in the left decubitus position (not to scale). Orientation: AP: anterior-posterior; LR: left-right; and FH: foot-head. The target slice is denoted in red, and the near field and far field are denoted in orange. (B&D) transverse view of T2-weighted MRI overlaid with a schematic illustration of the beampath and the transducer in an example superficial (B) and deep (D) HT session.
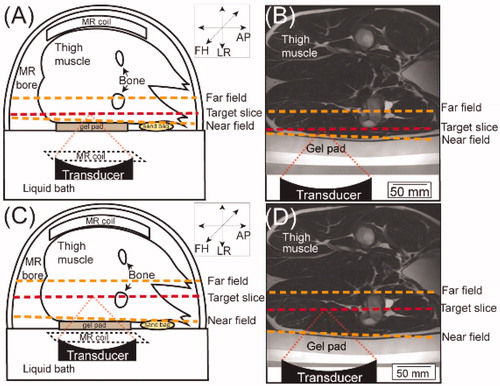
Treatment planning: A T2-weighted MRI [Citation40,Citation42,Citation43] was acquired to image hind leg muscle for treatment planning: echo time =130 ms, repetition time =1300 ms, flip angle =90°, number of signal averages =1, field-of-view =250 × 259-mm2, acquisition matrix =250 × 217, spatial resolution (voxel size) =1.12 × 1.19 × 2.5-mm3, scan time =190-s. We maintained a safety margin of ≥10-mm [Citation45–47] between the HIFU beampath and any bone.
Temperature monitoring: The proton resonance frequency shift (PRFS) method was used for temperature mapping [Citation39,Citation40,Citation42,Citation43] using field of view =400 × 400mm2, voxel size =2.5 × 2.5 × 7mm3, temporal resolution =3.7s. Briefly, a dynamic, RF-spoiled, fast-field-echo echo-planar imaging (FFE-EPI) sequence was utilized to obtain magnitude and phase images. Six FFE-EPI planes (5 coronal, 1 sagittal relative to the transducer, as shown in ) were used for temperature monitoring. Coronal slices include: a ‘target’ slice, placed at the target depth; 2 slices, one each 7-mm distal and proximal to the ‘target’ slice, respectively; and user-positioned ‘near field’ and ‘far field’ planes proximal and distal to the target slice relative to the transducer, respectively. The sagittal slice was along the ultrasound beampath perpendicular to the target slice.
The depths of the target slices were separated into two groups: (1) Superficial HT targeted depths of approximately 20-mm from skin surface (average = 18.8 ± 1.8mm, N = 11) (); (2) Deep HT targeted depths of approximately 60-mm from the skin surface (average = 62.2 ± 2.3mm, N = 8) (). For superficial HT, the near-field-only (N = 4), the far-field-only (N = 3), or both the near- and far- fields (N = 4) were selected as the threshold-plane. In deep HT, the near-field-only (N = 4), or both near- and far-field (N = 4) were selected as the threshold-plane. Since we detected some skin damage when using the far-field only as the threshold-plane for superficial targets, we did not apply the far-field-only as the threshold-plane for deep HT, although later analysis revealed those changes were not related to the heating. In all sessions, attempts were made to keep the distance between the near-field and tROI, and the distance between the far-field and tROI as consistent as possible. Of note, some adjustments (generally <1.0-cm) were made on a case-by-case basis to avoid placement of the tROI/near-field/far-field on a blood vessel or nerve as well as to address practical access limitations of the system. The distance between the near field and tROI was 54.55 ± 4.95mm and 11.80 ± 3.75mm for deep and superficial HT, respectively. The distance between the far field and the tROI was 32.60 ± 4.41mm and 28.73 ± 11.62mm in deep and superficial HT, respectively.
HT treatment: Prior to HT treatment in each animal, the MR thermometry sequence was performed for 10–15 min to bring the magnet hardware to a thermal steady-state [Citation48]. HT treatment then proceeded with the following parameters for all sonications: acoustic power 100 W; treatment duration 30 min; target temperature 42 °C; maximum threshold temperature 44 °C (applied to threshold-plane). A frequency of 1.0 MHz was used, producing an ellipsoidal focal point with length and width of 14.65 mm and 1.90 mm, respectively [Citation43]. All sonications employed continuous ultrasound waves.
Post-sonication scanning: After HT completion, two T1-weighted 3D high resolution isotropic volume excitation (THRIVE) sequences were acquired before and after the injection of gadobenate dimeglumine (MultiHance; Bracco Imaging, Milan, Italy) at a dose of 0.2 mL/kg [Citation43]. Potential thermal damage was presented as abnormally-perfused areas. To avoid the temperature raise or the thermal damages from a completed HT session affecting a subsequent HT session, we took the following steps. First, we waited for the temperature to decrease to below 39 °C before starting planning of the subsequent HT session. Second, five out of the eight pigs received 2 HT sessions or less. In four of these pigs, we heated both legs to avoid the overlapping of heated tissue in the second HT session. Third, for the three pigs who received ≥3 HT sessions, we changed the pigs’ positions/orientations to target a separate portion of the same leg or the other leg in a consecutive HT session, so that there was enough cooling down time. Due to a relatively smaller heated volume in superficial HT, when a pig received ≥3 HT sessions, the 3rd or 4th HT sessions were performed to superficial targets, so that the decreased heated volume could help to minimize any heating overlap in the previously heated deep-seated regions.
Animal euthanasia: After the MR scanning, pentobarbital (150 mg/kg) was administered intravenously for euthanasia.
tROI temperature quantification
The acquired FFE-EPI images were processed after the experiment using a previously-described method [Citation42,Citation43]. The reliability of the MR thermometry of Sonalleve® system has been verified by a comparison between the temperature measured by fiberoptic sensors and the Sonalleve® reported temperature. Only through a discrepancy of those two measurements by less than 1 °C, was the system deemed reliable for clinical use [Citation40]. This accuracy has also been similarly validated in our system. Therefore, the temperature reported by Sonalleve® system was used for the temperature analysis in this study. Temperature statistics within the tROI were evaluated and a number of factors tabulated [Citation43]. Briefly, the spatial average temperature (Tavg), the maximum temperature of all the voxels within the tROI (Tmax), the minimum temperature of all the voxels within the tROI (Tmin), the temperature that only highest tenth percentile of voxels reached (T10), the temperature that only the lowest tenth percentile of voxels reached (T90), and the spatial standard deviation of all voxels within the tROI (σT) were all evaluated. Each metric was first calculated in each ‘dynamic’, i.e., one of the temperature maps acquired every 3.7 s, and the metrics were then averaged across all dynamics. Additionally, the maximum value of Tmax,d in tROI of each session was calculated, as well as the Tavg, Tmax, and T90 values in the cross-section within the beampath of the near field slice. Cumulative equivalent minutes at 43 °C of T90 (CEM43T90), a thermal dose predictor of clinical efficacy for RT + HT in cancer patients in several clinical trials [Citation12,Citation49–59], was also analyzed. We chose to evaluate the CEM43T90 since it characterizes the lower end (T90) of the of the temperature distribution over extended time, which was reported to have the most relevance for the tumor response [Citation50,Citation53,Citation57,Citation58]. Additionally, in order to benchmark the achieved thermal dose with previous studies for the muscle tissue damage [Citation60–64], cumulative equivalent minutes at 43 °C of Tavg (CEM43Tavg) at the tROI was also characterized.
The following four parameters were calculated for evaluation of the system performance [Citation39,Citation40,Citation43]: temperature accuracy, precision, temporal variation, and heating uniformity. Temperature accuracy was calculated by subtracting the desired temperature of 42 °C from the achieved Tavg. Precision was quantified by σT, an estimate of the spatial temperature variance of each voxel across the tROI. Temporal variation was gauged by the standard deviation of the Tavg,d across all dynamics. Heating uniformity was assessed by the temporal average of the largest difference between the Tavg,d and either the T10,d or T90,d, which was for evaluating the degree of spatial temperature homogeneity. We chose to quantify the above-mentioned parameters due to the fact that they have been calculated in our previous study [Citation43] and others’ work [Citation39,Citation40], thus allowing benchmarking of our results against the existing data. For clinical management purposes, it was also crucial to evaluate how quickly the temperature increases to within 1 °C of the target temperature (here, 42 °C), as this is generally considered a reasonable benchmark for successful heating with these MRgHIFU systems. Thus, the duration required for the Tavg,d in the 58-mm tROI (not any subcell in the tROI) to reach ≥41 °C after the start of sonication was also recorded.
Two-tailed Mann-Whitney U tests were used to assess significant statistical differences (p < 0.05) among different heating conditions for the tROI temperature metrics and/or the time for temperature increase.
Three-dimensional temperature characterization
The volume and shape of temperature distributions within the tROI were evaluated using the time-in-range (TIR) concept in both the sagittal and the coronal slices. Briefly, the TIR values of 10-min/20-min/25-min (TIR10/TIR20/TIR25) were calculated, which were presented as maps of voxels that stay within 40–45 °C for 10-min/20-min/25-min during the whole 30-min sonication. The dimensions of TIR maps along the ultrasound beampath were analyzed in the sagittal plane. The dimensions of the TIR maps perpendicular to the ultrasound beampath were analyzed in the target plane along both horizontal and vertical axes to characterize the beam profiles at the target depth.
We benchmarked the size and shape of the HT heating distributions against those seen in a commercial HIFU cylindrical gel phantom filled with proprietary hydrogel (Profound Medical Inc., Mississauga, ON, Canada) using the same sonication parameter as in vivo. The inclusion of HT in phantom served as a model with few external heating artifacts (i.e., animals’ cardiovascular, respiratory, and digestive motions) to help validate any potential changes in temperature distributions caused only by the different positions of the threshold-plane. All TIR maps of HT sessions both in vivo and in phantom were processed in MATLAB (R2018a, MathWorks, Natick, MA, USA).
One-tailed Mann-Whitney U test was used to assess significant statistical differences (p < 0.05) in penetration depth since the depths of the TIR dimensions were expected to be more superficial when the threshold-plane for temperature regulation included the far-field compared to when it excluded the far-field. Two-tailed Mann-Whitney U test was used to assess significant statistical differences (p < 0.05) for comparison of the TIR dimensions along the horizontal and the vertical axes. GraphPad Prism (Version 8.3.1, La Jolla, CA, USA) was used for all statistical analyses in this study.
Tissue damage characterization
Tissue damage caused by HT was assessed by contrast-enhanced MRI, gross pathology, and histopathology using hematoxylin and eosin (H&E) staining. After euthanasia, the locations of HT targets were identified using anatomical landmarks (greater trochanter of the femur and tibial tuberosity) to identify the hip, knee, and orientation of the femur as reference. A gross examination of the skin surface overlaid on the treatment area was done and then each lesion was collected for histopathological analysis.
The pathological samples were taken by referring the distances (measured in the MR images) from the tROI to palpable and recognizable anatomical landmarks (greater trochanter of the femur and tibial tuberosity) and its location relative to the femur during gross dissection. During the dissection, we located the center of the tROI by finding the point that has the same distances to the above-mentioned bony landmarks and its geometrical location relative to the femur as seen in the MR images. This method resulted in a collected tissue section (25 × 20 mm) smaller than the heated volume, so this study did not quantify the geometrical boundaries of the tissue damage. The following tissues were collected: (1) muscles within the heated volume (including the center of the tROI and tissues 20-mm distal to the HT targets relative to the transducer), (2) tissues within ultrasound beampath, including skin and subcutaneous fat (i.e., representative of the near field), (3) muscles from the non-heated contralateral leg (as negative controls). Tissues were processed as previously described [Citation43].
Results
tROI temperature characterization
An illustration of the HT and off-line processing is shown in , including the tROI denoted by the red dashed circle overlaid on a representative axial MRI target slice (), the corresponding Tavg map of the target slice (), and the TIR10/TIR20/TIR25 maps in this target slice (). For this treatment, presents the temporal Tavg,d/Tmax,d/Tmin,d of all voxels within the tROI during the 30-min HT session and 15-min cooling period. The spatial homogeneity () and temporal stability () evident in this case were seen in all other HT sessions. The temporal-average metrics in the tROI for all HT sessions (Tavg, Tmax, Tmin, T10, T90, σT, CEM43T90 and time for Tavg to reach 41 °C) are shown in , along with the Tavg, Tmax, and T10 in the cross-section of the near-field. Across all HT sessions, the Tavg in the tROI were 41.7 ± 0.4 °C and 41.6 ± 0.5 °C for deep and superficial HT, respectively. The maximum Tmax in tROI averaged 51.3 ± 2.2 °C and 49.4 ± 2.1 °C for deep and superficial HT, respectively. Tavg, T10, and Tmax in the near-field cross-section were 39.1 ± 0.7 °C, 42.0 ± 0.8 °C, and 44.8 ± 1.2 °C across all deep HT sessions, and 40.2 ± 1.0 °C, 42.1 ± 1.0 °C, and 45.4 ± 3.7 °C across all superficial HT sessions. The average CEM43T90 and CEM43Tavg was 0.9 ± 0.7 min and 12.7 ± 6.1 min, respectively. Among all HT sessions, Tavg reached 41 °C at 247.8 ± 66.5 s.
Figure 2. Workflow of the HT to thigh muscle and off-line temperature processing. (A) Illustration of the target region-of-interest (tROI) selection indicated by the red dashed circle on the axial MRI (target slice). (B) For the treatment in (A), a representative average temperature map of the target slice with the tROI outlined in red dashed circle is shown. (C) Time-in-range map (TIR) map of 10-min, 20-min, and 25-min of the target slice for the 30-min HT for this treatment. (D) The representative average temperature (Tavg), maximum temperature (Tmax), and minimum temperature (Tmin) of all voxels within the tROI during the 30-min HT session and 5-min cooling period.
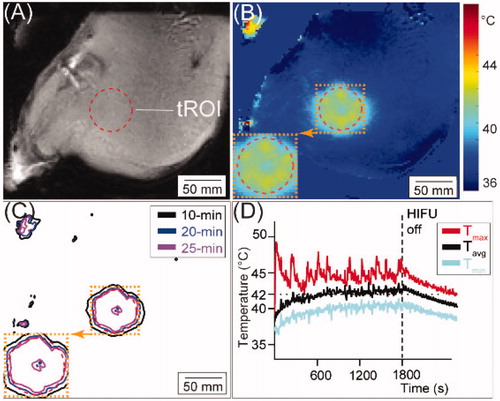
Table 1. Temperature statistics in the target region-of-interest (tROI) within the target slice and the beam cross-section in the near field (NF).
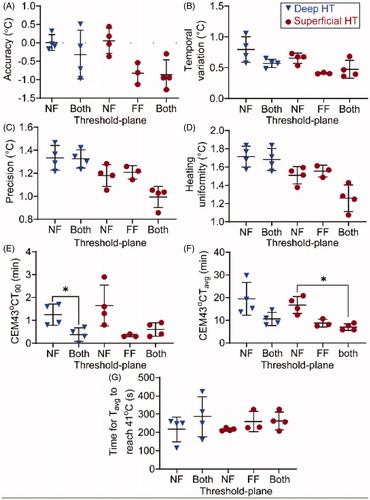
shows the metrics within the tROI in different locations of threshold-plane in both deep and superficial HT, including the accuracy (A), temporal variation (B), precision (C), heating uniformity (D), CEM43T90 (E), CEM43Tavg (F), and the time needed for the Tavg,d to reach 41 °C (G). Across all HT sessions, accuracy was −0.4 ± 0.5 °C, precision was 1.2 ± 0.2 °C, temporal variation was 0.6 ± 0.2 °C, and heating uniformity was 1.5 ± 0.2 °C. For deep HT, the CEM43T90 was significantly lower when the threshold-plane included both the near- and far-fields, compared to the threshold-plane applied for the near-field only (). For superficial HT, the CEM43Tavg was significantly lower when the threshold-plane included both the near- and far-field, compared to the threshold-plane applied for the near-field only ().
Figure 3. Temperature metrics for both deep HT (blue triangles) and superficial HT (red dots) in thigh muscle. Plane(s) included in the threshold-plane are shown on the abscissa: near-field only (NF), far-field only (FF), or both near- and far-fields (Both). (A) Difference between Tavg and desired temperature of 42 °C. (B) Temporal variation. (C) Temperature precision. (D) Temperature uniformity. (E) The CEM43T90 (min) calculated based on the T90 for the 30-min in each voxel within the target region-of-interest (tROI). (F) The CEM43Tavg (min) for the 30 min calculated based on the Tavg in each voxel within the target region-of-interest (tROI). (G) Time (s) for the average temperature of the tROI to reach 41 °C after the start of the sonication. All results are presented in form of mean (central black line) ± standard deviation (black range bars). The statistically significant differences (p < 0.05) are indicated by asterisk (*).
Three-dimensional temperature characterization
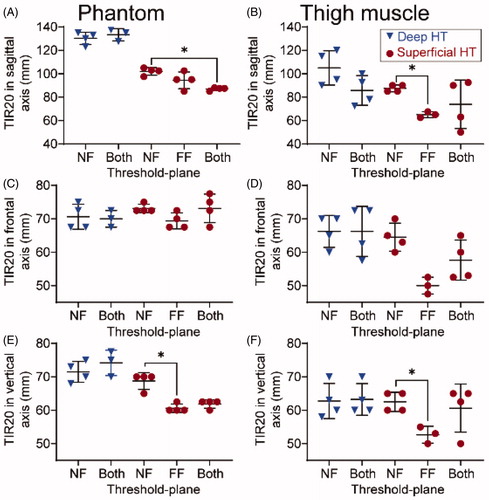
presents the average dimensions of the TIR10/TIR20/TIR25 along the sagittal axis, the horizontal axis, and the vertical axis in both porcine thigh muscle and phantom. Across all 19 sessions in the thigh muscle, the widths of TIR10, TIR20, and TIR25 within the treatment plane averaged 73.7 ± 13.2 mm, 61.1 ± 7.5 mm, and 53.3 ± 6.9 mm, respectively. Due to its high conformality to the target cell size (58 mm), TIR20 was selected as a representative metric for describing the heating dimensions. show representative examples of HT performed in phantom and pig thigh muscle in vivo, respectively. The red dashed lines indicate the locations of the target slice, and the orange dashed lines represent the locations of threshold-plane. Threshold-plane was the near-field only in , while it was both the near- and far-fields in . Visually, the TIR maps appear to be more superficial in compared to . This is evident in both the porcine thigh muscle () and phantom ().
Figure 4. Representative time-in-range (TIR)-10/20/25 min maps overlaid on the Tavg maps acquired from MR thermometry in the sagittal plane for superficial targets in both phantom (A&B) and porcine thigh (C&D). The target slice is denoted by the red dashed line and the near- and/or far-fields denoted by orange dashed lines. The threshold-plane included only the near-field in (A, C), while the threshold-plane included both the near- and far-field in (B, D). Orientation: AP: anterior-posterior; LR: left-right; FH: foot-head.
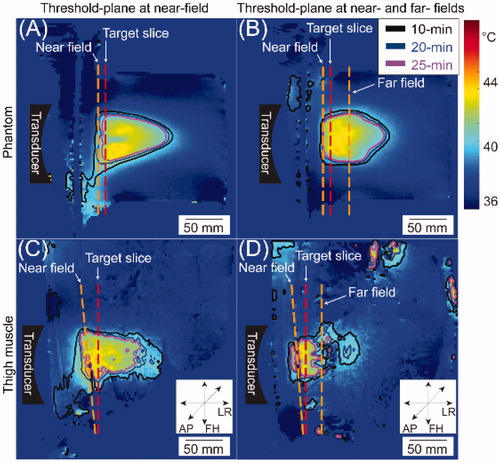
Table 2. The dimensions of the time-in-range (TIR)-10/20/25 min in the sagittal axis (along LR orientation shown in ), the frontal axis (along the AP axis in ), and the vertical axis (along the HF axis in ) in both the porcine thigh muscle and the commercial phantom.
An analysis of TIR20 dimensions for different locations of threshold-plane is shown in . In superficial HT in vivo, a 22.5-mm difference (sagittal; ) and a 10.2-mm difference (vertical; ) in TIR20 dimensions were observed when the threshold-plane included only the far-field () or both fields () compared to when threshold-plane included only the near-field (p < 0.05). Such differences were also observed in phantom; the TIR20 dimensions along sagittal and vertical axes were significantly decreased (p < 0.05) when far-field or both fields were included in the threshold-plane compared to when the near-field alone was included (). For deep HT, no statistically significant difference was found in TIR20 dimensions either in the thigh muscle or in phantom. Similar results were also observed in the dimensions of TIR10 () and TIR25 () when the threshold-plane was applied to only the near-field compared to when the threshold-plane was applied to the far-field only or both fields.
Figure 5. Dimensions of time-in-range (TIR)-20min on the sagittal axis, frontal horizontal axis, and vertical axis in both phantom and porcine thigh muscle, respectively. All results are presented in form of mean (central black line) ± standard deviation (black range bars). Results of deep HT is denoted by blue triangles and results of superficial HT is denoted by red dots. The plane included in the threshold-plane is shown on the abscissa: near-field only (NF), far-field only (FF), or both near- and far-fields (Both). Statistically significant differences (p < 0.05) are indicated by asterisk (*).
Characterization of tissue damage
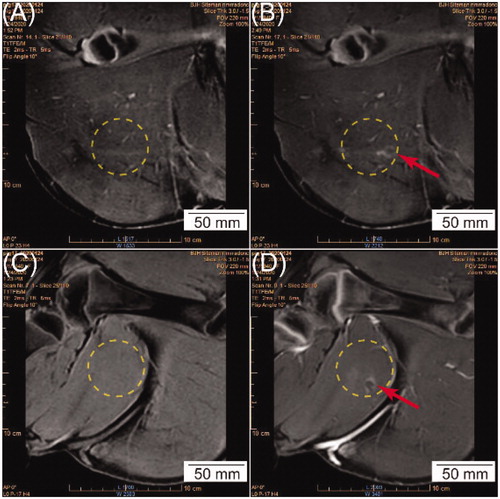
Post sonication, contrast-enhanced MRI showed abnormally-perfused, hyper-intense regions within the tROIs in ten out of nineteen sessions (). The nature of the post-HT MRI changes in the heated volume (both in the tROI and 2-cm distal to the tROI) was very subtle and only presented in sub-volumes in the heated area (as represented in ) in some cases. Although some image quantification techniques were attempted, they were not able to capture these changes. Therefore, whether there was post-HT MRI enhancement in T1 MR images was evaluated based on observer-scoring. No abnormally-perfused area was observed in the contrast-enhanced MRI for subcutaneous tissues or skin that overlayed the tROI. In gross analysis, no abnormalities were observed on the skin surface for any HT session.
Figure 6. (A, C) A representative T1-weighted 3D high resolution isotropic volume excitation (THRIVE) MRI in the coronal view before HT. (B, D) Contrast-enhanced MRI in the coronal view in the same plane as (A, C) at the completion of HT, respectively. The yellow circle represents the cross-section of the beam path, and the red arrow shows hyper-intense regions detected in the cross-section of the beam path, indicating potential tissue damage.
For the histopathological analysis, heated muscle tissues from sixteen HT sessions were examined and classified based on the target depth and the location of the threshold-plane, as summarized in . Tissues from the other three sessions were destroyed before they could be collected for analysis. Overall, the pathological changes in the muscle tissues within the heated volume (including tissues at the center of the tROI at the heating depth and 20-mm distal to heating depth) could be classified into three categories. First, acute myofiber degeneration and necrosis, characterized by swelling and hypereosinophilia of myofibers, with variable vacuolation, fragmentation, contraction band necrosis, loss of cross-striations, or nuclear pyknosis or loss, as shown in , were detected in ten sessions (62.5%, as shown in ). Second, mild to moderate edema, characterized as mild separation of myofibers, were captured in two sessions (as shown in and ), both of which were accompanied by the acute myofiber degeneration and necrosis as we described previously. Third, no significant lesions compared to the negative control tissues were detected in the rest of the sections, as shown in Supplementary Figure 3(E,F). For the skin and subcutaneous tissue, in all HT sessions, compared with the control tissue, we did not detect any acute changes related to experimental procedure, as shown in Supplementary Figure 3(G,H).
Table 3. Categorization of the post-HT histological analysis based on the target depth, threshold-plane slice, and different tissue types.
Discussion
This study was the first to characterize tempeature statistics and tissue damage of large-volume MRgHIFU-mediated HT in both superficial and deep targets in vivo. The satisfactory temperature profile within the tROI indicates its potential clinical utility. Most HT sessions showed irreversible tissue damage within the heated (target) volume. The skin and subcutaneous tissues presented no significant damage. Overall, these results demonstrate that large-volume MRgHIFU HT could be clinically feasible and safe with some precautions. Furthermore, the characterization of the heating distributions imply that applying the threshold-plane to different locations/orientations might provide another potential way of shaping temperature distributions.
Generally speaking, temperature statistics obtained from the tROI agree with published studies [Citation39,Citation40,Citation43]. The difference between the achieved and the desired temperature averaged −0.4 °C, and agrees with the requirement in published guidelines of less than 1 °C [Citation42,Citation48,Citation65]. Precision averaged 1.2 °C and uniformity averaged 1.5 °C, both showing that the tROI temperature remained within the hyperthermic range. Temperature uniformity was consistent with the previous studies at 1.4 °C [Citation39].
Some subtle differences were seen when comparing the results to those of previous studies. For example, the higher (worse) precision here compared to previous studies (0.3 °C [Citation39] and 0.8 °C [Citation40]) was expected due to the larger cell size [Citation39,Citation40], more subcells [Citation39], and/or a lower MRI field strength used in our study [Citation39]. The use of mechanical + electronic beam steering to manage seven subcells for the 58-mm target compared to electronic steering only for the 18-mm cell likely lead to an increase in the standard deviation of temperature in the tROI, and thus worse precision. Nonetheless, any differences between this and previous studies may not have clinical significance. The absence of statistically significant differences in temperature metrics between different HT heating conditions (in ) implies the robust and reliable performance of large field HT heating with the MRgHIFU system for both deep and superficial targets.
The CEM43T90 averaged 0.9 min in our study. This is slightly lower than a published clinical guideline of CEM43T90≥1 min for superficial HT [Citation66]. However, a different randomized trial [Citation12] by Jones et al. deemed superficial tumors treatable if a CEM43T90 ≥0.5 min over 1-h HT. Eighteen out of 19 sessions in our study would pass Jones’s threshold. The concept of CEM43T90 has been widely used as a treatment goal in many studies [Citation12,Citation49,Citation51,Citation52,Citation54–56,Citation59] and is one of the quality indicators in guidelines [Citation66,Citation67]. It has been reported to be correlated with positive outcomes: significantly higher complete response [Citation12,Citation54], longer or improved local control [Citation49,Citation52], and longer disease-free survival [Citation54,Citation56] in the treatment of various types of cancer. Therefore, its relationship with the clinical outcome has been mostly revealed. For example, CEM43T90≥1 min was shown to be correlated with longer local survival in cervical cancer [Citation54]. In our study, a relatively higher CEM43T90 (1.2 min in our case) was considered more preferable to a lower one (i.e., 0.4 min). In this way, the characterization of CEM43T90 facilitates the benchmark of this work with previous studies and can potentially help us to determine a clinically-relevant and achievable thermal dose in our subsequent clinical trial. Additionally, as an incorporation of time and temperature, heating duration and target temperature might have a stronger impact for the CEM43T90/CEM43Tavg than the selection of threshold-plane(s). However, part of the goal of the study was to investigate the impact of the threshold-plane on temperature distribution. Thus we presented CEM43T90/CEM43Tavg as an illustration of the impact of the selection of the threshold-plane on the thermal dose. A relatively higher CEM43T90/CEM43Tavg could be obtained by the modification of T90/Tavg, the number of HT fractions, and/or the durations of each fraction, all of which were potentially adjustable. On another note, the selection of threshold-plane may influence the thermal dose (including CEM43T90 and CEM43Tavg) in some cases. For deep HT, CEM43T90 became significantly lower when the threshold-plane included both fields (0.4 ± 0.3 min) compared to the near-field alone (1.2 ± 0.4 min, ). For superficial HT, CEM43Tavg was significantly lower when both near- and far- fields were included in the threshold-plane (16.7 ± 3.7 min) compared to including the near-field alone (7.0 ± 1.5 min, ). The explanation for this might be that when including far-field plane into the threshold-plane, sonication will be disabled more frequently due to the Tmax in the far-field reaching the cutoff temperature, thus providing a lower overall energy delivered to the tROI. The significance of the lower CEM43T90/CEM43Tavg is that it demonstrated using two temperature threshold planes instead of one might decrease the delivered thermal dose (CEM43T90/CEM43Tavg) in the target level. In other words, if CEM43T90/CEM43Tavg were used as the treatment objective, then when using two threshold planes for temperature regulation, a lengthened heating duration or a relatively higher target temperature (i.e., 42.5 °C) might be needed to achieve a preferable CEM43T90 (i.e., 1.2 ± 0.4 min). However, despite a statistical significance, this difference seen in this study might not have clinical significance, potentially due to the relatively smaller sample size and possible anatomical differences between animals and humans. Ultimately, this study highlights that thermal dose may be dependent on several different factors for MRgHIFU systems although clinically-relevant CEM43T90 values may be reasonably achievable for both deep and superficial MRgHIFU HT.
The time to reach the therapeutic HT temperature range is an important practical consideration. Protracted ‘warm up’ times could add considerably to treatment time (i.e., >15–20 min), resulting in more patient discomfort, motion artifacts in the MR thermometry, and challenges in maintaining good patient-to-transducer coupling. Severe instances could warrant interventions such as mild or moderate sedation. Our results show that the overall time needed for Tavg to reach 41 °C was around 4 min, with the longest time of approximately 7 min ( and ). These reasonably short times imply that minimal patient interventions may be needed when treating patients with large field MRgHIFU-mediated HT.
For superficial HT, both in hind leg muscle and in phantom, the dimensions of the TIR10/TIR20/TIR25 along sagittal, horizontal, or vertical axes were found to be significantly shorter (, ) when the far-field or both fields are included in threshold-plane, compared to only applying the threshold-plane to the near-field. This finding informs a potential strategy to adjust the penetration or shape of the heated region by manipulating the locations of the threshold-plane. In cancer treatment, the shape, size, and/or location of the tumor could be variable, depending on its properties (i.e., tumor type, stage, previous treatment, degree of malignancy, invasiveness, route of metastasis, etc.) and its surrounding anatomy. Due to these differences, ideal implementation of HT would have the ability to modulate the heated volume to be conformal to the tumor lesion, while avoiding heating of the normal surrounding areas. Since the tumor volume could vary from patient to patient, the intended heated volume needs to be discussed on a case-by-case or a tumor-by-tumor basis, based on the specific property of each tumor as described above. General speaking, for heating of a large tumor extended from the superficial subcutaneous tissue to the deep-seated region (with a depth of approximately up to 80 mm), then using only near field as the threshold-plane might be optimal. For heating a more superficial tumor, then using both near- and far-field as the threshold planes might be optimal for the protection of the more deep-seated normal tissue. For extremely large tumors, multiple heating events could be used to cover the whole target volume. Although additional work is needed to assess the feasibility and actualization of this approach, manipulation of both the position and threshold values for the threshold-plane might provide the ability to shape the thermal dose distribution, achieving more conformal HT to desired targets and/or avoiding the heating of nearby sensitive tissue structures.
This study found acute myofiber degeneration/necrosis within the heated volume in ten out of sixteen HT sessions. This result differs from our previous work [Citation43], where no permanent tissue damage was found in pelvic targets treated with 18-mm-diameter HT. However, that previous study had a lower average Tmax of 44.7 °C and an average of the maximum temperature of each HT session of 48.7 °C compared to this study (averaged Tmax of 45.7 °C and averaged maximum temperature in each HT session of 50.2 °C), that may explain the observed tissue damage. As for the thermal dose-damages relationship, our CEM43Tavg averaged 12.7 ± 6.1 min in our study, which is slightly lower than the reported thermal damage threshold in normal muscle tissue [Citation60,Citation63]. It was previously reported that CEM43 between 41 and 80 min cause acute but minor damage to muscle, while significant and chronic damage (i.e., hemorrhage and necrosis) resulted from thermal dose of CEM43 higher than 80 min [Citation63]. Ichinoseki-Sekine et al. [Citation60] reported CEM43 of 26 min using microwave did not cause thermal damage, evaluated by blood creatine kinase levels and tissue histology. Several studies have detected muscle damage with a higher thermal dose. At higher thermal dose level, Zderiz et al. reported CEM43 of 283 min caused thermal damage to muscle immediately after the heating; and Mahoney et al. [Citation61] reported that a higher CEM43 (between 650 and 1.9 × 1010 min) caused tissue necrosis in rabbit thigh. Compared to these two studies, our results showed the tissue necrosis or degeneration at a relatively lower thermal dose level (CEM43Tavg of 5.8 min −29.7 min). The reason for the difference might be due to different treatment systems, different temperature measurement methods, and/or a different targeting temperature, among many other factors. Ultimately, it would be desirable to correlate thermal dose with tissue damage, but this relationship might be more comparable when using the same heating modality/system, targeting similar heating dimension, with the same temperature measurement modality, and heating the same type of tissue. To our knowledge, this is the first study that evaluated the large-volume (with the diameter of 58 mm) HT using the MRgHIFU system. In addition, more advanced technology incorporating with a higher spatial/temporal resolution of the MR thermometry, more temperature monitoring plane, and/or better heating control algorithm, might be needed. Additionally, the mild to moderate edema, as seen in two sessions, were all accompanied by myofiber degeneration/necrosis, so we considered it as an accompanying lesion which might not have always been captured in the examined section. Future study is needed to more fully characterize the damage when using large fields, its severity, and potential selective effects on tumor tissue. In terms of clinical utilization, permanent damage in tumor tissues were deemed desirable for cancer therapy, since the necrotic cell death by HT was reported to be able to release tumor antigens in complexes, taken up by dendritic cells, and induce specific anti-tumor immunity [Citation68–71]. These results imply that large-volume MRgHIFU-mediated HT could be clinically implemented with some precautions, such as placement of the target volume largely within the tumor volume.
For the skin and subcutaneous tissues, we did not detect any changes either in contrast-enhanced MRI, gross observation, or histopathological analysis compared to the negative control tissue of each pig. These results imply that the damage to the skin and/or subcutaneous tissue might not be a concern for the HT therapy with target depths utilized in our study. Nevertheless, future work is needed to assess any potential skin damages at other targeting depths or delivered thermal dose, and explore any other potential alternative image-based strategies to assess skin damage in MRgHIFU therapies.
Several limitations need to be mentioned. First, this study only provided histopathology analysis of the tissues within, and 2-cm beyond the tROI and the skin and subcutaneous tissues, without providing quantified geometrical boundaries of the irreversible/reversible damage. This was due to limitations on the volume and geometries of tissues examined in the histological analysis, as H&E staining of the entire large heated volume was not practical. Nonetheless, we believe that this study provides critical information for those wanting to perform such treatments, and these results may serve as a basis for further research. Second, we did not explore the full scope of the six adjustable parameters (acoustic power, HT duration, frequency, HT cell size, target temperature, and different target threshold temperature) provided by the system, or assess the effects of different geometrical arrangements of the different MR planes (distances of the near-field and/or far-field planes from the tROI). Since this study aimed to compare heating effects caused only by the location of threshold-plane, all other factors were kept the same. However, these six parameters could potentially affect the heating performance. For example, theoretically, for deep HT, the acoustic power might need to be increased to compensate for the intensity lost due to the increased depth. This adjustment was not considered in this study, as the aim here was to characterize the temperature distribution and tissue damage with all the parameters maintained the same, and our previous published work [Citation43] and other study [Citation40] suggest 100 W is a reasonable power level to use in this work. Regarding the selection of target temperature, in clinical practice, the target temperature might vary when HT is used for different applications, such as RT, chemotherapy, chemoradiation, or for the delivery of thermo-sensitive agents. For RT + HT, published target goal temperature included 41.0 °C [Citation72], 42.0 °C [Citation73], 42.5 °C [Citation13,Citation74], 43.0 °C [Citation14,Citation75,Citation76]; for chemotherapy, the objective temperatures included 42.0 °C [Citation7,Citation77,Citation78] or the range of 41 °C–43 °C [Citation79,Citation80]; for chemotherapy + RT + HT, one study has the target temperature of 42.5 °C–43 °C (T90) [Citation81]. However, for HT assisted localized drug delivery, the target temperature could be slightly lower (i.e., higher than 40 °C [Citation38,Citation82]), since the blood flow and oxygenation start to raise at a lower temperature (i.e., 39 °C), reach the maximum and decrease again after the temperature become higher than the thermal threshold of stasis [Citation83]. Also, the target temperature is related to the composition of the lipid layer [Citation82,Citation84], with the main purpose of allowing the maximal release of therapeutic agents into the target area. Investigation of the impact of the parameter space on the performance of the MRgHIFU system is outside the scope of this study, although an area for future research.
Third, this study only analyzed the effect of large field HT on porcine muscle and other nearby tissues, and does not provide specific guidance for HT in different tissue types. Different organs and/or disease types might show different responses. Further investigations are needed to assess each organ/tissue of interest in a case-by-case basis.
Fourth, we demonstrated a proof-of-concept in changing the shape of the temperature distribution by manipulating the threshold-plane. To fully understand this strategy and move toward clinical implementation, additional studies are needed to better characterize the temperature/dose distribution and optimize the methodology for conforming thermal dose distributions to desired targets/regions. Some technological limitations of the MRgHIFU system also need to be mentioned. The values of the threshold temperature could not be set to be different in the near-field and the far-field, preventing the ability to use different near- and field-far field temperature thresholds to control the thermal dose distributions. Also, the accessible depth of the MRgHIFU system in HT is 8-cm, limiting the application of MRgHIFU HT in deeper-seated tumors.
Furthermore, the average temperature map shown in presented some heterogeneity within the tROI that mimicked the shape of the subcell. This might be due to the feedback-based temperature control algorithm which only directly heated the tROI by targeting seven subcells subsequently, while the heating to the space outside the subcells were through heating diffusion. Therefore, as a result, the subcells within the tROI seems to have a relatively higher average temperature. However, as we benchmarked the temperature uniformity with previous studies [Citation39,Citation40,Citation43], despite some subtle difference that might be due to the larger tROI size and/or lower MRI field strength, the result is generally in line with previous work, which spoke for the satisfactory temperature profile obtained from this work.
Last but not least, this study did not observe any significant correlation (data not shown) between the existence of the observed contrast-enhanced MRI changes and the thermal damages shown in the histopathology, Tmax, or any quantified thermal dose. There are several potential reasons for this. First, the contrast enhanced MR images immediately after the HT session (within 10 min) might not be sensitive enough to manifest all the histological changes. Second, the collection of the section (in the 2.5 × 2.0 cm tissue cassette) at the heated volume did not cover all the corresponding heated area, so it is possible that the sectioned tissue did not capture the sub-volume changes present in the MR images. Third, the relatively coarsely-sampled pixel size (2.5 mm ×2.5 mm ×7mm) or temporal resolution (3.7 s) of the MR thermometry of the system may impair the ability to correlate the MR thermometry measurement with tissue damage. Future studies are needed to investigate techniques to correlate thermal damage with contrast enhanced MRI and/or temperature measurements.
Nonetheless, this study demonstrates that large-volume MRgHIFU-mediated HT may be feasible for both superficial and deep targets, implying that such devices may be able to treat a broader range of targets and disease sites. Furthermore, the potential ability to achieve spatial temperature control by adjusting the position of threshold-plane might provide a new way of improving the ability to conform the heated volume to the tumor shape and/or modulating the heating pattern to increase the thermal dose to desired target sub-volumes while decreasing/avoiding thermal dose to neighboring healthy tissues. This strategy might allow conforming spatial thermal dose delivery to complement the delivered RT and/or chemotherapy dose distribution when HT is combined with RT and/or chemotherapy. Although additional work is needed to actualize/optimize this strategy, this work could open new horizons in the control and precision of HT as an adjuvant therapy to RT and/or chemotherapy.
Conclusion
MRgHIFU-induced large-volume HT is feasible in both deep (i.e., 6-cm) and superficial (i.e., 2-cm) targets with satisfactory temperature characteristics. Target tissue histology showed some localized tissue damage within the HT volume. No thermal-related skin or subcutaneous tissue damage was observed, implying a satisfactory safety profile of MRgHIFU HT of the skin tissues. The results of this study also demonstrated the potential of using feedback control planes to tailor the spatially delivered thermal dose to clinical targets. This study lays the foundation for the future development of spatially controlled HT.
Disclosure statement
Ari Partanen is a paid consultant for Profound Medical Inc. The other authors have no relevant conflicts of interest to declare.
Additional information
Funding
References
- Januszewski A, Stebbing J. Hyperthermia in cancer: is it coming of age? Lancet Oncol. 2014;15(6):565–566.
- Chicheł A, Skowronek J, Kanikowski M. Thermal boost combined with interstitial brachytherapy in breast conserving therapy - assessment of early toxicity. Rep Pract Oncol Radiother. 2011;16(3):87–94.
- Woeber K. Combination of ultrasound and X-ray radiation in the treatment of cancer. Int J Phys Med. 1960;4:10–19.
- Xia T, Sun Q, Shi X, et al. Relationship between thermal parameters and tumor response in hyperthermia combined with radiation therapy. Int J Clin Oncol. 2001;6(3):138–142.
- Leopold KA, Harrelson J, Prosnitz L, et al. Preoperative hyperthermia and radiation for soft tissue sarcomas: advantage of two vs one hyperthermia treatments per week. Int J Radiat Oncol Biol Phys. 1989;16(1):107–115.
- De Jong MAA, Oldenborg S, Bing Oei S, et al. Reirradiation and hyperthermia for radiation-associated sarcoma. Cancer. 2012;118(1):180–187.
- Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11(6):561–570.
- Overgaard J, Gonzalez DG, Hulshof MCCM, et al. Randomized trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant-melanoma. Lancet. 1995;345(8949):540–543.
- Emami B, Myerson RJ, Cardenes H, et al. Combined hyperthermia and irradiation in the treatment of superficial tumors: results of a prospective randomized trial of hyperthermia fractionation (1/wk vs 2/wk). Int J Radiat Oncol Biol Phys. 1992;24(1):145–152.
- Myerson RJ, Straube WL, Moros EG, et al. Simultaneous superficial hyperthermia and external radiotherapy: report of thermal dosimetry and tolerance to treatment. Int J Hyperth. 1999;15(4):251–266.
- Dunlop PR, Hand JW, Dickinson RJ, et al. An assessment of local hyperthermia in clinical practice. Int J Hyperthermia. 1986;2(1):39–50.
- Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Cin Oncol. 2005;23(13):3079–3085.
- Harari PM, Hynynen KH, Roemer RB, et al. Development of scanned focussed ultrasound hyperthermia: clinical response evaluation. Int J Radiat Oncol Biol Phys. 1991;21(3):831–840.
- Corry PM, Spanos WJ, Tilchen EJ, et al. Combined ultrasound and radiation therapy treatment of human superficial tumors. Radiology. 1982;145(1):165–169.
- Corry PM, Barlogie B, Tilchen EJ, et al. Ultrasound-induced hyperthermia for the treatment of human superficial tumors. Int J Radiat Oncol Biol Phys. 1982;8(7):1225–1229.
- Oleson JR. Eugene Robertson special lecture hyperthermia from the clinic to the laboratory: a hypothesis. Int J Hyperthermia. 1995;11(3):315–322.
- Stauffer PR. Evolving technology for thermal therapy of cancer. Int J Hyperthermia. 2005;21(8):731–744.
- Schlemmer M, Lindner LH, Abdel-Rahman S, et al. [Principles, technology and indication of hyperthermia and part body hyperthermia]. Radiologe. 2004;44(4):301–309.
- Eckert F, Braun LH, Traub F, et al. Radiotherapy and hyperthermia with curative intent in recurrent high risk soft tissue sarcomas. Int J Hyperth. 2018;34(7):980–987.
- Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3(8):487–497.
- Fatehi D, van der Zee J, de Bruijne M, et al. RF-power and temperature data analysis of 444 patients with primary cervical cancer: deep hyperthermia using the Sigma-60 applicator is reproducible. Int J Hyperth. 2007;23(8):623–643.
- Weihrauch M, Wust P, Weiser M, et al. Adaptation of antenna profiles for control of MR guided hyperthermia (HT) in a hybrid MR-HT system. Med Phys. 2007;34(12):4717–4725.
- Wu L, Mcgough RJ, Arabe OA, et al. An RF phased array applicator designed for hyperthermia breast cancer treatments. Phys Med Biol. 2006;51(1):1–20.
- Johnson JE, Neuman DG, MacCarini PF, et al. Evaluation of a dual-arm Archimedean spiral array for microwave hyperthermia. Int J Hyperthermia. 2006;22(6):475–490.
- Juang T, Stauffer PR, Neuman DG, et al. Multilayer conformal applicator for microwave heating and brachytherapy treatment of superficial tissue disease. Int J Hyperthermia. 2006;22(7):527–544.
- Kangasniemi M, McNichols RJ, Bankson JA, et al. Thermal therapy of canine cerebral tumors using a 980 nm diode laser with MR temperature-sensitive imaging feedback. Lasers Surg Med. 2004;35(1):41–50.
- McNichols RJ, Kangasniemi M, Gowda A, et al. Technical developments for cerebral thermal treatment: water-cooled diffusing laser fibre tips and temperature-sensitive MRI using intersecting image planes. Int J Hyperthermia. 2004;20(1):45–56.
- Jordan A, Wust P, Fähling H, et al. Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. Int J Hyperthermia. 2009;25(7):499–511.
- Tasci TO, Vargel I, Arat A, et al. Focused RF hyperthermia using magnetic fluids. Med Phys. 2009;36(5):1906–1912.
- Das P, Colombo M, Prosperi D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf B Biointerfaces. 2019;174:42–55.
- Spirou SV, Basini M, Lascialfari A, et al. Magnetic hyperthermia and radiation therapy: radiobiological principles and current practice. Nanomaterials. 2018;8(6):401.
- Rao W, Deng ZS, Liu J. A review of hyperthermia combined with radiotherapy/chemotherapy on malignant tumors. Crit Rev Biomed Eng. 2010;38(1):101–116.
- Zhu L, Altman MB, Laszlo A, et al. Ultrasound hyperthermia technology for radiosensitization. Ultrasound Med Biol. 2019;45(5):1025–1043.
- Diederich CJ, Hynynen K. Ultrasound technology for hyperthermia. Ultrasound Med Biol. 1999;25(6):871–887.
- Datta NR, Ordóñez SG, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742–753.
- Bakker A, Holman R, Rodrigues DB, et al. Analysis of clinical data to determine the minimum number of sensors required for adequate skin temperature monitoring of superficial hyperthermia treatments. Int J Hyperth. 2018;34(7):910–917.
- Winter L, Oberacker E, Paul K, et al. Magnetic resonance thermometry: methodology, pitfalls and practical solutions. Int J Hyperthermia. 2016;32(1):63–75.
- Partanen A, Yarmolenko PS, Viitala A, et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia. 2012;28(4):320–336.
- Chu W, Staruch RM, Pichardo S, et al. Magnetic resonance-guided high-intensity focused ultrasound hyperthermia for recurrent rectal cancer: MR thermometry evaluation and preclinical validation. Int J Radiat Oncol Biol Phys. 2016;95(4):1259–1267.
- Tillander M, Hokland S, Koskela J, et al. High intensity focused ultrasound induced in vivo large volume hyperthermia under 3D MRI temperature control. Med Phys. 2016;43(3):1539–1549.
- Kothapalli SVVN, Partanen A, Zhu L, et al. A convenient, reliable, and fast acoustic pressure field measurement method for magnetic resonance-guided high-intensity focused ultrasound systems with phased array transducers. J Ther Ultrasound. 2018;6(1):1–8.
- V. V. N. Kothapalli S, Altman MB, Zhu L, et al. Evaluation and selection of anatomic sites for magnetic resonance imaging-guided mild hyperthermia therapy: a healthy volunteer study. Int J Hyperth. 2018;34(8):1381–1389.
- Zhu L, Partanen A, Talcott MR, et al. Feasibility and safety assessment of magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU)-mediated mild hyperthermia in pelvic targets evaluated using an in vivo porcine model. Int J Hyperthermia. 2019;36(1):1147–1159.
- Köhler MO, Mougenot C, Quesson B, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys. 2009;36(8):3521–3535.
- Seward MC, Daniel GB, Ruth JD, et al. Feasibility of targeting canine soft tissue sarcoma with MR-guided high-intensity focused ultrasound. Int J Hyperth. 2018;35(1):205–215.
- Hurwitz MD, Ghanouni P, Kanaev SV, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014;106(5):1–9.
- Shim J, Staruch RM, Koral K, et al. Pediatric sarcomas are targetable by MR-Guided High Intensity Focused Ultrasound (MR-HIFU): anatomical distribution and radiological characteristics. Pediatr Blood Cancer. 2016;63(10):1753–1760.
- Bing C, Staruch RM, Tillander M, et al. Drift correction for accurate PRF-shift MR thermometry during mild hyperthermia treatments with MR-HIFU. Int J Hyperthermia. 2016;32(6):673–687.
- De Bruijne M, Holt B, Van Der Van Rhoon GC, et al. Evaluation of CEM43°CT90 thermal dose in superficial hyperthermia: a retrospective analysis. Strahlenther Onkol. 2010;186(8):436–443.
- Sneed PK, Gutin PH, Stauffer PR, et al. Thermoradiotherapy of recurrent malignant brain tumors. Int J Radiat Oncol Biol Phys. 1992;23(4):853–861.
- Hand JW, Machin D, Vernon CC, et al. Analysis of thermal parameters obtained during phase III trials of hyperthermia as an adjunct to radiotherapy in the treatment of breast carcinoma. Int J Hyperthermia. 1997;13(4):343–364.
- Kroesen M, Mulder HT, van Holthe JML, et al. Confirmation of thermal dose as a predictor of local control in cervical carcinoma patients treated with state-of-the-art radiation therapy and hyperthermia. Radiother Oncol. 2019;140:150–158.
- Leopold KA, Dewhirst MW, Samulski TV, et al. Cumulative minutes with T90 greater than Tempindex is predictive of response of superficial malignancies to hyperthermia and radiation. Int J Radiat Oncol Biol Phys. 1993;25(5):841–847.
- Ohguri T, Harima Y, Imada H, et al. Relationships between thermal dose parameters and the efficacy of definitive chemoradiotherapy plus regional hyperthermia in the treatment of locally advanced cervical cancer: data from a multicentre randomised clinical trial. Int J Hyperth. 2018;34(4):461–468.
- Thrall DE, LaRue SM, Yu D, et al. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res. 2005;11(14):5206–5214.
- Yahara K, Ohguri T, Yamaguchi S, et al. Definitive radiotherapy plus regional hyperthermia for high-risk and very high-risk prostate carcinoma: thermal parameters correlated with biochemical relapse-free survival. Int J Hyperth. 2015;31(6):600–608.
- Dewhirst MW, Sim DA. The utility of thermal dose as a predictor of tumor and normal tissue responses to combined radiation and hyperthermia. Cancer Res. 1984;44(10):4772–4781.
- Dewhirst MW, Connor WG, Sim DA, et al. Importance of minimum tumor temperature in determining early and long-term responses of spontaneous canine and feline tumors to heat and radiation. Cancer Res. 1984;44(1):43–50.
- Oleson JR, Samulski TV, Leopold KA, et al. Sensitivity of hyperthermia trial outcomes to temperature and time: implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys. 1993;25(2):289–297.
- Ichinoseki-Sekine N, Naito H, Saga N, et al. Changes in muscle temperature induced by 434 MHz microwave hyperthermia. Br J Sports Med. 2007;41(7):425–429.
- Mahoney K, Fjield T, Chopra R, et al. MRI-guided gas bubble enhanced ultrasound heating in in vivo rabbit thigh MRI-guided gas bubble enhanced ultrasound heating in in vivo rabbit thigh. Phys Med Biol. 2003;48:223–241.
- Rabkin BA, Zderic V, Crum LA, et al. Biological and physical mechanisms of HIFU-induced hyperecho in ultrasound images. Ultrasound Med Biol. 2006;32(11):1721–1729.
- Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia. 2011;27(4):320–343.
- Zderic V, Foley J, Luo W, et al. Prevention of post-focal thermal damage by formation of bubbles at the focus during high intensity focused ultrasound therapy. Med Phys. 2008;35(10):4292–4299.
- Craciunescu OI, Stauffer PR, Soher BJ, et al. Accuracy of real time noninvasive temperature measurements using magnetic resonance thermal imaging in patients treated for high grade extremity soft tissue sarcomas. Med Phys. 2009;36(11):4848–4858.
- Trefná HD, Crezee H, Schmidt M, et al. Quality assurance guidelines for superficial hyperthermia clinical trials: I. Clinical requirements. Int J Hyperthermia. 2017;33(4):471–482.
- Di Dia A, Maggio A, Gabriele D, et al. Quality indicators for hyperthermia treatment: Italian survey analysis. Phys Med. 2020;70:118–122. (July 2019):
- Frey B, Weiss E-MM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28(6):528–542.
- Cirincione R, Di Maggio FM, Forte GI, et al. High-intensity focused ultrasound– and radiation therapy–induced immuno-modulation: comparison and potential opportunities. Ultrasound Med Biol. 2016;43(2):1–14.
- Ho YJ, Li JP, Fan CH, et al. Ultrasound in tumor immunotherapy: current status and future developments. J Control Release. 2020;323:12–23.
- Mallory M, Gogineni E, Jones GC, et al. Therapeutic hyperthermia: the old, the new, and the upcoming. Crit Rev Oncol Hematol. 2016;97:56–64.
- Varma S, Myerson R, Moros E, et al. Simultaneous radiotherapy and superficial hyperthermia for high-risk breast carcinoma: a randomised comparison of treatment sequelae in heated versus non-heated sectors of the chest wall hyperthermia. Int J Hyperthermia. 2012;28(7):583–590.
- Kapp DS, Petersen IA, Cox RS, et al. Two or six hyperthermia treatments as an adjunct to radiation therapy yield similar tumor responses: results of a randomized trial. Int J Radiat Oncol Biol Phys. 1990;19(6):1481–1495.
- Algan O, Fosmire H, Hynynen K, et al. External beam radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate carcinoma. Cancer. 2000;89(2):399–403.
- Marmor JB, Hahn GM. Combined radiation and hyperthermia in superficial human tumors. Cancer. 1980;46(9):1986–1991.
- Guthkelch AN, Carter LP, Cassady JR, et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial. J Neurooncol. 1991;10(3):271–284.
- Issels RD, Prenninger SW, Nagele A, et al. Ifosfamide plus etoposide combined with regional hyperthermia in patients with locally advanced sarcomas: a phase II study. J Clin Oncol. 1990;8(11):1818–1829.
- Colombo R, Da Pozzo LF, Salonia A, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol. 2003;21(23):4270–4276.
- Wessalowski R, Schneider DT, Mils O, et al. Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non-testicular malignant germ-cell tumours: an open-label, non-randomised, single-institution, phase 2 study. Lancet Oncol. 2013;14(9):843–852.
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Cin Oncol. 2003;21(20):3737–3743.
- Hua Y, Ma S, Fu Z, et al. Intracavity hyperthermia in nasopharyngeal cancer: a phase III clinical study. Int J Hyperthermia. 2011;27(2):180–186.
- Negussie AH, Yarmolenko PS, Partanen ARI, et al. Formulation and characterisation of magnetic resonance imageable thermally sensitive liposomes for use with magnetic resonance-guided high intensity focused ultrasound. Int J Hyperther. 2011;27(2):140–155.
- Van Rhoon GC. Is CEM43 still a relevant thermal dose parameter for hyperthermia treatment monitoring? Int J Hyperth. 2016;6736(May):1–13.
- Mazzotta E, Tavano L, Muzzalupo R. Thermo-sensitive vesicles in controlled drug delivery for chemotherapy. Pharmaceutics. 2018;10(3):150.
