Abstract
Purpose
To evaluate the feasibility, effectiveness, and treatment outcomes of percutaneous radiofrequency ablation (RFA) in the application of intrahepatic recurrent hepatocellular carcinoma (r-HCC) after liver transplantation (LT).
Methods
From April 2008 to December 2019, a total of 37 patients (34 male and 3 female, mean age: 48.7 ± 10.5 years) with 61 r-HCCs after LT treated by RFA as a first-line option were enrolled. The technical success, recurrence-free survival (RFS), overall survival (OS) and complications were evaluated.
Results
After the first session of RFA, three patients were detected with residual foci. All of them received additional session of RFA and two tumors were successfully ablated. Therefore, the technical success was 97.3% (36/37). During the follow-up period, a total of 7 tumors developed local tumor progression (LTP) after 2.2–10.8 months. The LTP rate was 11.7% for r-HCC in the transplanted liver. The median RFS was 4.8 months (95% confidence interval [CI]: 2.2–7.3 months). The 1-, 3-, and 5-year cumulative OS rates were 68.5%, 40.3%, and 40.3%, respectively. Multivariate analyses revealed that tumor size was the only independent predictor for RFS (hazard ratio [HR] = 2.557, 95% CI, 1.015–6.444; p = .046) and limited extrahepatic metastasis was the only independent prognostic factors of OS after RFA for post-LT r-HCC (HR = 4.031, 95%CI, 1.218–13.339; p = .022). Major complications after RFA occurred in two patients (2/37, 5.4%).
Conclusion
Percutaneous RFA is safe and effective for intrahepatic r-HCC after LT, especially for those without limited extrahepatic metastasis.
Introduction
Liver transplantation (LT) is regarded as one of the first-line treatments for patients with early-stage hepatocellular carcinoma (HCC) within Milan criteria [Citation1]. Theoretically, liver transplantation not only eradicates the existing tumors, but also the diseased liver parenchyma in which the tumor developed [Citation2]. When the Milan criteria are fulfilled, the long-term survival after LT for HCC is similar to the LT in patients without HCC [Citation3,Citation4]. However, with the expansion of standard LT criteria, tumor recurrence after LT has increased [Citation5–7]. Previous studies reported that HCC recurrence in the transplanted liver is at 8–20% patients and denotes a poor prognosis of survivals [Citation8,Citation9].
Once recurrent HCC (r-HCC) is diagnosed in the LT patients, clinicians and surgeons face a difficult decision-making process. Treatments for r-HCC after LT have been discussed by previous studies, including surgical resection, transarterial chemoembolization (TACE), and tyrosine kinases inhibitor such as Sorafenib [Citation8,Citation10]. Surgical resection should be the first curative attempt when it is technically feasible, while Sorafenib can be used as systemic therapy in cases with multi-organ recurrence [Citation10]. Both of them have been reported to improve survival in r-HCC patients after LT, but are only applicable to a limited patient cohort due to unresectability or systemic side effects [Citation9,Citation11]. Moreover, the morbidity rate after surgery for post-LT r-HCC is high [Citation9], while TACE produces poor survivals for patients with r-HCC after LT [Citation12].
Percutaneous thermal ablation has been increasingly accepted for its advantages of minimal invasion, favorable efficacy, good reproducibility and cost-effectiveness [Citation13]. According to the Barcelona Clinic Liver Cancer (BCLC) staging system, ablation has been considered as the first-choice treatment to early-stage HCC [Citation1]. Several studies have shown that radiofrequency ablation (RFA), one of the most common used thermal ablation techniques, may provide similar survivals compared to liver resection for patients with HCC or r-HCC no larger than 5.0 cm in diameter [Citation14,Citation15]. Given the difference in oncological characteristics and patterns of recurrence between non-LT and post-LT situations, as well as the specific immunosuppression status of LT patients, whether RFA is still effective for r-HCC after LT remains indefinite. Nevertheless, few published studies focused on this topic [Citation16–19].
The purpose of this study was to evaluate the feasibility, effectiveness, and treatment outcomes of percutaneous ultrasound-guided RFA in the application of intrahepatic r-HCC after LT.
Materials and methods
This retrospective study was performed according to the guidelines of the Helsinki Declaration. The study was approved by the Ethical Committee of the First Affiliated Hospital of Sun Yat-sen University (Guangzhou, China) with the written informed consent waived for this retrospective study.
Patients
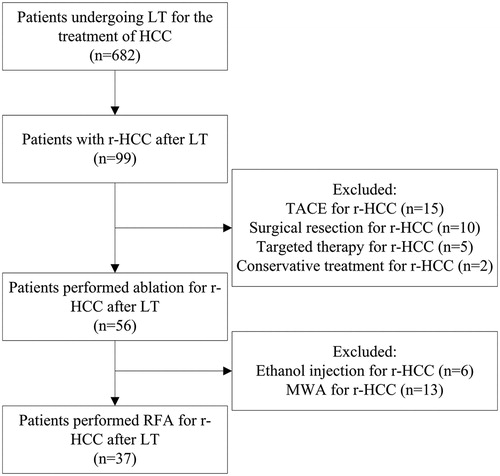
From April 2008 to December 2019, a total of 682 patients underwent LT for the treatment of HCC in our hospital. Among them, 37 patients with 61 intrahepatic r-HCCs after LT treated by percutaneous RFA were included (). The diagnosis of r-HCC was based on pathological analysis or imaging, according to the European Association for the Study of the Liver (EASL) guidelines [Citation20].
The inclusion criteria for RFA were as follows:
Adult patient (18–75 years) with intrahepatic r-HCC after LT.
No more than five tumors with a maximum diameter ≤5 cm.
Absence of significant direct tumor invasion of adjacent organs, or tumor thrombi in the main or lobar portal system.
No extrahepatic metastasis (EM) or limited EM with good control.
Tumors visible by ultrasound and accessible via a percutaneous approach for RFA (i.e., tumors were not adjacent to important structures or organs such as large vessels, biliary ducts, diaphragm, heart or gastrointestinal tract).
Liver function status at Child-Pugh class A or B.
East Coast Oncology Group (ECOG) performance status value 0 or 1.
No severe coagulopathy (e.g., platelets ≥50,000/mL, prothrombine time ratio ≥50%).
The baseline characteristics of the patients are listed in . There were 34 male and 3 female patients. Patients’ ages ranged from 18 to 72 years (mean, 48.7 ± 10.5 years). All of them received deceased donor liver transplantation (DDLT), whereas no patient received living donor liver transplantation (LDLT). Based on explant histopathology, tumors were classified according to Milan criteria, including 10 patients within Milan criteria and 29 patients beyond Milan criteria. All patients had previous liver cirrhosis, which was caused by hepatitis B in 20 patients, hepatitis C in 1 patient, and others in 16 patients. The tumor size of r-HCC in the transplanted liver was 1.9 ± 1.1 cm (0.8–5.0 cm). There were 20 patients with an elevated AFP level before RFA therapy. The mean value of AFP was 2239.6 ± 5519.1 μg/L (range, 2.03–23241.0 μg/L). Nineteen patients had intrahepatic r-HCC within 12 months, and the other 18 patients had intrahepatic r-HCC beyond 12 months. Twenty-three patients had a single HCC and 14 had multiple HCCs. Before RFA, 18 patients suffered limited EM, which were summarized in . Limited EM was defined that tumor had been controlled by systematic therapy before RFA or could be ablated simultaneously [Citation21,Citation22].
Table 1. The baseline characteristics of the patients.
Table 2. The location and treatment for limited EM.
RFA
Percutaneous RFA was guided and monitored by real-time ultrasound (US) using Acuson Sequoia 512 (Siemens Medical Solutions, Mountain View, CA, USA) equipped with a 4V1 vector transducer (frequency range, 1.0–4.0 MHz), or Aplio 500 (Toshiba Medical Systems, Tokyo, Japan) equipped with a PVT-375 BT convex transducer (frequency range, 1.9–6.0 MHz). RFA devices used in this study were LeVeen electrodes (Boston Scientific, Natick, MA), Starburst XL electrodes (RITA Medical Systems, Mountain View, CA) and Cool-tip electrodes (Valleylab, Boulder, CO).
All procedures of RFA were performed under the guidance of real-time ultrasound by two experienced operators (M.K. and X.Y.X., both had experience in tumor ablation over 20 years). RFA was performed with conscious analgesic sedation (intravenous administration of 0.1 mg of fentanyl, 5 mg of droperidol and 0.1 mg of tramadol hydrochloride) and local anesthesia (5 mL of 1% lidocaine). Vital signs were continuously monitored during the procedure. The selection of device was based on the size and location of the tumor. Number of electrodes to be used in ablation was determined according to the tumor size, shape, and location with the aim to achieve an ablative margin at least 0.5 cm beyond the tumor boundary. Multiple overlapping ablation techniques were conducted as appropriate. For tumors adjacent to potentially vulnerable structures, ethanol injection using 21-gauge needle was used to achieve tumor coagulative necrosis, because it is very difficult to create an extensive ablative margin. After ablation procedure was completed, the needle track was carefully treated with the electrode by retracting by 1 cm increments to prevent bleeding and tumor seeding.
Treatment efficacy and follow-up
One month after ablation, contrast-enhanced ultrasound (CEUS) and contrast-enhanced computed tomography (CECT) was performed to evaluate the technical effectiveness. Complete ablation (CA) was defined as a complete non-enhancement of treated tumor on CECT. In the case with viable residual tumor, incomplete ablation (ICA) was defined and additional session of ablation was given. If the residual tumor was still viable after the additional session of RFA, percutaneous ablation therapy was considered a failure and the patients were referred to other therapies.
Thereafter, all the patients were followed up and monitored by conventional ultrasound/CEUS, serum AFP and liver function 3 monthly for the first 2 years, then 6 monthly from 2 to 5 years and 12 monthly after 5 years. Abdominal contrast-enhanced CT or Magnetic Resonance Imaging (MRI) scan was performed if suspicious recurrence was detected on CEUS. Chest radiography, CT of the chest, MRI or bone scan was performed when clinically indicated. When local tumor progression (LTP), intrahepatic distant recurrence or new EM was detected during the follow-up, corresponding treatments were given according to the tumor characteristics, liver function and requests of patients, etc. LTP was defined as the reappearance of enhancing tumor tissue adjacent to the ablated zone after achievement of ablation success [Citation23]. Overall survival (OS) was defined as the time interval from the date of RFA for r-HCC after LT to the date of death or to the date of the last follow-up visit, and ‘event’ associated with it was defined as death. Recurrence free survival (RFS) was defined as the time interval from the date of RFA for r-HCC after LT to new recurrence after RFA or to the date of death or to the date of the last follow-up visit, and ‘event’ associated with it was defined as new recurrence or death [Citation16].
Assessment of complications
Complications were reported using the Dino-Clavien classifications [Citation24]. Major complications were defined as clinical events leading to additional therapeutic interventions or prolonged hospitalization [Citation25]. All other complications are considered as minor complications.
Statistical analysis
Continuous variables are presented as means ± standard deviation with range and 95% confidence interval (CI), and categorical variables were presented as numbers and percentages. Survival analysis of RFS and OS rates were calculated by the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazard model with forward elimination was used to calculate multivariate regression analysis, where the risk factors were selected by univariate analysis determined by the log-rank test. A two-side p value < .05 was considered as statistical significant difference. All the analyses were carried out by SPSS software (version 16.0, SPSS Inc., Chicago, IL, USA).
Results
Treatment efficacy
Forty sessions of percutaneous RFA procedures were performed on 37 patients with 61 post-LT r-HCCs (). The mean session was 1.1 ± 0.3. An adjuvant small dose of ethanol injection was administered in 8 tumors, with the mean ethanol volume at 6.8 ± 3.9 mL (range 3–15 mL). After the first session of RFA, 3 patients were detected with residual foci. Details of the 3 patients were listed in . All of the 3 patients received additional session of RFA and2 tumors were successfully ablated. As a result, the technical success was 97.3% (36/37). Finally, the one remaining patient failed CA after two sessions of RFA was referred to liver resection.
Figure 2. Images in a 60-year-old man received radiofrequency ablation (RFA) for a recurrent hepatocellular carcinoma (r-HCC) in the transplanted liver. A, Pre-interventional B-mode ultrasound showed a tumor sized 1.5 cm × 1.4 cm (arrow). B and C, Contrast-enhanced ultrasound (CEUS) before RFA showed obvious enhancement in the artery phase (B) and washout in the late phase (C) (arrow). D and E, Contrast-enhanced computed tomography (CECT) before RFA showed obvious enhancement in the artery phase (D) and washout in the late phase (E) (arrow). F, Electrode placement during the ablation (arrowhead). G, Tumor appeared completely hyperechoic after the beginning of RFA. H, CEUS obtained 1 month after RFA showed no contrast enhancement, suggesting complete ablation.
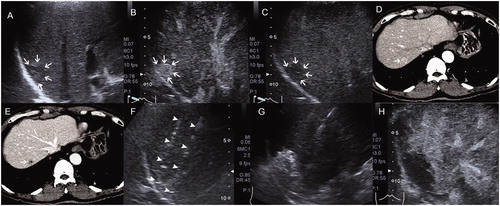
Table 3. The details of the patients with incomplete ablation after the first session of RFA.
Recurrence and treatments
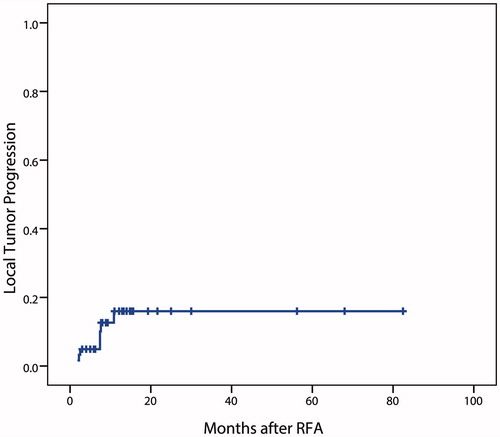
The mean follow-up period after RFA was 17.3 ± 18.8 months (range, 3–82.5 months). The tumors with technical success were evaluated of LTP (n = 60). During the follow-up period, 7 tumors of 7 patients developed LTP after 2.2–10.8 months (mean, 5.7 months). The LTP rate after RFA for r-HCC in the transplanted liver was 11.7% (). Of the patients with LTP, additional RFA was performed on 6 patients and achieved CA in 5 of them. The patient who failed CA received a further session of RFA for LTP. Unfortunately, CA was not achieved after total of two additional sessions of RFA on this patient. Finally, surgical resection was performed. The one remaining patient was performed TACE due to simultaneous multiple intrahepatic recurrences when LTP was detected.
Intrahepatic distant recurrences occurred in 12 patients, EM occurred in 6 patients, and EM plus intrahepatic distant recurrences in 9 patients. The treatments for initial intrahepatic distant recurrences were: RFA in 11 patients, TACE in 4 patients, TACE plus RFA in 2 patients, and TACE plu Sorafenib in 4 patients. Locations of the initial EM after RFA were as follows: lung (n = 6), bone (n = 3), abdominal wall (n = 1), adrenal gland (n = 1), and multiple organs (n = 4). The median RFS was 4.8 months (95% CI: 2.2–7.3 months) (). The RFS rates after RFA at 1 and 2 years were 36.7% and 18.3%, respectively. Univariate analysis showed that tumor size (p = .038) were significantly associated with RFS after RFA in the transplanted liver (). Multivariate analysis demonstrated that tumor size (hazard ratio [HR] = 2.557, 95% CI, 1.015–6.444; p = .046) was the only independent predictor for RFS ().
Figure 4. Recurrence-free survival in patients performed RFA for r-HCC after liver transplantation. A, in all patients; B, comparison between patients with maximum tumor diameter ≤ 3 cm and > 3 cm.
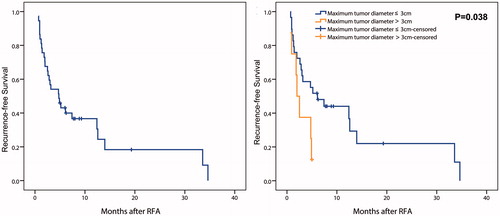
Table 4. Prognostic factors of RFS in patients performed RFA for r-HCC after LT.
Overall survivals
At the end of follow-up, a total of 14 patients died. The causes of death were tumor progression (n = 10, 71.4%), liver failure (n = 3, 21.4%), and gastrointestinal bleeding (including varices rupture) (n = 1, 7.1%). The median OS time was 30.0 months (95% CI: 8.1–51.9 months), with 1-, 3-, and 5-year cumulative OS rates of 68.5%, 40.3%, and 40.3%, respectively (). The median OS of intrahepatic r-HCC only patients (68.0 months, 95% CI: 4.2–131.8 months) was significantly longer than those with intrahepatic r-HCC plus limited EM before RFA (12.0 months, 95% CI: 7.4–16.6 months; p = .019) (). The 1-, 3-, and 5-year OS rates in patients without limited EM were 87.7%, 62.4%, and 62.4%, respectively; while the 1- and 2-year OS in patients with limited EM were 55.6% and 37.1%, respectively. There was a significant difference in OS between patients with and without therapy before LT (p = .032). No significant difference of OS after RFA was detected between patients with LT to recurrence ≤12 months and those with LT to recurrence >12 months (p = .584). Multivariate analyses revealed that only patients with limited EM was the independent prognostic factors of OS after RFA for post-LT r-HCC (HR = 4.031, 95% CI, 1.218–13.339; p = .022) ().
Figure 5. Overall survival in patients performed RFA for r-HCC after liver transplantation. A, in all patients; B, comparison between patients with and without limited extrahepatic metastasis.
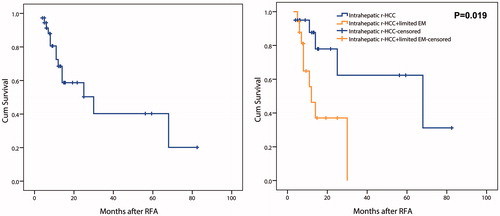
Table 5. Prognostic factors of OS in patients performed RFA for r-HCC after LT.
Complications
There was no procedure-related death. Major complications were observed in two patients (5.4%) including: symptomatic pleural effusion requiring percutaneous drainage in one patient, and liver abscess associated with treatment-related bile duct infection requiring percutaneous drainage in the other patient. Minor complications occurred in 10 (27.0%) patients, including four (10.8%) with fever ≥38.5 °C, two (5.4%) with severe local pain, two (5.4%) with asymptomatic pleural effusion and two (5.4%) with biloma.
Discussion
The results showed that percutaneous US-guided RFA in the treatment of r-HCC after LT can be safely and effectively accomplished, with acceptable local tumor control and survivals. Limited EM before RFA was the independent prognostic factors of OS after RFA, even the EM was under control. It is therefore deemed that RFA is a promising technique for intrahepatic r-HCC after LT, especially for those without limited EM.
Recently, some studies of RFA in the treatment of r-HCC after LT have been published. Dai et al. [Citation18] reported that RFA was adopted in the treatment of 12 patients with intrahepatic r-HCC after LT. No LTP was found one month after treatment in the follow-up. The results of the study conducted by Dai et al. showed that percutaneous RFA in the treatment of r-HCC after LT had the advantages of good efficacy, easy operation, and minimal invasion. In another study, Huang et al. [Citation16] reported 1-, 3-, and 5-year OS after RFA were 87%, 51%, and 28%, respectively. RFA was considered preferable when liver resection was contraindicated or technically infeasible, and RFA provided comparable long-term survival compared with surgery. Other studies also demonstrated that RFA was feasible and effective for r-HCC after LT [Citation16,Citation17]. However, all the reports had either limited number of samples or short period of follow-ups. Recently, in the study of Shi et al. [Citation26], it was reported that a total of 51 patients who underwent thermal ablation for r-HCC after LT, but only 21 patients were treated by RFA. When consider a limited number of patients receiving LT, the present study is considered a relatively large cohort of patients who received RFA for r-HCC after LT.
In present study, we investigated and analyzed the treatment outcome of RFA and the prognostic factors in 37 patients with intrahepatic r-HCC after LT. The 1-, 3- and 5-year OS rates were 68.5%, 40.3%, and 40.3%, respectively. The results were comparable with previous studies, of which 3-year OS rate at 24%–51% was reported [Citation16,Citation17,Citation26]. Moreover, the results of this study revealed that a limited EM before RFA was an independent prognostic factors of OS after RFA. When the analysis was limited to patients without limited EM, the 5-year OS rate was 62.4%, which was superior to the aforementioned studies [Citation16,Citation17,Citation26]. Perhaps, as palliative therapy, there is a limited benefit of RFA for intrahepatic tumors, even the metastases are under control. On the other hand, local therapy such as ablation for intrahepatic tumors with vascular invasion or EM can reduce the patients’ tumor burden, which may improve the survivals [Citation27]. Therefore, further studies are required to evaluate the treatment value of RFA for patients with limited EM. Although time to recurrence after LT and stage of HCC before LT are shown to be a risk factor for survivals in many previous studies [Citation5,Citation26,Citation28], however they were not predictors of survival after RFA in the present study. The difference might be caused by the limited sample size in present study.
For r-HCC after LT, liver resection is considered as the first curative attempt when it is technically feasible. In a systematic review, the reported median survival after liver resection for r-HCC in the transplanted liver was 42 ± 24.45 months [Citation9]. To date, RFA has been studied as a bridge to transplantation in HCC patients in many studies, with generally favorable results when patients are appropriately selected [Citation29,Citation30]. Being demonstrated by the results of this study, RFA has also emerged as an optimal tool in the treatment of r-HCC after LT. Although RFA was not compared with liver resection for r-HCC in the transplanted liver, the survivals after RFA in the present study were comparable with those after liver resection reported by previous studies [Citation9]. In addition, it is worth to mention that RFA is able to be easily and safely repeated many times to treat residual tumors, local recurrence or intrahepatic distant tumors. Compared with surgery, one advantage of RFA is good repeatability. In the further, a case-control study to be performed to compare RFA with liver resection for r-HCC in the transplanted liver.
There were two patients occurred major complications after RFA, one developed symptomatic pleural effusion and required percutaneous drainage and of the other developed liver abscess. Liver abscess is a rare complication of RFA for HCC [Citation31]. Patients with Roux-en-Y reconstruction may be at elevated risk of infection [Citation32]. Welch et al. [Citation32] reported, liver abscess is rare and indeed can occur after percutaneous thermal ablation in the LT,. Perhaps, liver abscess after RFA in the transplanted liver is associated with specific immunosuppression status.
The present study has some limitations. First, it was a retrospective study involving the experience from a single center. It was not able to completely exclude the influence of selection bias in the study. A multicenter study with a larger number of patients is required. Second, this study did not compare the patients performed RFA with other treatments such as liver resection for r-HCC after LT in the preliminary study. Finally, due to advanced stage of HCC, an array of therapies, including target therapy using Sorafenib, chemotherapy, and iodine-125 radioactive seed implantation, were used to control multifocal progression in the extrahepatic sites before and after RFA. Thus, the observed survivals could not be attributed solely to RFA for intrahepatic r-HCC.
In conclusion, percutaneous RFA is safe and effective for intrahepatic r-HCC after LT, especially for those without limited EM. The survival after RFA in patient with limited EM is poor, even though the EM has been controlled.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255.
- Xu DW, Wan P, Xia Q. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria: a review. World J Gastroenterol. 2016;22(12):3325–3334.
- Schwartz M. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127(5):S268–S76.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8(9):765–774.
- Kornberg A, Kupper B, Tannapfel A, et al. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol. 2010;36(3):275–280.
- Roayaie S, Frischer JS, Emre SH, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235(4):533–539.
- Roayaie S, Llovet JM. Liver transplantation for hepatocellular carcinoma: is expansion of criteria justified? Clin Liver Dis. 2005;9(2):315–328.
- Sposito C, Mariani L, Germini A, et al. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59(1):59–66.
- de'Angelis N, Landi F, Carra MC, et al. Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World J Gastroenterol. 2015;21:11185–11198.
- Gabutti A, Bhoori S, Cascella T, et al. Hepatocellular carcinoma recurrence after liver transplantation. Oncology. 2020;34(3):692516.
- Staufer K, Fischer L, Seegers B, et al. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl Int. 2012;25(11):1158–1164.
- Ko HK, Ko GY, Yoon HK, et al. Tumor response to transcatheter arterial chemoembolization in recurrent hepatocellular carcinoma after living donor liver transplantation. Korean J Radiol. 2007;8(4):320–327.
- Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–307.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802.
- Liang HH, Chen MS, Peng ZW, et al. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol. 2008;15(12):3484–3493.
- Huang J, Yan L, Wu H, et al. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? J Surg Res. 2016;200(1):122–130.
- Yang W, Wu H, Zhang ZY, et al. Long-term outcome of percutaneous radiofrequency ablation in recurrent hepatocellular carcinoma after liver transplantation. Int J Hyperthermia. 2018;34(1):68–76.
- Dai X, Zhao HQ, Liu RH, et al. Percutaneous radiofrequency ablation guided by contrast-enhanced ultrasound in treatment of metastatic hepatocellular carcinoma after liver transplantation. Asian Pac J Cancer Prev. 2012;13(8):3709–3712.
- Kim SS, Kang TW, Song KD, Cho SK, et al. Radiofrequency ablation and transarterial chemoembolisation as first-line treatment for recurrent hepatocellular carcinoma or isolated intrahepatic recurrent hepatocellular carcinoma in transplanted livers. Clin Radiol. 2017;72(2):141–149.
- European Association for the Study of the Liver. Electronic address: [email protected]; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Byam J, Reuter NP, Woodall CE, et al. Should hepatic metastatic colorectal cancer patients with extrahepatic disease undergo liver resection/ablation? Ann Surg Oncol. 2009;16(11):3064–3069.
- Poston GJ, Figueras J, Giuliante F, Nuzzo G, et al. Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol. 2008;26(29):4828–4833.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Omary RA, Bettmann MA, Cardella JF, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol. 2003;14(9 Pt 2):S293–S5.
- Shi Y, Chi J, Wang T, et al. Mid-term outcome of percutaneous thermal ablation for intrahepatic recurrent hepatocellular carcinoma after liver transplantation. Clin Radiol. 2019;74:735.e1–735.e7.
- Long J, Zheng JS, Sun B, et al. Microwave ablation of hepatocellular carcinoma with portal vein tumor thrombosis after transarterial chemoembolization: a prospective study. Hepatol Int. 2016;10(1):175–184.
- Sapisochin G, Goldaracena N, Astete S, et al. benefit of treating hepatocellular carcinoma recurrence after liver transplantation and analysis of prognostic factors for survival in a large Euro-American Series. Ann Surg Oncol. 2015;22(7):2286–2294.
- Lee MW, Raman SS, Asvadi NH, et al. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: a 10-year intention-to-treat analysis. Hepatology. 2017;65(6):1979–1990.
- Lu DSK, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41(5):1130–1137.
- Maeda M, Saeki I, Sakaida I, et al. Complications after radiofrequency ablation for hepatocellular carcinoma: a multicenter study involving 9,411 Japanese Patients. Liver Cancer. 2020;9(1):50–62.
- Welch BT, Schmitz JJ, Kurup AN, et al. Feasibility and outcomes of percutaneous thermal ablation of hepatocellular carcinoma in a transplanted allograft. Abdom Radiol. 2018;43(6):1478–1481.