Abstract
Aim
The purpose of this study is to observe the volume change of prostate and laser-ablated lesions in the canine and to explore the mechanism and clinical significance through histopathology.
Materials and methods
Transperineal laser ablation (TPLA) was performed under the guidance of transrectal ultrasound (TRUS) in eight canines. Two canines were sacrificed 1 day and 1 week after TPLA, respectively. The remaining six canines were sacrificed after finishing transrectal contrast-enhanced ultrasound (TR-CEUS) at three phases.
Results
The prostatic volumes immediately following TPLA and 1 week later were larger than before TPLA (20.1 ± 3.9 vs 17.1 ± 3.8 ml; 21.7 ± 3.6 vs 17.1 ± 3.8 ml, p < 0.05), but 1 month later, returned to the preoperative level (17.4 ± 3.2 ml). At three time points, the mean volumes of laser-ablated lesions at 3 W/600 J were 0.6 ± 0.2, 1.1 ± 0.4, and 1.7 ± 0.5 ml, respectively, while those of laser-ablated lesions at 3 W/1200 J were 1.2 ± 0.2, 1.6 ± 0.3, and 2.2 ± 0.5 ml, respectively. The mean volumes of laser-ablated lesions increased significantly over time after TPLA (p < 0.050).
Conclusion
The prostate volume transient enlarges after TPLA, which prompts for clinical application that it should prolong appropriately the duration of urinary catheterization to avoid acute urinary retention. Many inflammatory cells were observed in the laser-ablated lesions and adjacent normal prostate parenchyma through histopathology. It is speculated that the inflammatory response is involved in the progression of tissue damage.
Introduction
Prostate cancer (PCa) is the most commonly diagnosed solid cancer among men, and remains a leading cause of cancer death [Citation1]. For the low risk and localized PCa, focal therapy could reduce the complication of radical prostatectomy, including incontinence, impotence, and damage to surrounding organs [Citation2]. Focal therapy aims to maintain the oncological benefit of active treatment options and reduces the risk of side effects through preserving noncancerous tissues, which possesses advantages of minimally invasiveness, local anesthesia, shorter length of stay in hospital, and fast recovery [Citation3]. Focal therapy of the prostate could be administered by several modalities: high-intensity focused ultrasound (HIFU) [Citation4], cryoablation [Citation5], photodynamic therapy [Citation6], laser ablation [Citation7], radiofrequency ablation (RFA) [Citation8], and microwave ablation (MWA) [Citation9].
Percutaneous laser ablation is performed by delivering the laser light inside the tissue usually via a flexible optical fiber with a small diameter (0.2−0.8 mm). The light absorbed by the tissue is converted into heat, and the short-interval exposure of tissue cells to a temperature of higher than 60 °C causes irreversible damages [Citation10,Citation11]. The percutaneous laser ablation could create accurate, predictable, and reproducible ablation zones and induce minimal changes to the tissues outside the targeted ablation zone. Prostate tissue is particularly well suited for laser ablation due to its optical absorption rate and lack of excessive vascularity [Citation12]. The laser ablation for moderate-risk PCa was found safe and feasible in phases I and II clinical trial [Citation7,Citation13].
Previous literature reported that the application of laser in benign prostate hyperplasia (BPH) mostly focused on the transurethral approach [Citation14], which may result in retrograde ejaculation and complications, such as hematuria and urinary retention, while urethral and bladder neck strictures still occur. Transperineal laser ablation (TPLA) is a novel option for minimally invasive treatment of benign prostatic obstruction [Citation15,Citation16]. TPLA could be performed under local anesthesia. Besides, the transperineal approach avoids damage to the urethral channel. Approximately 50% of men over 50 years of age will show pathological evidence of BPH, and this number increases to 80% in men with an age of more than 80 years old [Citation17]. BPH is a common cause of lower urinary tract obstruction in men, which reduces the quality of life [Citation18]. The symptoms of lower urinary tract obstruction in patients who underwent TPLA were significantly relieved at 3 and 6 months [Citation19]. However, previous studies have reported that the laser-ablated volume in liver tumors has increased immediately after the laser ablation operation [Citation20,Citation21]. Little is known about the volume change of prostate and laser-ablated lesions in a short time after TPLA. The transient enlargement of prostate volume will result in acute urinary retention. The catheterization time for patient underwent prostatic focal therapy varied from 1 to 15 days [Citation19,Citation22].
In this study, it was attempted to observe the dynamic volume change of prostate gland and laser-ablated lesions in canine treated by TPLA and provide valuable information for determining the duration of urinary catheterization and the evaluation time of laser-ablated lesions after TPLA. The volume can be evaluated by transrectal contrast-enhanced ultrasound (TR-CEUS), which had been verified to assess hyperthermal lesion volume in the prostate [Citation23,Citation24].
Materials and methods
Animals
The study protocol was approved by the Ethics Committee of the Center for Drug Safety Evaluation of Zhengzhou University (Zhengzhou, China). Eight adult male beagles with a mating history were purchased from Xi'an Dilepu Biomedical Co., Ltd (Xian, China). The mean age of the beagles was 6.5 ± 0.35 years, and their mean weight was 16.4 ± 2.05 kg. The beagles were kept in the animal center for the Drug Safety Evaluation of Zhengzhou University (Zhengzhou, China). All surgical procedures and experiments were performed under anesthesia, and every effort was made to minimize animal suffering.
Equipment
An ultrasound system equipped with a transrectal dual-plane probe (E14CL4b) and contrasted tuned imaging mode (CnTI) (BK3000; B-K Medical Systems, Inc., Peabody, MA) was used in this study. The frequency of the probe was 4–14 MHz, and the mechanical index was adjusted to 0.06 in the contrast imaging mode.
The Echolaser X4 (Esaote SpA, Genoa, Italy) with an Nd: YAG laser emission source (at a wavelength of 1064 nm) was used for TPLA. The optical laser fiber with a diameter of 300 μm and a length of 20 cm was inserted through the sheath of the guidance needles, and the tip of the laser fiber was in direct contact with the tissue.
Preoperative preparation
The animals were fasted for 12 h before the experiment. Their perineal region was prepared, and a clean enema was performed using glycerin (Hubei Ketian pharmaceutical co. LTD, Hubei, China). An indwelling needle was inserted into the forearm vein for saline infusion.
Anesthesia was induced by the intramuscular injection of dexmedetomidine (8 mg/kg) and Zoletil 50 (2 mg/kg). After 5 min, propofol (3 mg/kg) was injected intravenously. The beagle was then endotracheally intubated and connected to a respirator (Ruiwode life technology co. LTD, Shenzhen, China). Anesthesia was maintained with 2–3% aerosolized sevoflurane (Shanghai Hengrui Pharmaceutical Co., Ltd, Shanghai, China) in oxygen during the procedure. All beagles were placed in a supine position and the body temperature was maintained with a warmed blanket. An animal-specific urinary catheter was placed into the bladder of each beagle. The disinfection and draping of the local perineal area were then performed.
Ablation experiment
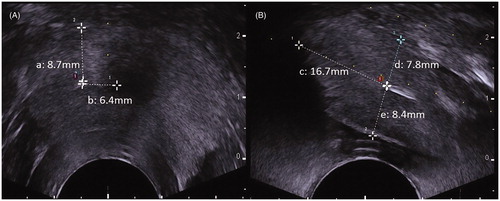
TPLA was performed by a physician with over 5 years of ablation experience. Under the transrectal ultrasound (TRUS) guidance, a 21-Gauge guidance needle was first inserted into each lobe of the prostate via the transperineal approach. The optical laser fiber was placed through the guidance needle, and 10 mm of the nude fiber tip was exposed to achieve direct contact with the prostate gland tissue before the fiber was connected to a continuous-wave emission source. The distance from the tip of the laser fiber to the outer wall of the urethra, the rectum, and the bladder wall was more than 6 mm, while the distance from the tip of the laser fiber to the bladder floor was more than 15 mm (). The ablation process was continually monitored by TRUS. Each treatment was performed with a fixed power (3 W), and the total delivered energy was 600 joule (J) and 1200 J in the left and right prostate lobes, respectively. Therefore, the ablation time was determined by the fixed power and the total output energy. The TPLA was performed in eight adult beagles resulting in sixteen laser-ablated lesions. Two beagles were sacrificed to observe the pathological information 1 day after TPLA and 1 week later, respectively. Twelve laser-ablated lesions in remaining six beagles were used to observe the dynamic volume change immediately, 1 week and 1 month after TPLA. Therefore, there were six samples available for comparison volume at different periods in each group, including prostate gland and laser-ablated lesions group (3 W/600J and 3 W/1200J).
Figure 1. The position and orientation of the laser applicator on transrectal ultrasound images (A, transverse section; B, longitudinal section). The distances from the tip of the laser applicator to prostatic capsule (a, d), the outer wall of the urethra (b), the rectum wall (e), and the bladder floor (c).
Transrectal contrast-enhanced ultrasound (TR-CEUS) examinations were conducted to image the prostate and laser-ablated lesions at about 10 min after TPLA. The details of TR-CEUS examinations were described in the previous article [Citation25]. Briefly, the machine was adjusted to the contrast mode with low mechanical index (0.06). A bolus injection of 0.06 ml/kg of SonoVue (Bracco Group, Milan, Italy) was carried out through the indwelling catheter at the forearm vein, and 5 ml of normal saline was used to flush the catheter. TR-CEUS examinations were repeated 1 week and 1 month after TPLA.
The range of laser-ablated lesions was bounded by no enhancement area on the TR-CEUS images. Three biggest dimensions (a, b, c) of each prostate and laser-ablated lesion were measured on the TR-CEUS images, respectively. The volume (V) of the prostate gland and laser-ablated lesions were calculated according to the formula: V = 1/6 × π × a × b × c.
During the TPLA procedure and recovery from anesthesia, the heart rate, breathing, and body temperature of the animals were closely monitored. The beagles were fed regularly after 6 h of monitoring. Each beagle was intramuscularly administered with 80 mg/kg of gentamicin (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) once a day in the morning for 1 week following TPLA to avoid infection. The signs of hematuria and haematochezia were closely monitored. Two beagles were sacrificed 1 day and 1 week after TPLA, respectively, and the remaining six beagles were sacrificed 1 month after TPLA by using an intravenous injection of sodium pentobarbital (30 mg/kg).
Histopathological examination
Necropsy and gross pathological inspections were performed immediately after each beagle was sacrificed. The prostate gland and its surrounding structures (bladder, seminal vesicle gland, and rectum) were carefully inspected. The prostate was removed and fixed in the 10% buffered formaldehyde solution for 24 h. The prostate glands of the first two beagles were sliced in the sagittal section along the laser fiber path. However, the prostate glands of the six remaining beagles were sliced in the transverse section at 5 mm intervals perpendicular to the urethra. Then, the sliced specimens were paraffin-embedded, and 5-μm-thick sections were prepared and stained with hematoxylin-eosin (HE) for later optical microscopic evaluation, including necrosis, hemorrhage, and hyperemia.
All histopathological specimens were evaluated by two experienced pathologists. In addition, the liquid in all the laser-ablated lesions was aspirated and examined by HE staining, bacterial culturing (Becton, Dickinson and Company, America), and complete blood counting. The quantity of white blood cell, polykaryocyte, and monocyte in the cystic fluid was analyzed using automatic blood cell analyzer (Shanghai Xiesen Meikang Medical Electronics Co. Ltd, China).
Statistical analysis
The SPSS 22.0 software (IBM, Armonk, NY) was used to perform the statistical analysis. Quantitative data were expressed as mean ± standard deviations (M ± SDs). The comparison of volumes of prostate and laser-ablated lesions at different time points was done using paired t-tests. p < 0.050 was considered statistically significant.
Results
TPLA procedures in all beagles were performed successfully with no major complications. No signs of hematuria, haematiochezia, and infection were found during the 1-month follow-up.
Characterization of laser-ablated lesions on TRUS and TR-CEUS
Real-time TRUS can be used to visualize needle insertion and guide laser fiber deployment. The laser-ablated lesions appeared as gradually expanded hyperechoic areas located in front of the laser fiber tip during the ablation progress. Immediately after TPLA, the shape of the laser-ablated lesion was irregular without a clear boundary (). However, 1 week and 1 month after TPLA, the laser-ablated lesions become hypoechoic areas with relatively clear margins, which may be contributed by the formation of lesion cyst ().
Figure 2. The prostate gland and laser-ablated lesions in beagles prostate were evaluated by TRUS Images (A, B, C, D) and TR-CEUS images (a, b, c, d) before and after TPLA. TPLA: transperineal laser ablation.
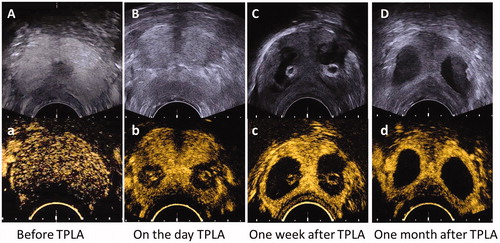
After TPLA, each laser-ablated lesion was observed as an avascular defect area within the normal perfused gland on TR-CEUS image. The shape of laser-ablated lesions was round on the transverse and elliptic on the longitudinal section images, so the shape of it was ellipsoidal on the whole, which has been described in the previous study [Citation25]. The follow-up TR-CEUS examination carried out 1 week and 1 month after TPLA displayed the laser-ablated lesions with a demarcated boundary ().
Dynamic volume changes of the prostate gland and laser-ablated lesions over time
The volumes of prostate glands and laser-ablated lesions were measured by TRUS and TR-CEUS in the remaining six beagles, respectively. Before TPLA, the mean volume of prostate glands was 17.1 ± 3.8 ml. The mean volumes of prostate glands were 20.1 ± 3.9 ml and 21.7 ± 3.6 ml immediately and 1 week after TPLA, respectively, which were significantly larger than the baseline value (p < 0.050). The volume of the prostate returned to the baseline level (17.4 ± 3.2 ml) 1 month after TPLA ().
Table 1. Volumes of the prostate gland and laser-ablated lesions before and after TPLA in beagles.
At the three time points, the mean volumes of the laser-ablated lesions at 3 W/600J were 0.6 ± 0.2, 1.1 ± 0.4, and 1.7 ± 0.50 ml, respectively, while the mean volumes of lesions at 3 W/1200J were 1.2 ± 0.2, 1.6 ± 0.3, and 2.2 ± 0.5 ml, respectively. The volumes of all laser-ablated lesions increased significantly over time within 1 month after TPLA (p < 0.050) ().
Histopathological evaluation of laser-ablated lesions in canine prostate
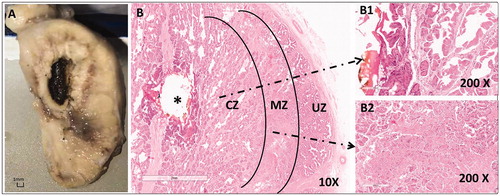
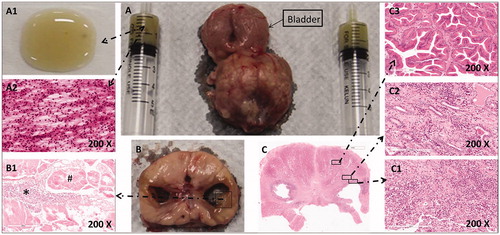
Regarding the first beagle sacrificed 1 day after TPLA, the gross inspection revealed that the urethra, the rectum, and the seminal vesicle appeared normal in situ, and the prostate capsule showed bleeding bands indicating acute hyperemia. The laser-ablated thermal lesion within the prostate appeared as an oval-shaped dark brown area in the center surrounded by a yellowish band (). The laser-ablated lesions were shown in three zones: the coagulative zone (CZ), marginal transition zone (MZ), and untreated zone (UZ), in the direction from the lesion center () to the peripheral normal prostate tissue in the images of HE staining observed under the microscope ().
Figure 3. The pathological images of beagle prostate were captured 1 day after TPLA (3 W/600J). (A) A photo of the formalin-fixed specimen showing a coagulated/necrotic lesion. (B) A microscopic photo of the prostate with HE staining demonstrating three zones of the laser-ablated lesion, i.e. the coagulative zone (CZ), the marginal zone (MZ), and the untreated normal zone (UZ), under low magnification (10 ×). Note that B1 and B2 are photos of CZ and MZ observed under high magnification (200 ×). TPLA: transperineal laser ablation.
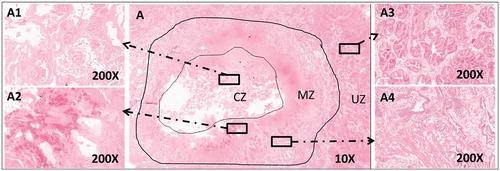
The gross inspection of the beagle sacrificed 1 week after TPLA showed findings similar to those of the first beagle. However, a small amount of fluid was seen in the center of the laser-ablated lesion. The microscopic images of the HE staining of the specimens showed the three zones of lesions. The inner layer of MZ close to CZ showed a bleeding band, while the out layer of MZ close to UZ was infiltrated with inflammatory cells, including neutrophils, macrophages, and lymphocytes ().
Figure 4. The microscopic images of HE staining of beagle prostate one week after TPLA (3 W/600J). (A) A low magnification (10 ×) view of the prostate showed three zones (CZ, MZ, UZ) of the ablated lesion. A1–A4: A high magnification (200 ×) view from A demonstrated the structure of each zone in details. CZ: coagulative zone; MZ: marginal zone; TPLA: transperineal laser ablation; UZ: untreated zone.
Regarding the remaining six beagles scarified 1 month later, the gross specimens of the prostate appeared plumpness. All laser-ablated lesions eventually developed into liquefaction cavity. The liquid was yellow-brown and viscous (). Many inflammatory cells were found in the liquid on the HE staining images, including neutrophils, lymphocytes, and macrophages (). No bacterial growth was seen in the fluid after 72 h of culturing. There were many inflammatory cells infiltrated through the cavity wall and the normal prostate tissue (). The quantity of white blood cell, polykaryocyte, and monocyte in the cystic fluid were 50.1 ± 54.9 × 109/L, 7.8 ± 1.3 × 109/L, and 1.5 ± 0.9 × 109/L, respectively.
Figure 5. The pathological images of a beagle prostate were captured 1 month after TPLA (left 3w/600J, right 3w/1200J). (A) A prostate specimen with urinary bladder and fluid aspirated from the cavity of the ablated lesion. A1. The liquid appeared yellowish and sticky on the glass slide. (A2) The HE staining image of the liquid under high magnification (200 ×) showed unstructured necrotic debris and inflammatory cells. (B) A cross-sectional view of the prostate gland with an ablated lesion on each lobe. (B1) The HE staining image of the cavity wall showed necrotic tissues (#) and inflammatory cells (*) without a definite structure. (C) The HE staining image of a prostate section under low magnification (10 ×). C1. Inflammatory cells were seen on the wall of the cavity under high magnification (200 ×). C2. Inflammatory cells and normal prostate gland were seen in the MZ. C3. Normal prostate gland structures were seen in the NZ under high magnification (200 ×).
Discussion
The TPLA operation the prostate in all beagles was guided by TRUS and was performed successfully. There were no signs of hematuria and haematochezia in these beagles during the 1-month follow-up. Besides, the organs adjacent to the prostate appeared normal.
In this study, real-time TRUS was able to precisely guide the laser fiber placement and monitor the entire ablation procedure, which was consistent with the previous report [Citation26]. The prostate gland was clearly observed and could be precisely evaluated through the TRUS. However, the region of the laser-ablated lesion appeared as a hyperechoic area without a regular shape or a clear boundary in the TRUS during the process of TPLA. One week and 1 month later, the laser-ablated lesions were demarcated from surrounding normal tissue and were observed as low echo region in the follow-up TRUS examination, which may be contributed by the formation of lesion cyst. Previous studies have shown that the TR-CEUS imaging can be used to accurately evaluate the size of the radiofrequency ablated lesion in the prostate, which was consistent with pathological results [Citation27]. The microbubble-based contrast agent could enhance the contrast of normal viable prostate tissues but could not enter the ablated coagulated or necrosis tissues [Citation28]. In this experiment, the peripheral blood vessels of prostate, prostatic capsule, and prostatic parenchyma enhanced gradually in sequence after ten seconds injection of the SonoVue, and the intensity peaked around thirty seconds. At the same time, the laser-ablated lesions were observed as an avascular defect area around with enhanced rims in the normal perfused gland. The enhanced rims may be the hyperemia zone according to our histopathology results and previous literatures [Citation21]. The shape of laser-ablated lesions was ellipsoidal, which has been described in previous study [Citation25]. In addition, the TPLA needle channel was bright in TR-CEUS, which was the same echo as the prostatic capsule in the two-dimensional ultrasonography and was verified as the carbonized tissue through the histopathology, so this “enhanced” was false appearance. This result reminds that the bright needle channel was not really enhanced and there was no survival tissue in clinical application. Some low and high echo tissue was observed in transrectal two-dimensional ultrasonography but did not enhance in TR-CEUS, which was proved to be necrotic and carbonized tissue through histopathology. TR-CEUS is a feasible tool to evaluate the transperineal laser-ablated lesion in prostate operation and follow up.
It was found that the volume of the prostate gland became enlarged immediately after TPLA, reached the peak 1 week later, and returned to the baseline level 1 month later. The initial findings of this animal experiment were analyzed based on histopathological examinations. According to the histopathological examinations carried out 1 day and 1 week after TPLA, the laser-ablated tissues became coagulated and necrotic, and the tissues were clearly demarcated from the surrounding normal tissues. There were bands of bleeding and inflammatory responses between the laser-ablated lesions and normal tissues. The findings of this study were consistent with those in previous reports of laser ablation on canine prostate [Citation29] and other solid organs [Citation30]. Previous studies suggested that inflammatory reactions occurred after thermal ablation, along with increased vascular permeability in normal liver tissues and resulting leakage of the plasma, platelets, and inflammatory cells into the interstitial tissues [Citation31]. Therefore, the increased volume of the prostate observed after thermal ablation may be caused by these reasons. In addition, the pathological findings of this study revealed that the inflammatory cells not only appeared around the laser-ablated sites but also in the normal prostate tissues, which verified the fact that inflammatory cells leaked through blood vessels. In previous reports, the prostate volume was closely related to acute urinary retention, lower urinary tract symptoms, and bladder outlet obstruction from [Citation32–34]. The enlargement of the prostate volume could cause urinary compression, resulting in the symptoms of urinary retention. Based on the results of this study, the urinary catheter should be placed and kept for at least 1 week or longer after TPLA to avoid acute urinary retention.
The volume of laser-ablated lesions gradually increased in the beagles’ prostate in the 1-month period. Previous literature reported that the volume of laser-ablated liver tumors increased in a short period [Citation20,Citation21]. The heat-ablated lesions can be considered as having three zones: the CZ, which is located immediately beyond the application tip and undergoes ablation-induced coagulative necrosis, the marginal zone (MZ) of the sublethal hyperthermia, which is mostly caused by the thermal conduction of the central area either undergoing apoptosis or recovering from reversible injury, and the untreated normal zone (UZ) of surrounding tissues [Citation35]. Chu et al. [Citation36] elaborated that the heat-ablated lesions are associated with direct cellular damages and indirect or delayed cellular damages. The direct cellular damages may be immediate and caused by the hyperthermal conditions. The definition of hyperthermal was to heat target tissue rapidly, to achieve ablative temperatures (> 55 °C, but often > 80 °C) in a few seconds. The indirect or delayed cellular damages after hyperthermal had been reported in a previous study. Mehrdad Nikfarjam et al. reported that an increase in tissue injury following laser ablation in mouse liver, with finding that sublethal injury to the endothelium may lead to alterations in blood flow and microvascular damage and microthrombus formation, which participated in the progression of tissue injury [Citation37]. Besides, the pro-inflammatory cytokines that were released from the tissue of the MZ zone may aggravate the indirect damage. The histopathological observation that many inflammatory cells were in the liquefaction and cavity wall may support this hypothesis. These need further study.
All laser-ablated lesions in the beagle prostate eventually developed into a cystic cavity in this study. This phenomenon was observed in the canine prostate according to previous study [Citation38]. Cowan et al. [Citation39] believed that the canine prostate contains a large amount of glandular tissues which produce and store prostatic secretions, which is different from human. The tissues undergo coagulative necrosis to destroy the vascular system. Therefore, the necrotic materials and the prostatic secretions cannot be transported and absorbed in time. Ultimately, the ablated lesions develop into the liquefaction cavity. However, this phenomenon was also observed in clinical application. Madersbacher et al. [Citation40] discovered that intraprostatic cystic lesions were observed in some patients from 6 to 12 weeks after high-intensity focused ultrasound, and 92% of patients underwent transient urinary retention. Zvara et al. [Citation41] demonstrated that the cavity formed at 1 to 3 weeks after intraprostatic absolute ethanol injection. A clinical trial of the management of BPH with high-energy transurethral microwave therapy [Citation22] showed that intraprostatic cavities were found in 35 of 83 patients (42%) and the presence of a cavity was positively correlated positively with improvement in urinary performance and relief of bladder outlet obstruction. The mean improvement in peak urine flow rate in patients with a cavity was better than in patients without a cavity at 3-month follow-up evaluation (8.5 ± 7.3 vs 4.8 ± 5.4 ml/s) [Citation42]. So, the dynamic changes after TPLA should be monitored closely. Once the ablated lesions develop into liquefaction, the aspiration or drainage should be performed as soon as possible to reduce the pressure on the urethra and to reduce the inflammatory response. One question that remains is whether or not anti-inflammatory drugs can be used to reduce the inflammatory response after TPLA. Dexamethasone was administered postoperative to reduce edema according to the recent protocol about TPLA in BPH [Citation16]. This question needs to be verified in the clinical application.
Several points can be learned from this study. Firstly, the CEUS was able to measure the volume of TPLA for the energy levels investigated in this study. The laser-ablated lesions in prostate were demarcated from surrounding normal prostate tissue. Secondly, the changes in the prostate volume after TPLA will provide a reference for determining the duration of urinary catheterization. The transient enlargement of the prostate size could cause urinary compression, resulting in the symptoms of acute urinary retention, which prompts for clinical application that it should prolong appropriately the duration of urinary catheterization to avoid acute urinary retention. Thirdly, this study showed that the volume of laser-ablated lesions increased over time. The inflammatory response may be involved in the process of laser ablated lesion enlargement post-procedure, and possibly the administration of anti-inflammatory drugs may reduce this effect. However, the above assumptions have not been proven in patients, so further clinical trials should be carried out.
There were some limitations to this study. The sample size of the animals was small, and there was only one canine used for the histopathological examinations done at 1 day and 1 week after TPLA. Besides, the observation time in this study was only up to 1 month, which was not long enough to predict long-term changes.
Disclosure statement
All authors have completed and submitted the ICMJE form for disclosures of potential conflicts of interest and none were reported.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30.
- Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436–445.
- Sanchez-Salas R, de la Rosette J, Polascik TJ, et al. Focal therapy for prostate cancer: a more vehement view of the approach could translate into real benefits for our patients. Eur Urol. 2018;74(5):537–539.
- Ghai S, Perlis N, Lindner U, et al. Magnetic resonance guided focused high frequency ultrasound ablation for focal therapy in prostate cancer - phase 1 trial. Eur Radiol. 2018;28(10):4281–4287.
- Guo Z, Si T, Yang X, et al. Oncological outcomes of cryosurgery as primary treatment in T3 prostate cancer: experience of a single centre. BJU Int. 2015;116(1):79–84.
- Azzouzi AR, Vincendeau S, Barret E, et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017;18(2):181–191.
- Natarajan S, Raman S, Priester AM, et al. Focal laser ablation of prostate cancer: phase I clinical trial. J Urol. 2016;196(1):68–75.
- Shariat SF, Raptidis G, Masatoschi M, et al. Pilot study of radiofrequency interstitial tumor ablation (RITA) for the treatment of radio-recurrent prostate cancer. Prostate. 2005;65(3):260–267.
- Strohmaier WL, Bichler KH, Bocking A, et al. Histological effects of local microwave hyperthermia in prostatic cancer. Int J Hyperthermia. 1991;7(1):27–33.
- Ben-Tuvim N. Laser-light amplification by stimulated emission of radiation. Harefuah. 1963;65:191–194.
- Izzo F. Other thermal ablation techniques: microwave and interstitial laser ablation of liver tumors. Ann Surg Oncol. 2003;10(5):491–497.
- Lindner U, Trachtenberg J, Lawrentschuk N. Focal therapy in prostate cancer: modalities, findings and future considerations. Nat Rev Urol. 2010;7(10):562–571.
- Eggener SE, Yousuf A, Watson S, et al. Phase II evaluation of magnetic resonance imaging guided focal laser ablation of prostate cancer. J Urol. 2016;196(6):1670–1675.
- Costello AJ, Bowsher WG, Bolton DM, et al. Laser ablation of the prostate in patients with benign prostatic hypertrophy. Br J Urol. 1992;69(6):603–608.
- Patelli G, Ranieri A, Paganelli A, et al. Transperineal laser ablation for percutaneous treatment of benign prostatic hyperplasia: a feasibility study. Cardiovasc Intervent Radiol. 2017;40(9):1440–1446.
- van Kollenburg RAA, van Riel L, Bloemen PR, et al. Transperineal laser ablation treatment for lower urinary tract symptoms due to benign prostatic obstruction: protocol for a prospective in vivo pilot study. JMIR Res Protoc. 2020;9(1):e15687.
- Berry SJ, Coffey DS, Walsh PC, et al. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132(3):474–479.
- Lepor H. Pathophysiology of benign prostatic hyperplasia in the aging male population. Reviews in Urology. 2005;7 (Suppl 4):S3–S12.
- Pacella CM, Patelli G, Iapicca G, et al. Transperineal laser ablation for percutaneous treatment of benign prostatic hyperplasia: a feasibility study. Prostate Cancer Prostatic Dis. 2020;23(2):356–363.
- Vogl TJ, Naguib NN, Eichler K, et al. Volumetric evaluation of liver metastases after thermal ablation: long-term results following MR-guided laser-induced thermotherapy. Radiology. 2008;249(3):865–871.
- Sequeiros RB, Kariniemi J, Ojala R, et al. Liver tumor laser ablation - increase in the subacute ablation lesion volume detected with post procedural MRI. Acta Radiol. 2010;51(5):505–511.
- Tay KJ, Cheng CWS, Lau WKO, et al. Focal therapy for prostate cancer with in-bore MR-guided focused ultrasound: two-year follow-up of a phase I trial-complications and functional outcomes. Radiology. 2017;285(2):620–628.
- Atri M, Gertner MR, Haider MA, et al. Contrast-enhanced ultrasonography for real-time monitoring of interstitial laser thermal therapy in the focal treatment of prostate cancer. Can Urol Assoc J. 2009;3(2):125–130.
- Cheng HL, Haider MA, Dill-Macky MJ, et al. MRI and contrast-enhanced ultrasound monitoring of prostate microwave focal thermal therapy: an in vivo canine study. J Magn Reson Imaging. 2008;28(1):136–143.
- Liu R, Duan S, Cao H, et al. A pilot study of the shapes of ablation lesions in the canine prostate by laser, radiofrequency and microwave and their clinical significance. PLoS One. 2020;15(4):e0223229.
- Onik GM, Cohen JK, Reyes GD, et al. Transrectal ultrasound-guided percutaneous radical cryosurgical ablation of the prostate. Cancer. 1993;72(4):1291–1299.
- Feng C, Hu B, Hu B, et al. Comparative study of conventional US, contrast enhanced US and enhanced MR for the follow-up of prostatic radiofrequency ablation. Exp Ther Med. 2017;13(6):3535–3542.
- Liu JB, Merton DA, Wansaicheong G, et al. Contrast enhanced ultrasound for radio frequency ablation of canine prostates: initial results. J Urol. 2006;176(4 Pt 1):1654–1660.
- Stafford RJ, Shetty A, Elliott AM, et al. Magnetic resonance guided, focal laser induced interstitial thermal therapy in a canine prostate model. J Urol. 2010;184(4):1514–1520.
- Di Matteo F, Martino M, Rea R, et al. EUS-guided Nd:YAG laser ablation of normal pancreatic tissue: a pilot study in a pig model. Gastrointest Endosc. 2010;72(2):358–363.
- Bulvik BE, Rozenblum N, Gourevich S, et al. Irreversible electroporation versus radiofrequency ablation: a comparison of local and systemic effects in a small-animal model. Radiology. 2016;280(2):413–424.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158(2):481–487.
- Vesely S, Knutson T, Damber JE, et al. Relationship between age, prostate volume, prostate-specific antigen, symptom score and uroflowmetry in men with lower urinary tract symptoms. Scand J Urol Nephrol. 2003;37(4):322–328.
- Kang MY, Ku JH, Oh SJ. Non-invasive parameters predicting bladder outlet obstruction in Korean men with lower urinary tract symptoms. J Korean Med Sci. 2010;25(2):272–275.
- Ahmed M, Brace CL, Lee FT, et al. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351–369.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- Nikfarjam M, Muralidharan V, Malcontenti-Wilson C, et al. Progressive microvascular injury in liver and colorectal liver metastases following laser induced focal hyperthermia therapy. Lasers Surg. Med. 2005;37(1):64–73.
- Peters RD, Chan E, Trachtenberg J, et al. Magnetic resonance thermometry for predicting thermal damage: an application of interstitial laser coagulation in an in vivo canine prostate model. Magn. Reson. Med. 2000;44(6):873–883.
- Cowan DF, Orihuela E, Motamedi M, et al. Histopathologic effects of laser radiation on the human prostate. Modern pathology: an official journal of the United States and Canadian Academy of. Pathology, Inc. 1995;8:716–721.
- Madersbacher S, Kratzik C, Susani M, et al. Tissue ablation in benign prostatic hyperplasia with high intensity focused ultrasound. J Urol. 1994;152(6 Pt 1):1956–1960; discussion 60.
- Zvara P, Karpman E, Stoppacher R, et al. Ablation of canine prostate using transurethral intraprostatic absolute ethanol injection. Urology. 1999;54(3):411–415.
- de Wildt MJ, Debruyne FM, de la Rosette JJ. High-energy transurethral microwave thermotherapy: a thermoablative treatment for benign prostatic obstruction. Urology. 1996;48(3):416–423.
