Abstract
Purpose
To compare the efficacy and safety of stereotactic body radiotherapy (SBRT) with radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC).
Materials and methods
PubMed, MedLine, EMBASE, the Cochrane Library and Web of Science were searched to identify potentially eligible studies comparing the efficacy and safety of SBRT with RFA for HCC from January 1990 to May 2020. Hazard ratios (HRs) or odds ratios (ORs) with 95% confidence intervals (CIs) were used to determine the effect size for overall survival (OS), local control (LC) and complications.
Results
Seven studies including 7928 patients were enrolled in this meta-analysis. The results showed that SBRT was not inferior to RFA based on the pooled HR for OS (HR = 1.09, 95%CI = 0.78–1.52, p = .62); however, the pooled HR for the LC rate showed the superiority of SBRT (HR = 0.54, 95%CI = 0.35–0.84, p = .006). Subgroup analysis showed that the pooled HR for the LC rate favored SBRT in patients with tumors sized >2 cm (HR = 0.41, 95%CI = 0.23–0.74, p = .003), but no significant difference was observed in patients with tumors sized ≤2 cm (HR = 0.56, 95%CI = 0.25–1.28, p = .17). In addition, no significant differences in the incidence of late severe complications were observed between the SBRT and RFA groups (OR = 1.01, 95%CI = 0.59–1.73, p = .97).
Conclusions
Based on the current data, we concluded that SBRT was well tolerated with an OS equivalent to that with RFA; SBRT was superior to RFA in terms of LC of HCC, especially in those with tumors sized >2 cm.
Introduction
The incidence of hepatocellular carcinoma (HCC) is continuously increasing worldwide, with 78,200 patients newly diagnosed per year; yet, the long-term prognosis remains poor [Citation1]. Surgical resection is still the preferred strategy for patients with HCC, but approximately 70% of patients are ineligible for resection at the preliminary diagnosis [Citation2,Citation3]. Radiofrequency ablation (RFA), a minimally invasive curative modality, has been recommended as an alternative to surgical resection in the current guidelines, especially for early HCC [Citation2,Citation3]. However, RFA has its disadvantages: (i) the treatment of subdiaphragmatic tumors is technically challenging [Citation4] and (ii) tumors located adjacent to the macrovasculature are much more likely to be influenced by the heat-sink effect [Citation5].
Stereotactic body radiotherapy (SBRT) is an emerging modality for HCC treatment showing excellent local control (LC) [Citation6–8]. Considering that SBRT is not limited by the anatomic position of the tumor and does not involve a heat-sink effect [Citation9], it can replace RFA as the optimal treatment. However, the results of a recent meta-analysis on this topic were beyond expectations [Citation10]. Two recent retrospective studies [Citation11,Citation12], including one multicenter study, reported results in contrast to the results of the former meta-analysis as they both reported better LC with SBRT. Hence, a systematic review and meta-analysis based on the current evidence are warranted to identify the optimal modality between SBRT and RFA.
Material and methods
This meta-analysis was conducted according to the guidelines of the preferred reporting items for systematic reviews and meta-analyses. No ethical approval was required because this study was based on published studies.
Literature search
A comprehensive search of the existing published medical literature on the comparison of the clinical efficacy of SBRT vs. RFA for HCC was conducted by Lei Wang and Qiao Ke independently. English electronic databases such as PubMed, MedLine, EMBASE, the Cochrane Library and Web of Science were searched for studies published from 1 January 1990 to 31 May 2020. The following keywords were used: (‘hepatocellular carcinoma’ or ‘HCC’ or ‘liver tumor’ or ‘liver cancer’ or ‘liver neoplasm’) AND (‘stereotactic body radiotherapy’ or ‘Stereotactic Body Radiation Therapy’ or ‘SBRT’ or ‘stereotactic ablative radiotherapy’ or ‘SABR’ or ‘stereotactic radiotherapy’ or ‘SRT’ or ‘stereotactic radiosurgery’ or ‘SRS’ or ‘hyperfraction radiotherapy’ or ‘HFRT’ or ‘Cyberknife’ or ‘Gammaknife’ or ‘TOMO’) AND (‘Radiofrequency ablation’ or ‘RFA’). Any potentially eligible studies were identified manually from the references of the included studies, reviews, letters and comments.
Selection criteria
Following were the inclusion criteria: (i) studies including patients with primary or relapsed HCC, (ii) studies in which HCC was diagnosed by clinical imaging or pathological biopsy and (iii) studies including SBRT and RFA groups.
Following were the exclusion criteria: (i) studies including patients with intrahepatic cholangiocarcinoma, mixed liver cancer, or metastatic liver cancer; (ii) studies including patients receiving simultaneous treatments, including transarterial chemoembolization (TACE), (iii) studies with unavailable data on long-term outcomes, (iv) studies based on overlapping cohorts derived from the same center, (v) reviews, comments, letters, case reports and conference abstracts.
Endpoints
Endpoints included overall survival (OS), LC and complications. In this meta-analysis, OS was defined as the time from the start of treatment to death or the last follow-up. LC was defined as the absence of infield recurrence in patients receiving SBRT or the absence of recurrence within or adjacent to the ablation area for RFA. Early complications were defined as complications occurring within 6 months of treatment or those described as acute complications in the relevant study. Late complications were defined as complications occurring after 6 months of treatment or those described as late complications in the relevant study.
Data extraction
Data on the author’s first name, year of publication, study methods, patient characteristics, interventions and outcomes were extracted and assessed by Lei Wang and Qiao Ke independently using predefined forms. The hazard ratios (HRs) for LC or OS were extracted directly from the original studies or indirectly from Kaplan–Meier curves according to the methods described in detail by Tierney et al. [Citation13] and Parmar et al. [Citation14]. The 1-, 2- and 3-year LC rates or survival rates were extracted from the original studies directly or calculated from Kaplan–Meier curves. In case of a disagreement, a third investigator, Qizhen Huang, intervened to reach a decision.
Quality assessment
The quality of non-randomized studies was assessed using the modified Newcastle-Ottawa Scale (NOS) [Citation15]: ≥7 stars, high quality; 4–6 stars, medium quality; and <4 stars, low quality.
Statistical analysis
The meta-analysis was registered at http://www.crd.york.ac.uk/PROSPERO/ (Review registry 183028) and was performed using RevMan Version 5.3. Heterogeneity was assessed using the χ2 test and I2 statistics: p > .10 and I2<50% were considered to indicate no heterogeneity. When the hypothesis of homogeneity was rejected, the random-effects model was used to estimate the effect size, and the fixed-effects model was used for studies without significant heterogeneity [Citation16]. Publication bias was not assessed because <10 studies were included in the meta-analysis [Citation17].
Results
Baseline characteristic of the included studies
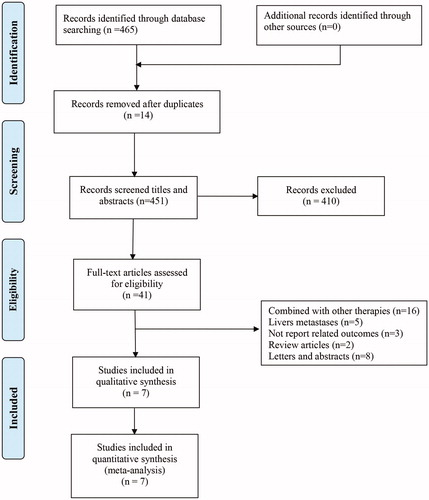
Initially, 465 studies were identified, of which 14 were excluded due to duplication. After screening the titles and abstracts, 410 studies were excluded. Finally, seven studies [Citation11,Citation12,Citation18–22] were included after the full-text review ().
In total, 7928 patients were enrolled in this meta-analysis, including 1170 patients in the SBRT group and 6758 patients in the RFA group. The characteristics and baseline demographic data of the patients in each study are listed in . The SBRT and RFA procedures in each primary study are depicted briefly in . The NOS scores for each included study are exhibited in ; two studies [Citation19,Citation20] had a score of 7, four [Citation12,Citation18,Citation21,Citation22] had a score of 8 and one [Citation11] had a score of 9.
Table 1. Characteristics of the clinical trials included in the meta-analysis.
Table 2. Interventions of the included studies.
Table 3. Literal quality assessment of the included studies.
Overall survival
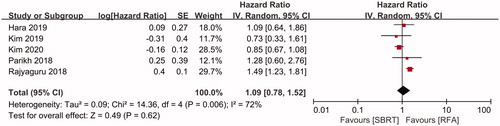
OS was compared between SBRT and RFA in five studies [Citation11,Citation12,Citation18–20], including 1092 patients in the SBRT group and 6559 patients in the RFA group. No significant difference was observed in the pooled HR for OS between the SBRT and RFA groups (HR = 1.09, 95%CI = 0.78–1.52, p = .62, ) using a random-effects model (I2=72%, p = .006, ). The 1-, 2- and 3-year OS rates were evaluated in six [Citation11,Citation12,Citation18–20,Citation22], five [Citation11,Citation12,Citation18–20] and four [Citation11,Citation12,Citation18,Citation19] studies, respectively. Similar results were found in terms of the pooled ORs for 1-, 2- and 3-year OS between the SBRT and RFA groups (1-year OS: OR = 0.83, 95%CI = 0.63–1.10, p = .19; 2-year OS: OR = 0.88, 95%CI = 0.54–1.43, p = .61; and 3-year OS: OR = 0.85, 95%CI = 0.45–1.59, p = .61; ).
Table 4. Pooled rates of local control and overall survival among studies.
Local control
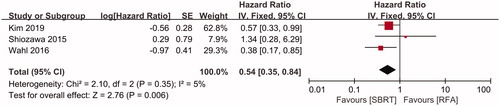
LC rates were compared between the SBRT and RFA groups in three included studies [Citation18,Citation21,Citation22], including 203 patients in the SBRT group and 867 patients in the RFA group. Using a fixed-effects model (I2=5%, p = .35, ), the pooled HR for the LC rate was in favor of SBRT (HR = 0.54, 95%CI 0.35–0.84, p = .006, ). Subgroup analysis showed that compared to those in the RFA group, the pooled ORs for 1-, 2- and 3-year LC in the SBRT group were 2.78 (95%CI 1.95–3.95, p < .001, ), 2.00 (95%CI 1.50–2.66, p < .001, ) and 1.97 (95%CI 1.51–2.57, p < .001, ), respectively.
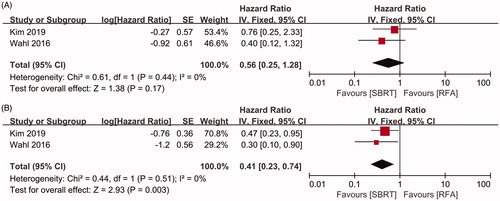
The efficacies of SBRT and RFA for HCC were compared according to tumor size in two studies [Citation18,Citation21]. In the subgroup of tumors sized ≤ 2 cm, including 76 patients in the SBRT group and 180 patients in the RFA group. The pooled HR showed that no significant difference was observed in the rate of LC between two groups (HR = 0.56, 95%CI 0.25–1.28, p = .17, ), using a fixed-effects model (I2=0, p = .44, ). However, in the subgroup of tumors sized > 2 cm, including 101 patients in the SBRT group and 164 patients in the RFA group. The pooled HR for LC was in favor of SBRT (HR = 0.41, 95%CI 0.23–0.74, p = .03, ), using a fixed-effects model (I2=0, p = .51, ).
Complications
Early complications were evaluated in four studies [Citation11,Citation12,Citation18,Citation21]. The incidence of early severe complications (grade ≥ 3) ranged from 0 to 5% in the SBRT group and from 0 to 11.0% in the RFA group (). A meta-analysis of three of the included studies [Citation11,Citation18,Citation21] showed that the pooled OR for the incidence of early severe complications in the SBRT group was 0.42 (95%CI 0.19–0.92, p = .03, ) compared to that in the RFA group.
Figure 5. Forest plot of the incidence of early and late toxicity between groups of SBRT and RFA. A, early toxicity. B, late toxicity.
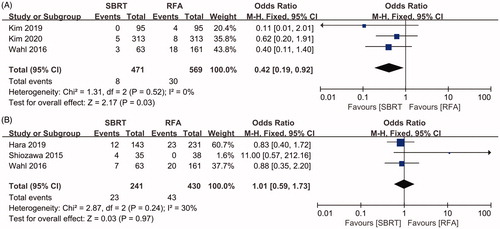
Table 5. Treatment-related complications in included studies.
Late complications were evaluated in five studies [Citation11,Citation12,Citation18,Citation21,Citation22]. The incidence of severe late complications (grade ≥3) ranged from 0 to 11.4% in the SBRT group and from 0 to 12.4% in the RFA group (). A meta-analysis of three of the included studies [Citation12,Citation21,Citation22] showed that the pooled OR for the incidence of late severe complications in the SBRT group was 1.01 (95%CI 0.59–1.73, p = .97, ) compared to that in the RFA group.
Discussion
As a minimally invasive treatment, RFA is the first-line strategy for early HCC [Citation2,Citation3], but it has always been challenged by SBRT [Citation2,Citation3,Citation23]. In this meta-analysis, seven studies with 7928 patients were identified to evaluate the clinical efficacy of SBRT versus RFA. The results showed that the OS with SBRT was comparable to that with RFA, but the LC rate with SBRT was superior to that with RFA; in addition, compared to RFA, SBRT decreased the incidence of early toxicities without increasing the incidence of late toxicities. Hence, SBRT is safe and efficient and should be considered as an alternative treatment for HCC.
RFA is not only a minimally invasive curative treatment but also a cost-effective treatment strategy for small HCCs [Citation20,Citation24]. RFA is recommended for single tumors sized <5 cm or in patients with less than four tumors among which the largest has a size of <3 cm. The short-term efficacy of RFA was confirmed to be non-inferior to that of radical resection [Citation2,Citation3]. In addition, intraoperative RFA combined with hepatectomy has been used for multiple tumors [Citation25]. However, the clinical application of RFA has been restricted by its inherent defects as previously mentioned.
As a modality of high-dose-rate external beam radiotherapy, SBRT typically exhibits a strong biological effect with an excellent LC rate (87–100%) [Citation6–8]. SBRT was also confirmed to be equivalent to proton or carbon ion therapies by a systematic review [Citation10]. Consequently, SBRT has been conducted prevalently in clinical settings as an alternative to difficult resection or RFA [Citation2], as a second-line treatment after failed TACE [Citation2,Citation23], as a downstaging strategy for large HCCs [Citation17], as a bridging therapy for liver transplantation [Citation26,Citation27], and as a palliative option for advanced HCC or distant metastasis [Citation2,Citation23]. However, SBRT has three drawbacks: (i) it is necessary to extend the clinical target volume and planning target volume beyond the tumor itself to avoid missing the target; an extended target volume results in more damage to normal tissues, especially in those with background cirrhosis [Citation28]; (ii) repeat SBRT may not be feasible for intrahepatic recurrent tumors; and (iii) more fractions are generally needed.
A previous meta-analysis found that that the efficacy of SBRT was equivalent to that of RFA in terms of LC but was inferior to that of RFA in terms of OS [Citation10]. This may be because SBRT is often performed in cases involving difficulties in RFA, failed TACE, large tumors, or relapsed HCC; these patients often show worse LC and poor prognosis. However, since 2019, two studies [Citation11,Citation12], including one international multicenter study [Citation11], have reported results in favor of SBRT using a propensity score matching (PSM) design. Hence, the current meta-analysis of updated data was warranted to help in clinical decision-making. In this meta-analysis, seven studies [Citation11,Citation12,Citation18–22] were eligible for the final synthesis. The results showed that the pooled HR for the LC rate was in favor of SBRT; significantly increases were also observed in the 1-, 2- and 3-year LC rates with SBRT. Furthermore, SBRT was found to be superior to RFA for tumors sized >2 cm but not for tumors sized ≤2 cm. Hence, we strongly recommend SBRT as the first-line treatment for unresectable HCC, especially in those with tumors sized >2 cm.
Excellent LC with SBRT was confirmed in this meta-analysis, but SBRT did not confer a long-term survival benefit. The reasons for this might be as follows: (i) patients in the SBRT group often had unfavorable factors, including advanced stage, larger tumor size and failure of initial treatment, which would affect the result regardless of PSM; (ii) recurrence was the bottleneck of HCC prognosis [Citation29]; and (iii) treatment after recurrence would affect the long-term outcome [Citation30]. Consequently, we speculate that SBRT would result in more favorable outcomes if it is applied as the first-line treatment; a well-designed multicenter randomized controlled trial is needed to verify this hypothesis.
SBRT is conducted in fewer fractions, and the dose to the target edge drops rapidly [Citation31]. Early toxicities related to SBRT, including radiation-induced liver disease, have rarely been reported, especially with advances in breathing-motion management strategies such as mechanical abdominal compression [Citation32], four-dimensional computed tomography [Citation33,Citation34], respiration gating systems [Citation35,Citation36], real-time target tracking and continuous patient position adjustment with robotic treatment couches [Citation37,Citation38]. As a noninvasive modality, SBRT can be used to avoid the adverse events caused by RFA, including hemorrhage, pneumothorax and biliary fistula. In this meta-analysis, no significant differences in the incidence of late severe toxicity were observed between the SBRT and RFA groups, but the pooled OR for the incidence of early severe toxicity was in favor of SBRT.
However, the accessibility of SBRT is not similar to that of RFA, especially in China, where SBRT is generally conducted in highly-volume radiotherapy centers. Quality of life (QoL) is an important posttreatment endpoint for evaluating different treatment modalities for HCC mainly because long-term survival is generally poor in this population. Klein et al. [Citation39] reported that liver SBRT was well tolerated and did not worsen the overall QoL, although it could lead to temporary appetite loss and fatigue. However, the QoL has not been compared between SBRT and other modalities, especially RFA. In reality, cost-effectiveness is also a concern in patients when they make decisions regarding treatment. Previous studies have found that if equal survival was assumed, SBRT was less cost-effective than RFA either for inoperable colorectal liver metastases or for inoperable localized HCC [Citation24]. Hence, more factors, in addition to efficacy and safety, should be taken into consideration when making treatment decisions.
There were several limitations to this meta-analysis. First, all the included studies were retrospective, and selection and recall bias were difficult to avoid. Second, the population in each included study was slightly different, which would result in apparent heterogeneity, even though we conducted subgroup analyses. Third, considering that patients receiving SBRT often have unfavorable prognostic factors in real-world settings, confounding factors were difficult to eliminate, although PSM was performed in five included studies [Citation11,Citation12,Citation18–20]. Fourth, LC included the absence of local progression and recurrence; however, only data on recurrence were reported in one of the included studies, which would lower the credibility of this meta-analysis. Fifth, the procedure of SBRT was not standardized, which might have resulted in treatment bias. Lastly, conference abstracts were excluded from this meta-analysis due to the inability to assess literature quality, which would have increased the publication bias. Hence, a well-designed randomized controlled trial is needed with unified dosage and fractionation regimens for SBRT and preset response criteria for treatment.
Conclusions
Based on the current data, we concluded that SBRT can be used as an alternative to RFA, especially for tumors sized >2 cm, tumors adjacent to the macrovasculature, tumors difficult to treat with RFA and cases involving TACE failure; however, SBRT does not exhibit a survival advantage. This conclusion needs to be validated in future prospective randomized controlled trials.
Acknowledgements
The authors thank Editage (www.editage.cn) for English language editing.
Disclosure statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Benson AB, D'Angelica MI, Abbott DE, et al. Guidelines insights: hepatobiliary cancers, version 2.2019. J Natl Compr Canc Netw. 2019;17(4):302–310.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
- Tanaka K, Kojima T, Hiraguchi E, et al. Laparoscopy-guided transthoracic transdiaphragmatic radiofrequency ablation for hepatic tumors located beneath the diaphragm. J Laparoendosc Adv Surg Tech A. 2016;26(3):180–184.
- Al-Alem I, Pillai K, Akhter J, et al. Heat sink phenomenon of bipolar and monopolar radiofrequency ablation observed using polypropylene tubes for vessel simulation. Surg Innov. 2014;21(3):269–276.
- Kim JW, Seong J, Lee IJ, et al. Phase I dose escalation study of helical intensity-modulated radiotherapy-based stereotactic body radiotherapy for hepatocellular carcinoma. Oncotarget. 2016;7(26):40756–40766.
- Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631–1639.
- Cardenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12(3):218–225.
- Hass P, Mohnike K. Extending the frontiers beyond thermal ablation by radiofrequency ablation: SBRT, brachytherapy, SIRT (radioembolization). Viszeralmedizin. 2014;30(4):245–252.
- Qi WX, Fu S, Zhang Q, et al. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2015;114(3):289–295.
- Kim N, Cheng J, Jung I, et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol. 2020;73(1):121–129.
- Hara K, Takeda A, Tsurugai Y, et al. Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: a propensity score analysis. Hepatology. 2019;69(6):2533–2545.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statist. Med. 1998;17(24):2815–2834.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.
- Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta‐analyses. In: Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions, Second Edition. Glasgow: John Wiley and Sons; 2019. Chapter 10, p. 241–284.
- Soin AS, Bhangui P, Kataria T, et al. Experience with LDLT in patients with hepatocellular carcinoma and portal vein tumor thrombosis postdownstaging. Transplantation. 2020; 104(11):2334–2345.
- Kim N, Kim HJ, Won JY, et al. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother Oncol. 2019;131:81–87.
- Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the national cancer database. J Cin Oncol. 2018;36(6):600–608.
- Parikh ND, Marshall VD, Green M, et al. Effectiveness and cost of radiofrequency ablation and stereotactic body radiotherapy for treatment of early-stage hepatocellular carcinoma: an analysis of SEER-medicare. J Med Imaging Radiat Oncol. 2018;62(5):673–681.
- Wahl DR, Stenmark MH, Tao Y, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Cin Oncol. 2016;34(5):452–459.
- Shiozawa K, Watanabe M, Ikehara T, et al. Comparison of percutaneous radiofrequency ablation and CyberKnife(®) for initial solitary hepatocellular carcinoma: a pilot study. World J Gastroenterol. 2015;21(48):13490–13499.
- Park HC, Yu JI, Cheng JC, et al. Consensus for radiotherapy in hepatocellular carcinoma from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014): current practice and future clinical trials. Liver Cancer. 2016;5(3):162–174.
- Pollom EL, Lee K, Durkee BY, et al. Cost-effectiveness of stereotactic body radiation therapy versus radiofrequency ablation for hepatocellular carcinoma: a markov modeling study. Radiology. 2017;283(2):460–468.
- Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018;68(4):783–797.
- Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67(1):92–99.
- Mohamed M, Katz AW, Tejani MA, et al. Comparison of outcomes between SBRT, yttrium-90 radioembolization, transarterial chemoembolization, and radiofrequency ablation as bridge to transplant for hepatocellular carcinoma. Adv Radiat Oncol. 2016;1(1):35–42.
- Rim CH, Kim HJ, Seong J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. Radiother Oncol. 2019;131:135–144.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314.
- Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955.
- Folkert MR, Timmerman RD. Stereotactic ablative body radiosurgery (SABR) or Stereotactic body radiation therapy (SBRT). Adv Drug Deliv Rev. 2017;109:3–14.
- Herfarth KK, Debus J, Lohr F, et al. Extracranial stereotactic radiation therapy: set-up accuracy of patients treated for liver metastases. Int J Radiat Oncol Biol Phys. 2000;46(2):329–335.
- Guckenberger M, Richter A, Boda-Heggemann J, et al. Motion compensation in radiotherapy. Crit Rev Biomed Eng. 2012;40(3):187–197.
- Guckenberger M, Wilbert J, Krieger T, et al. Four-dimensional treatment planning for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(1):276–285.
- Saito T, Sakamoto T, Oya N. Comparison of gating around end-expiration and end-inspiration in radiotherapy for lung cancer. Radiother Oncol. 2009;93(3):430–435.
- Kontrisova K, Stock M, Dieckmann K, et al. Dosimetric comparison of stereotactic body radiotherapy in different respiration conditions: a modeling study. Radiother Oncol. 2006;81(1):97–104.
- Guckenberger M, Meyer J, Wilbert J, et al. Precision of image-guided radiotherapy (IGRT) in six degrees of freedom and limitations in clinical practice. Strahlenther Onkol. 2007;183(6):307–313.
- Keall PJ, Cattell H, Pokhrel D, et al. Geometric accuracy of a real-time target tracking system with dynamic multileaf collimator tracking system. Int J Radiat Oncol Biol Phys. 2006;65(5):1579–1584.
- Klein J, Dawson LA, Jiang H, et al. Prospective longitudinal assessment of quality of life for liver cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(1):16–25.