Abstract
Introduction
Uterine fibroids are the most common benign neoplasms in women. The administration of intravenous oxytocin is known to increase the efficacy of a non-invasive thermal ablation method (MR-HIFU) for treating fibroids. However, it is not known whether this phenomenon is caused by the effect of the oxytocin on the myometrium or the fibroid itself. The objective of this study was to evaluate the influence of oxytocin on the blood flow of fibroids, myometrium and skeletal muscle using a quantitative perfusion MRI technique.
Materials and methods
17 premenopausal women with fibroids considered to be treated with MR-HIFU and 11 women with no fibroids were enrolled in the study. An extended MRI protocol of the pelvis was acquired for each subject. Later another MRI scan was performed with continuous intravenous infusion of oxytocin. The effect of oxytocin was analyzed from quantitative perfusion imaging. The study was registered in clinicaltrials.gov NCT03937401.
Results
Oxytocin decreased the blood flow of each fibroid; the median blood flow of fibroid was 39.9 ml/100 g tissue/min without and 3.5 mL/100 g/min with oxytocin (p ≤ 0.0001). Oxytocin did not affect the blood flow of the myometrium in either group. Oxytocin increased the blood flow of the skeletal muscle in both groups (p = 0.04).
Conclusion
Oxytocin is effective in decreasing the blood flow in fibroids while having minor or no effect on the blood flow of normal myometrium. Routine use of oxytocin in HIFU therapy may make the therapy suitable to a larger group of women in a safe manner.
Introduction
Uterine fibroids are the most common benign neoplasms in women affecting 2/3 of women by the age of 50 years [Citation1]. Symptoms caused by fibroids are menorrhagia, dysmenorrhea, abdominal pain, bladder dysfunction and pelvic pressure often resulting in a significantly decreased quality of life [Citation1]. Furthermore, fibroids can have an adverse impact on fertility by interfering with fertilization and implantation and leading to recurrent miscarriages and adverse perinatal outcomes [Citation2].
Magnetic resonance-guided high intensity focused ultrasound (MR-HIFU) is a noninvasive thermal ablation method based on high-intensity ultrasound waves causing heating of the target tissue and leading to coagulative necrosis [Citation3]. Real-time magnetic resonance imaging (MRI) is used for therapy guidance and temperature monitoring during the treatment.
Careful patient selection is important in treating fibroids with high intensity focused ultrasound (HIFU-therapy). The therapy is generally considered to be best suited for fibroids up to 10 cm in diameter or one to three smaller fibroids [Citation4]. Alongside anatomical factors such as fibroid location and thickness of the subcutaneous fat tissue, probably the most significant predictors of good treatment outcome are the structure and vascularization of the fibroid [Citation5]. The thermal response of the fibroid can be predicted based on the signal intensity of the T2-weighted MR images of the fibroid. An MRI-based Funaki classification is generally used to evaluate the suitability of a fibroid for HIFU therapy. The fibroids are divided into three Funaki types according to their average T2-signal intensity compared to those of the myometrium and skeletal muscle [Citation6].
Funaki type I–II fibroids are known to have a better thermal response and thus predict better treatment outcomes [Citation3]. Funaki type III fibroids have higher moisture content, lower collagen density, and tend also to have higher blood perfusion than other Funaki type fibroids and thus are more challenging to ablate with MR-HIFU [Citation7,Citation8]. Because of this heterogeneity, unfortunately not all fibroids are suitable for MR-HIFU treatment.
Oxytocin is a nonapeptide synthetized in the hypothalamus and it has many physiological functions [Citation9,Citation10]. It has an important role in parturition and lactation and is widely used in obstetric care to induce labor, create contractions and to decrease postpartum hemorrhage [Citation11–13].
The administration of intravenous oxytocin during the MR-HIFU treatment increases the efficiency of the therapy resulting in greater ablation volumes. It has also been shown that intravenous oxytocin administered before percutaneous microwave ablation can reduce blood flow in the fibroid and improve the therapeutic effect and that intraoperative infusion of oxytocin even reduces blood loss during myomectomy[Citation9,Citation14,Citation15]. Wang et al. showed that oxytocin reduces blood flow in uterine fibroids which can be seen using contrast-enhanced sonography [Citation16].
However, it has not been thoroughly investigated whether this phenomenon is caused by the effect of oxytocin on the myometrium or specifically on the fibroids.
Since the efficacy of oxytocin in improving the results of MR-HIFU treatments has also been observed in Turku University Hospital, we decided to evaluate this phenomenon in more detail. The objective of this study was to evaluate and quantify the influence of oxytocin on the blood flow of fibroids, normal myometrium and skeletal muscle of non-pregnant women using a quantitative perfusion MRI technique.
Materials and methods
Seventeen premenopausal women (age mean 38 ± 6, BMI mean 25 ± 5) with one or several fibroids were enrolled in the study. These women were consecutive patients referred to the gynecological outpatient clinic for a potential MR-HIFU treatment, that is, they represented a selected subgroup of fibroid patients. 12 patients had a fibroid of Funaki type II, and 5 patients a fibroid of Funaki type III. Inclusion criteria in this study were symptoms caused by fibroids, physical and mental health appropriate for an MRI scan, and premenopausal status. Exclusion criteria were a known allergy to the Syntocinon medicine or any of its adjuvants, high blood pressure, an ischemic heart condition, a long QT-syndrome, or medication that prolongs the QT segment. In addition, women with contraindications to an MRI scan were excluded. Details of the menstrual cycle and hormonal therapy were recorded for each woman. To evaluate whether the blood flow of the myometrium is affected by the presence of fibroids, additional 11 women (age median 26 [24–33]), (BMI median 21[20–22]) without uterine fibroids were enrolled in the study.
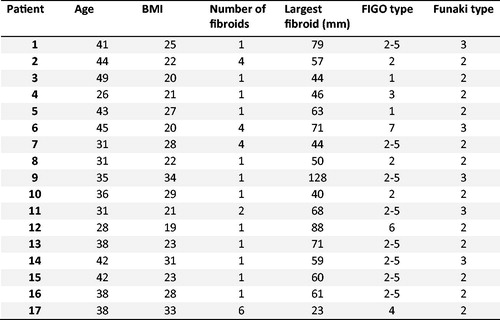
Written informed consent for the MRI and oxytocin administration was obtained from all patients and healthy volunteers. This study was performed in accordance with the ethical regulations of the Ethics Committee of the Hospital District of Southwest Finland and the National Committee of Medical Research Ethics (T366/2017 25.1.2018). The characteristics of the fibroid patients are shown in . The dominant fibroid was assessed if the woman had multiple fibroids.
All MRI images were acquired with the same MR scanner (Ingenia 3.0 T, Philips, Best, The Netherlands). Women were positioned in a prone position during the MRI scan of the pelvis using a 32-channel torso coil. An extended scanning protocol was acquired for each woman. Among other sequences, the imaging protocol included dynamic contrast-enhanced (DCE) imaging for quantitative perfusion analysis. Later (median 58, range 4–207 days) another MRI scan was performed with the same imaging protocol under continuous intravenous infusion of oxytocin. 40 IU of oxytocin (Syntocinon 8.3 μg/ml, Sigma-Tau Industrie Farmaceutiche Riunite S.p.A) diluted to 500 ml of 0.9% NaCl was infused at the rate of 5 ml/min resulting in an oxytocin infusion rate of 0.4 IU/min with a volumetric infusion pump (Infusomat® Space, B Braun, Germany, Melsungen) placed in MRI compatible docking station (SpaceStation® MRI, B Braun, Germany, Melsungen). The used dosage was considered safe since it’s widely used in obstetric care for the treatment of postpartum hemorrhage and inducing uterine contraction. During the infusion of oxytocin, women were regularly asked about possible symptoms caused by the oxytocin. In addition, their blood pressure and heart rate were monitored and recorded. The MRI scan was started approximately 15 min after the initiation of the oxytocin infusion and DCE imaging was obtained 30 min after the start of the oxytocin infusion.
MRI protocol included a dynamic contrast-enhanced (DCE) T1-weighted sequence, the parameters of which are shown in . A single dose (0.2 ml/kg) of contrast agent (Dotarem, Guebert, Roissy, France) was manually injected at a constant rate followed by 10 ml saline flush after the acquisition of the first five time frames of the dynamic scans. The DCE-MRI data were analyzed with NordicICE software v. 4.1.1 (NordicNeuroLab AS, Bergen, Norway). The arterial input function was determined from the iliac artery by placing a circular region of interest (ROI) onto the artery lumen. Blood flow values were calculated by standard model-independent deconvolution technique by means of contrast bolus tracking [Citation17]. Parametric blood flow values were obtained by T1 perfusion deconvolution arithmetic with the first pass of an AIF curve. Averaged blood flow values were obtained for the fibroids, myometrium and muscle. The fibroid ROIs were drawn within three middle slices of the fibroid to include most of the fibroid. The myometrium ROIs were drawn on the negative side of the fibroid in the three most representative slices of the myometrium and the muscle ROI’s were drawn on the abdominal muscle within the three middle slices as the fibroid ROIs.
Table 1. MR imaging parameters.
The Funaki type of the fibroids was determined by an experienced interventional radiologist (GK) by drawing the regions of interest (ROI’s) on the sagittal T2 weighted image. A round ROI was placed to cover most of the fibroid. Elliptical ROI’s were positioned on myometrium and skeletal muscle (abdominal muscle) so as not to include other tissues. Average signal intensity values for all three ROI’s were obtained and compared in order to determine the Funaki type of each fibroid.
Statistical analysis was performed using JMP Pro statistical software version 13.1.0 (SAS Institute Inc.). A p-value less than 0.05 was considered statistically significant.
Each dataset was analyzed for normal distribution with the Shapiro–Wilk W test. Normally distributed numerical data were presented as a mean ± standard deviation and data with a skewed distribution were presented as the median [interquartile range (IQR)]. Normally distributed data were compared with the Tukey–Kramer test for all pairs and data with skewed distribution were compared with a nonparametric Steel–Dwass method for all pairs. A retrospective power analysis was performed using standard least squares to ensure a sufficient number of patients in the fibroid group.
The primary clinical outcome was the change of blood flow in the fibroid during the infusion of oxytocin as compared to the baseline state as well as the change of blood flow in the normal myometrium and skeletal muscle.
The secondary outcome was the evaluation of the side effects during the use of oxytocin on non-pregnant women.
Results
Oxytocin significantly decreased the blood flow of all the fibroids analyzed. All the women in our study tolerated the infusion without any reported symptoms and no side effects were observed. The oxytocin infusion had no marked effect on the blood pressure or heart rate of the women.
The baseline blood flow of the fibroids varied greatly. Based on MRI images, in our study group, we had 12 patients with a fibroid of Funaki type II and 5 patients with a fibroid of Funaki type III. In Funaki type II fibroids, the median baseline blood flow was 36.5 [21.1–173.5] ml/100 g tissue/min and in Funaki type III the mean baseline blood flow was 16.5 ± 19.4 ml/100 gtissue/min. Thus, the highest measured blood flow values were in the Funaki type 2 fibroids. The difference between the baseline blood flow of Funaki type II and type III fibroids was not statistically significant (p = 0.49).
The oxytocin infusion had no effect on the size of the fibroid or the uterus. shows MRI scans of a fibroid without oxytocin and with oxytocin.
Figure 2. T2 weighted MR images of a uterine fibroid in (a) axial and (b) sagittal plane. Quantitative blood flow maps of the same fibroid in axial plane (c) without oxytocin and (d) with oxytocin. Contrast enhanced T1 weighted MR images of the same fibroid in axial plane (e) without oxytocin and (f) with oxytocin.
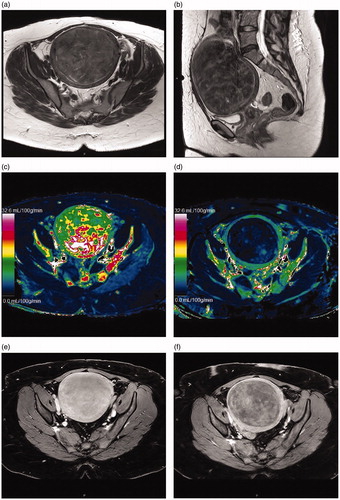
Oxytocin decreased the blood flow of every fibroid analyzed (). The median blood flow of all fibroids without oxytocin was 39.9 [21.9–134.5] ml/100 g/min. With oxytocin, the median blood flow was 3.5 [2.1–12.1] ml/100 g/min, (p ≤ 0.0001, power = 0.87). Oxytocin decreased the mean/median blood flow both in Funaki type II and type III fibroids. In Funaki type II the median blood flow with oxytocin was 3.4 [2.3–7.3] ml/100 g/min and Funaki type III the mean blood flow with oxytocin was 16.5 ± 19.4 ml/100 g/min. In Funaki type II fibroids the difference between the baseline blood flow and the blood flow with oxytocin was statistically significant (p = 0.0004 and power 0.65). Also in Funaki type III fibroids the difference between the baseline blood flow and the blood flow with oxytocin was statistically significant (p = 0.028 and power 0.65). The difference in the blood flow with oxytocin between the type II and III fibroids was not significantly different (p = 0.87).
Figure 3. (a) Blood flow of the largest fibroid of every patient without oxytocin and with oxytocin (b) The median blood flow of all fibroids without oxytocin and with oxytocin, *p-value < 0.0001.
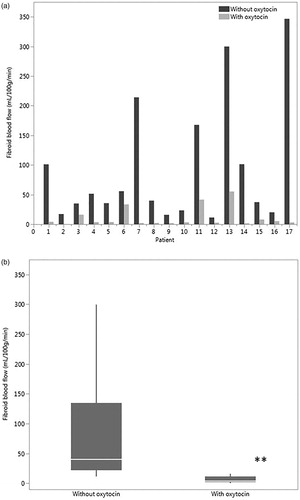
Oxytocin had no significant effect on the blood flow in the myometrium of the fibroid patients nor the control group (). In the fibroid group, the median myometrial blood flow without the oxytocin was 61.1 [26.4–165.2] ml/100 g/min and 69.4 [21.5–134.6] ml/100 g/min during oxytocin infusion (p = 0.81), whereas in the control group the blood flow values were 59.4 [43.6–109.2] and 68.6 [30.8–101.4] ml/100 g/min (p = 0.74). No difference was observed when comparing the myometrial blood flow between the groups with fibroids and without fibroids, neither with nor without the oxytocin infusion.
Figure 4. (a) Blood flow of the myometrium without oxytocin and with oxytocin in the fibroid patients and the control group (b) Blood flow of the skeletal muscle without oxytocin and with oxytocin in both groups, *p < 0.05.
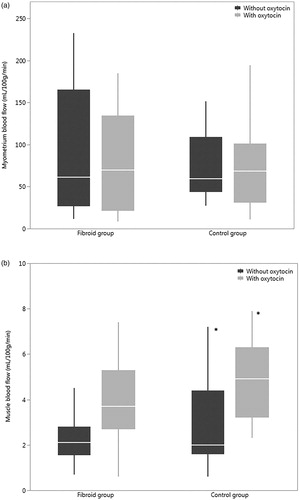
In our study, oxytocin had a minor, yet statistically significant effect on the skeletal muscle blood flow by increasing it (). In the fibroid patients, the median blood flow of the skeletal muscle was 2.1 [1.6–2.8] ml/100 g/min at baseline and 3.7 [2.7–5.3] ml/100 g/min during oxytocin infusion (p = 0.04). In the control group, the skeletal muscle blood flow was 2.0 [1.6–4.4] ml/100 g/min without oxytocin and 4.9 [3.2–6.3] ml/100 g/min with oxytocin, (p = 0.04).
Discussion
In this study, we demonstrated that oxytocin is very effective in decreasing the blood flow in the fibroid tissue; an effect that can be clearly demonstrated and quantified with MRI. Our results are in line with a previous study in which oxytocin was shown to decrease blood flow in fibroids with contrast-enhanced ultrasound and at the same time not affecting the uterine wall significantly [Citation16].
Fibroids are monoclonal tumors that arise from the smooth muscle tissue of the uterus. They borrow their vascularization from surrounding structures, usually the myometrium. The vessels in the fibroid tissue lack the normal structure compared to the myometrial vessels and are more narrow in diameter [Citation18]. Most fibroids are surrounded by a highly vascularized pseudocapsule which has its own gene expression profile and supplies the fibroid with peri- and intra-fibroid arteries [Citation19].
Oxytocin mainly exerts its effects through the oxytocin receptor, which has a tissue-specific expression pattern. There is little and somewhat contradictory data on the expression of the oxytocin receptors in the myometrium of non-pregnant women or in fibroids. In a study by Sendemir et al. oxytocin receptors were mainly found in fibroid tissue whereas only scarcely in the healthy myometrium [Citation20]. Lee et al., on the other hand, found oxytocin receptors to be more abundant in myometrium than fibroid tissue [Citation13].
The fibroids have their own hormonal environment, which may influence the amount of oxytocin and its receptors in fibroids. For example, there is local hyperestrogenism in fibroids [Citation21] and estrogens, in turn, have been demonstrated to increase the levels of oxytocin receptors and thus in part mediate the contractility of the myocytes. On the other hand, oxytocin receptors are also located in blood vessels, and commonly oxytocin is related to vasodilation [Citation22]. However, it is not known whether the aberrant blood vessels in the fibroids have oxytocin receptors or how they respond to oxytocin.
It seems also that the expression of oxytocin receptors is somewhat dependent on the menstrual cycle with some reports suggesting that the blood flow is higher during the follicular and ovulatory phase compared to the luteal phase [Citation23]. Despite our initial efforts, we could not assess this phenomenon in our study, since we were not able to synchronize the MR-imaging with the menstrual cycle of the women. However, the effect of oxytocin on the fibroid blood flow was strong in all women and it might be safe to speculate that the effect of oxytocin surpasses any possible effect of blood flow changes due to the menstrual cycle.
It must be kept in mind that the results of our study do not offer an explanation of the underlying mechanism for the rather dramatic decrease in blood flow in the fibroids in response to oxytocin. Since the blood flow in the healthy myometrium did not change during oxytocin infusion, it cannot be concluded that the decrease in blood flow in the fibroid is due to increased contractility of the uterus. Thus, it seems more likely that the effect is related to the fibroid itself or the supplying circulation. This, however, clearly needs to be explored in further studies.
Skeletal muscle was chosen as a negative control since during rest it exhibits constant blood flow levels. In our study, the infusion of oxytocin increased the blood flow of the skeletal muscle. Even though statistically significant, the rise in the perfusion value was fairly small in absolute terms (1.6 ml/100 g/min in the fibroid population and 2.9 ml/100 g/min in healthy volunteers) and may be explained by the small systemic vasodilatative effect of oxytocin [Citation22]. This effect was not strong enough to affect the blood pressure of the patients.
The study population comprised of women who were being evaluated for MR-HIFU therapy. Therefore, they represent a specific pre-selected subset of women with significant symptoms and fibroids initially considered treatable with MR-HIFU. Even though the fibroids were heterogeneous in size, location, Funaki type and blood flow, they represent a selected subgroup of fibroids and as such might not be representative of the general fibroid population. On the other hand, the observed great variety in blood perfusion in fibroids potentially suitable for HIFU therapy only emphasizes the importance of perfusion imaging. Nine of the women enrolled in our study underwent the HIFU treatment later. The effect of oxytocin on the results of the HIFU therapy was not in the scope of this study.
The effect of oxytocin was significant in all the fibroids studied and the results observed rather robust. Regardless of this robustness of the results, the study population was too small to assess whether the localization, classification or the size of the fibroid have an influence in the magnitude of the response to oxytocin. Further studies are needed to characterize whether the blood flow of the fibroid and the Funaki type are correlated and whether oxytocin response differs in various types of fibroids. It also remains to be studied whether the decrease in blood flow of the fibroid could be enhanced by increasing the dosage of oxytocin.
Our data suggest that oxytocin has a significant influence on blood flow in a non-pregnant uterus with fibroids. It has been shown that the use of oxytocin decreases blood loss during abdominal and laparoscopic myomectomies [Citation15,Citation24,Citation25]. Our results confirm that this is due to the very strong decrease in the blood flow of the fibroid rather than the myometrium itself. It is possible that with higher concentrations of oxytocin also the blood flow of the uterus would be affected. None of the women reported unpleasant sensations during oxytocin infusion, which may also reflect the relatively small contractive effect of the dose of oxytocin used.
MR-HIFU is an attractive non-invasive technology for treating symptomatic uterine fibroids. A major restriction of MR-HIFU, however, is that only a small proportion of fibroids respond to the therapy effectively. The Funaki classification is an important, yet often insufficient tool in predicting the efficacy of the MR-HIFU treatment. It is known that high blood flow in fibroids is one of the main predictors of a poor therapy outcome [Citation26]. Keserci et al. found that parameters related to blood perfusion of the fibroid have a strong correlation to the HIFU treatment outcome [Citation5]. Thus besides the Funaki type, also the blood perfusion of the fibroid should be measured for predicting the efficacy of MR-HIFU therapy. On the other hand, our results suggest that the effect of blood flow on the treatment efficacy can be overridden with the use of oxytocin during the MR-HIFU treatment, at least to some extent. It would be interesting to be able to define a threshold value for blood flow that would predict a good treatment outcome. Studying this would, however, require a much larger group of fibroids.
The results of our study demonstrate that oxytocin significantly and without side effects, decrease the blood flow of the fibroids, regardless of the Funaki type. This is probably why the administration of intravenous oxytocin during the MR-HIFU increases the efficacy of this treatment [Citation9]. Routine use of oxytocin in HIFU therapy may therefore make this therapy suitable to a larger group of women in a safe and inexpensive manner as well as result in shorter sonication times and overall duration of therapy in patients already suitable for HIFU.
Ethical approval and informed consent
This study was performed in accordance with the ethical regulations of the Ethics Committee of the Hospital District of Southwest Finland and the National Committee of Medical Research Ethics (T366/2017 25.1.2018). All the participants signed informed consent. The study was licenced by Finnish Medicines Agency (FIMEA) and reported to the EudraCT database (EudraCT: 2017-005022-38). The study was also registered in clinicaltrials.gov NCT03937401.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Stewart EA, Cookson CL, Gandolfo RA, et al. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501–1512.
- Laughlin-Tommaso SK. Non-surgical management of myomas. J Minim Invasive Gynecol. 2018;25(2):229–236.
- Silberzweig JE, Powell DK, Matsumoto AH, et al. Management of uterine fibroids: a focus on uterine-sparing interventional techniques. Radiology. 2016;280(3):675–692.
- Rueff LE, Raman SS. Clinical and technical aspects of MR-guided high intensity focused ultrasound for treatment of symptomatic uterine fibroids. Semin Intervent Radiol. 2013;30(4):347–353.
- Keserci B, Duc NM. Magnetic resonance imaging parameters in predicting the treatment outcome of high-intensity focused ultrasound ablation of uterine fibroids with an immediate nonperfused volume ratio of at least 90%. Acad Radiol. 2018;25(10):1257–1269.
- Andrews S, Yuan Q, Bailey A, et al. Multiparametric MRI characterization of funaki types of uterine fibroids considered for MR-guided high-intensity focused ultrasound (MR-HIFU) therapy. Acad Radiol. 2019;26(4):e9–e17.
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34(5):584–589.
- Zhao WP, Chen JY, Chen WZ. Effect of biological characteristics of different types of uterine fibroids, as assessed with t2-weighted magnetic resonance imaging, on ultrasound-guided high-intensity focused ultrasound ablation. Ultrasound Med Biol. 2015;41(2):423–431.
- Lozinski T, Filipowska J, Krol P, et al. Oxytocin administration in high-intensity focused ultrasound treatment of myomata. Biomed Res Int. 2018;2018:1–5.
- Lee HJ, Macbeth AH, Pagani JH, et al. Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88(2):127–151.
- Engel S, Klusmann H, Ditzen B, et al. Menstrual cycle-related fluctuations in oxytocin concentrations: a systematic review and meta-analysis. Front Neuroendocrinol. 2019;52:144–155.
- Gimpl G, Fahrenholz F, Gene C. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683.
- Lee KH, Khan-Dawood FS, Dawood MY. Oxytocin receptor and its messenger ribonucleic acid in human leiomyoma and myometrium. Am J Obstet Gynecol. 1998;179(3):620–627.
- Fu Y, Feng Q, Zhang S, et al. Application of oxytocin in ultrasound-guided percutaneous microwave ablation for treatment of hypervascular uterine fibroids: a preliminary report. Int J Hyperth. 2019;36(1):761–767.
- Atashkhoei S, Fakhari S, Pourfathi H, et al. Effect of oxytocin infusion on reducing the blood loss during abdominal myomectomy: a double-blind randomised controlled trial. BJOG: Int J Obstet Gy. 2017;124(2):292–298.
- Wang Y, Ren D, Wang W. The influence of oxytocin on the blood perfusion of uterine fibroids: contrast-enhanced ultrasonography evaluation. J Med Ultrasound. 2016;24(1):13–17.
- Østergaard L, Weisskoff RM, Chesler DA, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: mathematical approach and statistical analysis. Magn Reson Med. 1996;36(5):715–725.
- Fleischer R, Weston GC, Vollenhoven BJ, et al. Pathophysiology of fibroid disease: angiogenesis and regulation of smooth muscle proliferation. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):603–614.
- Nieuwenhuis LL, Keizer AL, Stoelinga B, et al. Fibroid vascularisation assessed with three-dimensional power Doppler ultrasound is a predictor for uterine fibroid growth: a prospective cohort study. BJOG. 2018;125(5):577–584.
- Sendemir A, Sendemir E, Kosmehl H, et al. Expression of sex hormone-binding globulin, oxytocin receptor, caveolin-1 and p21 in leiomyoma. Gynecol Endocrinol. 2008;24(2):105–112.
- Bulun SE, Moravek MB, Yin P, et al. Uterine leiomyoma stem cells: linking progesterone to growth. Semin Reprod Med. 2015;33(5):357–365.
- Rosseland LA, Hauge TH, Grindheim G, et al. Changes in blood pressure and cardiac output during cesarean delivery: the effects of oxytocin and carbetocin compared with placebo. Anesthesiology. 2013;119(3):541–551.
- Meylaerts LJ, Wijnen L, Bazot M, et al. Perfusion of the uterine junctional zone in nulliparous and primiparous women assessed by DCE-MRI, as a function of menstrual cycle and hormonal contraception. Magn Reson Imaging. 2017;38:101–111.
- Zhang R, Shi H, Ren F, et al. Assessment of carboprost tromethamine for reducing hemorrhage in laparoscopic intramural myomectomy. Exp Ther Med. 2015;10(3):1171–1174.
- Wang C-J, Lee C-L, Yuen L-T, et al. Oxytocin infusion in laparoscopic myomectomy may decrease operative blood loss. J Minim Invasive Gynecol. 2007;14(2):184–188.
- Jeong J-H, Hong GP, Kim Y-R, et al. Clinical consideration of treatment to ablate uterine fibroids with magnetic resonance imaging-guided high intensity focused ultrasound (MRgFUS): sonalleve. J Menopausal Med. 2016;22(2):94–107.