Abstract
Background
Acute renal impairment (ARI) is a major complication after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) for cancer patients with peritoneal metastases. This study aimed to investigate the incidence and identify the risk factors of post-HIPEC creatinine increased.
Methods
From April 2015 to December 2019, demographic and perioperative data of 169 patients undergoing CRS/HIPEC with a preoperative creatinine level <1.5 mg/dL were retrospectively reviewed. Renal impairment was defined according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0. The risk factors of creatinine increased were analyzed using univariate and multiple logistic regression analyses.
Results
Among the 169 enrolled patients, 21 (12.4%) had postoperative creatinine increased (ARI group) and 148 (87.6%) did not (non-ARI group). Significantly more of the ARI group received a cisplatin HIPEC regimen than the non-ARI group (71.4 vs. 37.8%, p = 0.004). Multiple logistic regression analysis revealed that the patients who received a cisplatin HIPEC regimen (adjusted odds ratio [AOR] = 11.38, p < 0.001) and peritoneal dialysis solution as HIPEC perfusate (AOR = 7.07, p = 0.002) were more likely to develop post-HIPEC creatinine increased.
Conclusions
Identifying the risk factors of post-HIPEC creatinine increased can help to improve patient selection, a dose of HIPEC regimens modification and perioperative care. We also identified the detrimental renal effect of peritoneal dialysis solution as HIPEC perfusate. More prospective studies are warranted to confirm these findings.
Introduction
Peritoneal metastasis is a lethal manifestation in patients with metastatic malignancies, and extensive cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has become the treatment of choice [Citation1]. In this procedure, after maximal cytoreduction, chemotherapeutic drugs are introduced into the peritoneal cavity at a temperature of 41–43 °C and perfused for about 60–90 min. This results in the direct exposure of microscopic residual cancer cells to the drugs with the synergistic effect of hyperthermia [Citation1]. However, CRS/HIPEC is an aggressive treatment and is often associated with high-grade complications [Citation2].
Acute renal impairment (ARI), after CRS/HIPEC, is a major morbidity resulting in prolonged hospitalization and increased mortality [Citation3]. In the past decades, several studies have demonstrated an association between ARI after HIPEC and cisplatin-based regimens. A study reported an incidence of overall renal toxicity of 40.4% and an incidence of grade 3–4 renal impairment of 10.6% in ovarian cancer patients treated with cisplatin-based HIPEC regimens according to the criteria of National Cancer Institute – Common Terminology Criteria for Adverse Events (NCI-CTCAE) v.3.0 [Citation4]. Another study reported that 11.1% (11/99) of patients who received HIPEC had a significantly higher risk of ARI and that 10 of these 11 patients received a regimen containing cisplatin (60 mg/m2 infused for 60–90 min) [Citation5]. In addition, Hakeam et al. reported that 3.7% of patients developed ARI after cisplatin-based HIPEC regimens, and that the risk factors included low intraoperative urine output, the use of angiotensin II receptor antagonists, and hypertension [Citation6]. Moreover, the HIPEC procedure itself can be an independent risk factor for ARI after CRS/HIPEC. Arjona-Sanchez and colleagues compared different non-cisplatin-based HIPEC chemotherapy regimens with the same infusion time, and found that 30.5% of 141 patients had acute renal disease according to the RIFLE (Risk, Injury, Failure, Loss and End-stage kidney function) criteria [Citation3]. In addition, Dagel et al. compared three different treatment groups, patients receiving systemic cisplatin (75 mg/m2), surgery alone and HIPEC with cisplatin (75 mg/m2 infused for 60 min) and found that those who received HIPEC with cisplatin had a higher rate of ARI (31.2%, 10/32) compared to those treated with intravenous cisplatin (10.5%, 4/38) and surgery alone (11.7%, 4/34) (p = 0.04) [Citation7].
Other risk factors have also been reported in addition to the chemotherapy regimen, including older age, elevated baseline creatinine, pre-operative albumin, number of cycles of pre-operative carboplatin, the time between pre-operative chemotherapy and CRS/HIPEC, the volume of blood transfusions, a decrease in arterial blood pH after HIPEC, the use of ureteral catheters, a decrease in serum sodium after HIPEC, the intraoperative use of inotropic agents, and peritoneal cancer index (PCI) [Citation3–5]. However, the risk factors of creatinine increased after HIPEC are unclear and vary according to study design, heterogeneous HIPEC procedure, racial difference and experience in supportive care between hospitals.
Our institute is a teaching hospital in Taiwan, and we first used the HIPEC procedure in 2015, with multi-disciplinary teamwork (MDT) being established 1 year later. Our preliminary data showed that ARI developed in 2/45 cases [Citation2]. However, the number of cases of newly developed ARI after CRS/HIPEC has increased since then despite efforts to minimize the risk of ARI by following suggestions in the literature. Therefore, the aim of this study was to investigate the clinical associations and identify the risk factors of ARI after CRS/HIPEC.
Materials and methods
Study overview
From April 2015 to December 2019, a total of 181 patients with cancer peritoneal metastases who received CRS/HIPEC at Chang Gung Memorial Hospital, Chiayi, Taiwan were retrospectively analyzed. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (201701400A3).
Patient enrollment and image evaluation
All of the patients were discussed at MDT meetings before surgery [Citation2]. The following topics were decided by consensus: patient selection, pre-operative imaging evaluation, perioperative management and technique. The HIPEC procedure was indicated for: (1) curative intent of peritoneal metastases from primary or recurrent ovarian cancer, colorectal cancer, gastric cancer, peritoneal mesothelioma, pseudomyxoma peritonei and other malignancies with peritoneal metastases; and (2) palliation to control ascites; (3) adjuvant treatment for the prophylaxis of suspicious T4 disease from gastric cancer and colorectal cancer or tumor rupture during surgery. The exclusion criteria were as follows: (1) Eastern Cooperative Oncology Group (ECOG) performance status >2; (2) extensive tumor burden for which complete CRS could not be performed for curative intent according to imaging studies (unless to control ascites); and (3) unresectable extra-peritoneal metastases. Patients who had creatinine levels >1.5 mg/dL before CRS/HIPEC or ECOG >2 after surgery were also excluded to clarify the treatment effects.
All subjects underwent multidetector computed tomography (CT) or magnetic resonance imaging (MRI) before surgery. The preoperative CT/MRI PCI was scored by experienced radiologists according to the Sugarbaker classification. 18-fluoro-2-deoxy-D-glucose positron emission tomography-CT or chest CT was performed to exclude extraperitoneal disease. After the MDT discussion, the patients were scheduled for the CRS/HIPEC procedure.
Surgery and HIPEC technique
The MDT program members performed the surgery and HIPEC procedures. Before CRS with curative intent, diagnostic laparoscopy was performed for PCI evaluation if the results of pre-operative imaging studies were inconclusive, and this exploration ensured successful CRS. Procedures were canceled if the laparoscopic examination revealed that the disease was too extensive (). After CRS, completeness of cytoreduction score was assessed [Citation1]. For the indication of palliative HIPEC to control ascites or adjuvant HIPEC in a prophylactic setting, laparoscopy was used to release intra-abdominal adhesion and compartment before further HIPEC.
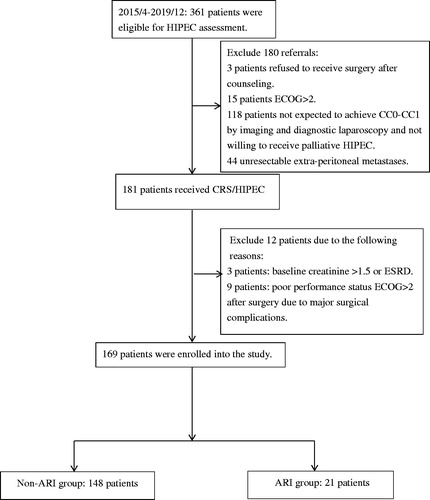
Figure 1. Flowchart of patient enrollment and study population. CC: completeness of cytoreduction score; CRS/HIPEC: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy; ECOG: Eastern Cooperative Oncology Group (ECOG) performance status; ESRD: end-stage renal disease; ARI: acute renal impairment.
After CRS, HIPEC was delivered using the closed method with a PerformerTM HT (RanD Biotech, Medolla, Italy). Three different perfusates were available: pure normal saline, a mixture of normal saline and pentastarch (Haes-steril, 60 mg/mL, Meda, Sweden) 10% (3:1), or Dianeal® PD4 peritoneal dialysis solution 1.5% Dextrose (Baxter). The perfusate was given at a dose of 2 L/m2 of the body surface. The chemotherapy was delivered after an intra-abdominal temperature of 41–43 °C had been reached, and the duration of HIPEC was 30–90 min. In all patients, urine output was monitored and maintained at 1 mL/kg/15 min during the HIPEC procedure. The choice of regimen and duration of HIPEC was according to the cancer types, status and corresponding references. The regimens included: (1) mitomycin 40 mg (30 mg at time 0; 10 mg at 60 min) delivered for 90 min or intraperitoneal oxaliplatin (460 mg/m2) and intravenous 5-FU 1 h before delivered for 30 min in colorectal cancer or pseudomyxoma peritonei [Citation8]; (2) cisplatin 100 mg/m2 (reducing dosage to 50–75mg/m2 if age ≥70 years-old or previous cisplatin exposure) and/or paclitaxel 175 mg/m2 delivered for 60 min in platinum-sensitive recurrent ovarian cancer or primary ovarian cancer [Citation9,Citation10]; (3) doxorubicin 35 mg/m2 and mitomycin 15 mg/m2 delivered for 60 min in platinum-resistant recurrent ovarian cancer [Citation9]; (4) mitomycin 15 mg/m2 and cisplatin 50 mg/m2 delivered for 60 min, doxorubicin 12.5 mg/m2 and cisplatin 50 mg/m2 delivered for 60 min or mitomycin 40 mg (30 mg at time 0; 10 mg at 60 min delivered for 90 min in gastric cancer [Citation11]. The intra-peritoneal chemotherapy drugs were drained out after HIPEC had been completed.
Perioperative care, monitoring, study parameters and data collection
Data on the patients’ characteristics, operative details, postoperative outcomes and pathology were recorded by the case manager and evaluated by the MDT committee. Intra-operative conditions, including fluid input/output, medications, blood loss and blood transfusion were evaluated from the anesthesia care records. The postoperative blood test included renal function, and electrolytes were regularly measured on postoperative days 1, 3 and 7 after April 2016 according to the MDT consensus. Postoperative complications were classified using a previously validated system, the NCI-CTCAE version 5.0. Renal impairment was graded according to the serum creatinine levels as follows: grade 1 represented > upper limit of normal reference (ULN)-1.5 times the ULN, grade 2 represented >1.5–3.0 times the baseline or >1.5–3.0 times the ULN, grade 3 represented >3.0 times the baseline or >3.0–6.0 times the ULN and grade 4 represented >6.0 times the ULN. Mortality was defined as any death occurring 30 days after surgery or during hospitalization. Follow-up CT/MRI was performed 3–6 months after surgery or when clinical symptoms recurred. Survival and disease recurrence was documented from medical records.
Statistical analysis
Descriptive statistics were reported as mean ± SD, median with minimum and maximum, or frequency with percentage as appropriate. Patients who developed post-CRS/HIPEC creatinine increased were defined as the ‘ARI’ group and those who did not were defined as the ‘non-ARI’ group. The associations between covariates and the occurrence of creatinine increased were examined using univariate analysis with the Chi-square test or Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. For those covariates with p < 0.15 in the univariate analysis, they were then considered in multiple logistic regression. The explanatory variables in the final model were identified manually by dropping the covariates with p-value >0.05 one at a time until all regression coefficients were significant. Besides, to adjust for baseline characteristics, insignificant variables, such as age and gender, were also included in the final model. All analyses were performed using SPSS statistical software (IBM SPSS Statistics for Windows, version 22.0, IBM Corp., Armonk, NY). All tests were two-sided, and a p-value <0.05 was considered to be statistically significant.
Results
Patients
The patient enrollment flowchart is shown in . From April 2015 to December 2019, 181 patients received CRS/HIPEC. Among these patients, high baseline serum creatinine (creatinine >1.5 mg/dL) were identified in 3 patients and major postoperative complications with poor performance status (ECOG > 2) were identified in another 9 patients. These 12 patients were excluded. Of the 9 patients with major postoperative complications, one refused early ambulation and suffered from unexpected sudden death, possibly due to pulmonary embolism or myocardial infarction. This patient had normal post-operative kidney function and creatinine, although creatinine levels could not be tested on the 7th postoperative follow-up. The other 8 patients had both postoperative rise in creatinine and postoperative complications, including intraabdominal abscess, bowel perforation, bladder and ureter injury, and other severe infection. In these 8 patients, their high creatinine levels were possibly related to sepsis, direct urinary tract injury or renal damage. The remaining 169 patients were eligible for analysis. The mean (± SD) age of these patients was 54.26 (±12.14) years, and the mean (± SD) follow-up time was 628 (±397) days. Of the 169 patients, 21 (12.4%) were identified as having postoperative creatinine increased. As shown in , before CRS/HIPEC, the ARI group used a higher proportion of cisplatin HIPEC regimens than the non-ARI group (71.4 vs. 37.8%, p = 0.004). The distribution of cancer types in these two groups was different (p = 0.04), and the ARI group had a higher rate of ovarian cancer. Other demographic variables were comparable between the two groups. The mean duration of hospital stay was also similar (13.43 ± 4.26 vs. 13.34 ± 8.61 days for the ARI group and non-ARI group, respectively, p = 0.962) ().
Table 1. Demographic and clinical data of the patients who underwent CRS/HIPEC.
Risk factors of acute kidney injury
The univariate analysis showed that the patients who had previously received systemic platinum-based chemotherapy (odds ratio [OR] = 4.30, 95% confidence interval [CI] = 1.63–11.35, p = 0.003), those with ovarian cancer (OR= 3.49, CI = 1.36–9.00, p = 0.010) and those who received cisplatin HIPEC regimens (OR= 4.11, CI = 1.51–11.20, p = 0.006) were at a higher risk of creatinine increased (). In contrast, albumin and electrolyte levels on postoperative day 1 were not related to the development of creatinine increased (). Furthermore, when significant variables in the univariate analysis were considered simultaneously, the adjusted odds ratios (AORs) revealed that the patients with the following risk factors had an elevated risk of postoperative creatinine increased: cisplatin HIPEC regimens (AOR = 11.38, CI = 3.07–342.13, p < 0.001) and peritoneal dialysis solution as the HIPEC perfusate (AOR = 7.07, CI = 2.03–24.26, p = 0.002) ().
Table 2. Analysis of risk factors for the occurrence of ARI using simple logistic regression models.
Table 3. Analysis of risk factors for the occurrence of ARI using simple logistic regression models (postoperative day 1 serum electrolytes and albumin level).
Table 4. Analysis of risk factors for the occurrence of ARI using multiple logistic regression models.
Characteristics of the 21 ARI patients
The cisplatin dose ranged from 50–100 mg/m2. Cisplatin regimen was administrated to 71 patients who received HIPEC. Of them, creatinine increased occurred in 44% (7/16) of patients following HIPEC with peritoneal dialysate solution and the prevalence of creatinine increased was 15% (8/55) after HIPEC with saline infusion. In the group of patients receiving cisplatin regimen, the patients who received peritoneal dialysate as perfusate were more likely to develop creatinine increased (OR= 4.57, p = 0.016). For patients with a non-cisplatin regimen (n = 98), the prevalence of creatinine increased was lower than those with a cisplatin regimen. Of them, only 10% (6/60) of patients reported creatinine increased following HIPEC with peritoneal dialysate solution whereas none reported creatinine increased following HIPEC with saline infusion. Of the 21 patients with creatinine increased, 14, 4, 2 and 1 developed grade 1, 2, 3 and 4, respectively (). None of the patients with postoperative creatinine increased needed renal replacement therapy. Patients with grade 1 and 2 creatinine increased recovered to a normal creatinine level. Of the patients with grade 1 creatinine increased, the median time to recovery to a normal creatinine level was 15.5 days, compared to 46 days for those with grade 2 creatinine increased. Three patients had grade 3–4 creatinine increased, in whom the creatinine level did not recover to normal range.
Table 5. Descriptive statistics of kidney function in the 21 ARI patients.
Discussion
In this study, 12.4% of our patients developed CTCAE grade 1–4 creatinine increased events after CRS/HIPEC, however, none of the patients needed renal replacement therapy. Cisplatin HIPEC regimens and perfusate with peritoneal dialysis solution were the major independent risk factors for developing ARI.
Chemotherapeutic agents have been associated with ARI after HIPEC. For example, cisplatin, oxaliplatin, mitomycin and doxorubicin are commonly used in HIPEC, and they have been associated with chemotherapy-induced renal toxicity and electrolyte metabolic disturbance [Citation4,Citation12–14]. Renal toxicity is one of the major side effects of using cisplatin. Cisplatin damages the proximal renal tubules and influences the function of renal regulation. Electrolyte imbalance, such as hypomagnesemia, hypocalcemia and renal salt wasting after cisplatin treatment has also been reported [Citation15]. In line with other reports, we found that the patients who received a cisplatin HIPEC regimen had a significantly higher incidence of creatinine increased compared to those who received non-cisplatin HIPEC regimens in this study [Citation4,Citation6,Citation16]. The choice of chemotherapy regimen for HIPEC was highly related to the type of cancer, and more of the ARI group had ovarian cancer and were treated with cisplatin. To prevent ARI, we have developed a few adoption strategies pre-, intra- and post-HIPEC, including aggressive perioperative hydration, close intraoperative urine output monitoring and routine preoperative albumin administration. Each patient was given 0.9% saline intravenously at 1.5 mL/Kg/h for at least 24 h before and after HIPEC. Intraoperative urine output was maintained at 1 mL/kg/15 min with either furosemide or saline hydration. An initial dose of 500 mL of the 5% albumin solution was given to the patient pre-operatively and an additional 500 mL of the 5% albumin solution was given if hypoalbuminemia was found postoperatively [Citation6]. Despite these efforts, some of our patients still developed grade 3–4 creatinine increased. In some HIPEC centers and in clinical trials, amifostine or sodium thiosulfate is routinely used to prevent cisplatin-induced severe renal impairment [Citation10,Citation17]. However, these agents are not currently available in Taiwan. We believe that the introduction of these agents may be helpful to prevent HIPEC-related ARI in Taiwan.
The HIPEC procedure requires a carrier solution as a medium in intra-abdominal circulation. There is currently no consensus on the choice of perfusate, and commonly used carrier solutions include peritoneal dialysis solution [Citation18] and saline solution [Citation19], which can be categorized as being colloid and crystalloid. We found a significantly higher risk of creatinine increased using peritoneal dialysis solution as the perfusate compared to normal saline-based solution. Peritoneal dialysis solution is a hypertonic agent and its main ingredient dextrose can be absorbed via peritoneal membranes, causing hyperosmolarity and hyperglycemia [Citation20]. Intraoperative hyperglycemia during HIPEC has been reported to be a common phenomenon, and it has been associated with an increased risk of infection [Citation21,Citation22]. In addition, hyperglycemia and intravenous compensation of sodium loss into the perfusate have been reported to lead to hyperosmolar hyponatremia and further influence the biochemical disturbance [Citation13]. This hyperosmotic stress is a potent inflammatory stimulus, and intravascular dehydration may worsen renal damage [Citation23]. In addition, hyperglycemia leads to the specific pathogenesis of tissue injury through oxidative stress by increasing the production of reactive oxygen species in the mitochondria. Lower renal cortical perfusion, severe morphologic renal damage, and lower oxygen delivery have been observed in patients with perioperative hyperglycemia [Citation24]. While cisplatin is preferred for HIPEC regimens, peritoneal dialysis solution perfusate should be used with caution due to the risk of aggravating renal toxicity. Perioperative strict glycemic control might also be helpful to minimize the risk of ARI [Citation24].
The severity of creatinine increased is related to long-term renal prognosis. In the current study, most of the patients’ serum creatinine levels returned to normal within 3 months. However, we found that the renal function did not recover in patients with advanced ARI that was greater than grade 3 after HIPEC, which is consistent with a previous study [Citation4]. Based on the finding that maximal creatinine elevation occurred within 7 days after the procedure, we suggest that the renal function of patients should be carefully monitored for 7 days after the operation.
There are several strengths to this study. First, all of the patients were from one single teaching hospital, and the CRS/HIPEC procedure was uniform. The surgical principle, technique and indications were consistent and the perioperative care was performed by the members of the peritoneal malignancy MDT program [Citation2]. Second, only a few studies have focused on ARI after CRS/HIPEC in an East Asian population [Citation4,Citation5], and this study further analyzed the risk of ARI after CRS/HIPEC in a larger Asian population. Our results broaden the understanding of this postoperative complication. Third, we identified that the use of peritoneal dialysis solution as the carrier solution was a significant risk factor for creatinine increased, which has not previously been reported in other studies. Fourth, we used NCI-CTCAE version 5.0 criteria to categorize the severity of renal complications, and the data were collected and reviewed by two experienced physicians. These results are reliable and this approach could be easily used in daily practice.
However, this retrospective study also had some limitations. The study is retrospective in nature and consisted of heterogeneous cancer types. The indication (curative intent or palliation) and CRS procedure varied from person to person. Patient selection bias and missing data were possible. Excluding patients with post-operative ECOG >2 from the analysis may result in selection bias. The aim of this study was to explore major HIPEC-related risk factors and therefore these patients were excluded to eliminate confounding variables. In addition, the incidence of severe ARI was relatively low in this study under MDT perioperative care. Patients with mild creatinine increased were classified into the ARI group, and overestimation of the risk factors identification may have occurred.
In conclusion, a cisplatin HIPEC regimen and HIPEC perfusate using peritoneal dialysis solution were independent risk factors for developing post-CRS/HIPEC creatinine increased. Identifying these risk factors may help to improve patient selection, a dose of HIPEC regimens modification, and perioperative care. Patient stratification based on these perioperative risk factors should be considered in further prospective studies. Furthermore, designing a prospective study to validate the new finding of the influence of the peritoneal dialysis solution is the future research aspect. The development of tools to detect ARI at an early stage remains an unmet medical need.
Acknowledgments
The authors are grateful to the members of the Peritoneal Malignancy Program of the Cancer Center Chang-Gung Memorial Hospital, Chiayi, and the case manager, Tzu-Ting Liao. The authors also thank ATS Medical Editing and Review Solutions for language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev. 2016;48:42–49.
- Wang TY, Chen CY, Lu CH, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal malignancy: preliminary results of a multi-disciplinary teamwork model in Asia. Int J Hyperthermia. 2018;34(3):328–335.
- Arjona-Sanchez A, Cadenas-Febres A, Cabrera-Bermon J, et al. Assessment of RIFLE and AKIN criteria to define acute renal dysfunction for HIPEC procedures for ovarian and non ovarian peritoneal malignances. Eur J Surg Oncol. 2016;42(6):869–876.
- Sin EI, Chia CS, Tan GHC, et al. Acute kidney injury in ovarian cancer patients undergoing cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. Int J Hyperthermia. 2017;33(6):690–695.
- Ye J, Ren Y, Wei Z, et al. Nephrotoxicity and long-term survival investigations for patients with peritoneal carcinomatosis using hyperthermic intraperitoneal chemotherapy with cisplatin: a retrospective cohort study. Surg Oncol. 2018;27(3):456–461.
- Hakeam HA, Breakiet M, Azzam A, et al. The incidence of cisplatin nephrotoxicity post hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery. Ren Fail. 2014;36(10):1486–1491.
- Dagel T, Misirlioglu S, Tanju S, et al. Hyperthermic intraperitonal chemotherapy is an independent risk factor for development of acute kidney injury. J Buon. 2018;23(5):1528–1533.
- Turaga K, Levine E, Barone R, et al. Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. 2014;21(5):1501–1505.
- Spiliotis J, Halkia E, Lianos E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015;22(5):1570–1575.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240.
- Roviello F, Caruso S, Neri A, et al. Treatment and prevention of peritoneal carcinomatosis from gastric cancer by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: overview and rationale. Eur J Surg Oncol. 2013;39(12):1309–1316.
- Rueth NM, Murray SE, Huddleston SJ, et al. Severe electrolyte disturbances after hyperthermic intraperitoneal chemotherapy: oxaliplatin versus mitomycin C. Ann Surg Oncol. 2011;18(1):174–180.
- De Somer F, Ceelen W, Delanghe J, et al. Severe hyponatremia, hyperglycemia, and hyperlactatemia are associated with intraoperative hyperthermic intraperitoneal chemoperfusion with oxaliplatin. Perit Dial Int. 2008;28(1):61–66.
- Lee VW, Harris DC. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology. 2011;16(1):30–38.
- Miller RP, Tadagavadi RK, Ramesh G, et al. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2(11):2490–2518.
- Cata JP, Zavala AM, Van Meter A, et al. Identification of risk factors associated with postoperative acute kidney injury after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: a retrospective study. Int J Hyperthermia. 2018;34(5):538–544.
- Bouhadjari N, Gabato W, Calabrese D, et al. Hyperthermic intraperitoneal chemotherapy with cisplatin: amifostine prevents acute severe renal impairment. Eur J Surg Oncol. 2016;42(2):219–223.
- Cioppa T, Vaira M, Bing C, et al. Cytoreduction and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis from pseudomyxoma peritonei. World J Gastroenterol. 2008;14(44):6817–6823.
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575–1581.
- Holmes CJ. Glucotoxicity in peritoneal dialysis-solutions for the solution!!. Adv Chronic Kidney Dis. 2007;14(3):269–278.
- Stewart CL, Gleisner A, Halpern A, et al. Implications of hyperthermic intraperitoneal chemotherapy perfusion-related hyperglycemia. Ann Surg Oncol. 2018;25(3):655–659.
- Torphy RJ, Stewart C, Sharma P, et al. Dextrose-containing carrier solution for hyperthermic intraperitoneal chemotherapy: increased intraoperative hyperglycemia and postoperative complications. Ann Surg Oncol. 2020. doi:10.1245/s10434-020-08330-y
- Brocker C, Thompson DC, Vasiliou V. The role of hyperosmotic stress in inflammation and disease. Biomol Concepts. 2012;3(4):345–364.
- Mendez CE, Der Mesropian PJ, Mathew RO, et al. Hyperglycemia and acute kidney injury during the perioperative period. Curr Diab Rep. 2016;16(1):10.
