Abstract
Background
Outcomes of high-intensity focused ultrasound (HIFU), as a non-surgical treatment option for benign symptomatic thyroid nodules, has mainly been based on single-center studies and short-term follow-up. Therefore, we assessed the safety, and long-term efficacy of HIFU in benign thyroid nodules among four centers with expertise in thyroid mini-invasive procedures.
Patients and methods
Retrospective three year follow-up study in four European centers, treating solid benign thyroid nodules causing pressure symptoms and/or cosmetic concerns. Nodule volume reduction was assessed at 1, 3, 6, 12, 24, and 36 months post-treatment. Technical efficacy, defined as a volume reduction rate (VVR) >50% was evaluated at 6, 12, 24 and 36 months. Predictive factors of efficacy were assessed using logistic models. Complications and side effects were classified according to the Interventional Radiology Guidelines and changes in local symptoms were scored on a visual-analog scale.
Results
Sixty-five patients (mean age 51.1 ± 14.0 years; 86.2% women) with a single thyroid nodule and a mean baseline nodule volume of 9.8 ± 10.3 mL were treated with a mean energy of 7.1 ± 3.1 kJ (range: 2.0 to 15.5 kJ). Median nodule volume reduction was 31.5% (IQR: −38.6% to −23.1%) at 12 months and 31.9% (IQR: −36.4% to −16.1%) at 36 months. Technical efficacy was obtained in 17.2% of cases at 6 months, 17.8% at 12 months, 3.4% at 24 months, and 7.4% at 36 months. The number of treated pixels and the mean energy delivered were positively correlated to VRR at 1, 6 and 12 months. The risk of treatment failure decreased by 4.3% for each additional unit of energy delivered. The procedure duration was inversely correlated with treatment failure (OR 1.043, 95% CI: 1.011–1.083; p = 0.014). Improvement of cervical pressure symptoms or cosmetic complaints were observed in less than 15% of the cases at 12, 24 and 36 months. Horner’s syndrome occurred in one case (1.5%) and minor complications, not requiring treatment, in three (4.6%) patients. No change in thyroid function was registered.
Conclusions
HIFU carried a low risk of complications. A single treatment resulted in a 30–35% thyroid nodule volume decrease within one year, reduction that remained stable for 2 years. Outcomes varied significantly between centers with different HIFU expertise. Focus on improved HIFU technology, adequate training, and appropriate selection of patients is needed to achieve efficacy comparable to other thermal ablation procedures.
Introduction
Most thyroid nodules (TNs) are benign and asymptomatic but 10–15% increase over time and become clinically relevant [Citation1,Citation2]. The management of symptomatic thyroid nodules poses three problems: (i) Identification and treatment of the minority of thyroid cancers, (ii) Improvement of local symptoms [Citation2], (iii) Preservation of thyroid function. However, the traditional surgical approach to symptomatic benign pathology should be modified because surgery is expensive [Citation3–5], confers a risk of complications [Citation6,Citation7], and is frequently followed by lifelong thyroid hormone replacement therapy [Citation8].
For these reasons, thermal ablation (TA) should be considered as a potential first-line treatment for selected patients with thyroid nodules, as recommended by the Korean Health Authorities (2012), the American Association of Clinical Endocrinologists, the American College of Endocrinology, and the Italian Society of Endocrinologists (AME) [Citation9–11]. Ultrasound (US)-guided percutaneous ablation using ethanol (EA), radiofrequency (RFA), laser ablation (LTA), microwaves (MWA), and high-intensity focused ultrasound (HIFU) are the techniques proposed for non-surgical management of thyroid nodules that cause local symptoms. Several retrospective and prospective randomized trials [Citation12–17] have demonstrated the efficacy and safety of RFA and LTA for the management of symptomatic thyroid lesions. A European Thyroid Association guideline on the use of minimally invasive procedures for thyroid nodules was recently released [Citation18], and the implementation of thyroid ablation techniques in Europe is currently suggested [Citation19].
HIFU is the most recent TA technique for thyroid lesions and is the only truly noninvasive method. HIFU does not require the insertion of any device into the neck and thereby prevents the risk of bleeding, infection, or thermal injury to the skin reported for the other TA procedures [Citation20–22]. HIFU, however, is still not a thoroughly assessed procedure for the management of thyroid lesions. The available evidence is based on a few retrospective and prospective single-center, mostly non-controlled, series of thyroid nodules with heterogeneous enrollment criteria, variable protocols of treatment, and short-term follow-up [Citation23]. Thus, the potential advantages and limitations of HIFU in comparison to the other, more comprehensively studied minimally invasive procedures remain to be established.
The aims of our study were: (i) To evaluate clinical efficacy and nodule volume changes over a follow-up period of up to 3 years; (ii) To assess tolerability and safety of HIFU ablation in a series of solid (≤10% of fluid component) non hyper functioning thyroid nodules; (iii) To assess the variability of results among thyroid reference centers using the same equipment; (iv) To analyze the outcomes according to the treatment parameters and baseline characteristics.
Patients and methods
Study design
A multicenter study was conducted in four accredited European thyroid referral centers (American Hospital of Paris (France), General Hospital of Livorno (Italy), Regina Apostolorum Hospital, Albano (Italy), and Azienda Sanatoria di Teramo Hospital (Italy). All institutions had a more than 3-year experience in the use of TA for thyroid nodules and an at least 3-month experience (range: 3–24 months) with HIFU treatment. The HIFU procedure was performed by operators with specific skill in TA and with the use of the same HIFU device. Clinical, biochemical and US follow-up was performed blindly by external thyroid experts. Follow-up was planned for 36 months. Data were registered by an external monitor and anonymously analyzed by an independent statistician.
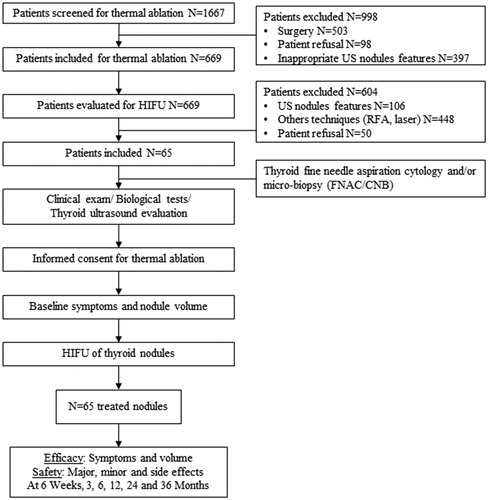
Patients
From January 2015 to December 2018, 1667 patients with symptomatic thyroid nodules and nodular goiters were referred to the four participating centers. After the initial diagnostic work-up, 669 patients (40.1%) were offered a TA procedure as a therapeutic option alternative to surgery. After full information, 65 of them (9.7%) asked for treatment with the HIFU procedure and were enrolled from January 2015 to December 2018. Inclusion criteria were: (i) Nodule benign at repeat cytological examination; (ii) Solid structure (≤10% of fluid component); (iv) Presence of pressure symptoms or cosmetic concerns; (v) Normal thyroid function; (vi) Written informed consent after dialogue with the operators.
Pressure symptoms were defined as persistent complaints of cervical constriction or dysphagia not related to cervical co-morbidity. Cosmetic complaints were defined as the presence of a visible nodule according to the WHO goiter classification [Citation24].
Exclusion criteria were: (i) Age <18 years or >80 years; (ii) Pregnant or lactating women; (iii) Inability to maintain a hyperextended neck; (iv) Presence of tattoos, skin moles, or scars on the path of the ultrasound beam passing; (v) Preexisting contralateral vocal cord palsy; (vi) History of neck irradiation; (vii) Presence of fluid collections or macro calcifications; (viii) Cytological or US suspicion of malignancy; (ix) Close contact of the nodule with heat-sensitive structures (trachea, esophagus, carotid artery) [Citation25].
Patient assessment before HIFU procedure
Nodule US features were classified according to the Thyroid Image Reporting and Data System (TI-RADS) classification [Citation26] and nodule vascularization was categorized by color flow Doppler as absent, peri-nodular, or peri- and intra-nodular. Two benign fine needle aspirations were obtained before enrollment [Citation27] and a pretreatment neck sonography was performed by the operator (Esaote MyLab Twice, LA435) for planning the procedure. Vagal and recurrent laryngeal nerves were considered as ‘danger zones’ and excluded from treatment. All imaging data were recorded on a map-drawing.
The following biochemical tests were performed: serum TSH (µIU/mL), fT3 (pg/mL), fT4 (ng/dL), anti-TPO antibodies (IU/L), calcitonin (pg/mL), blood calcium level (mg/L), blood count, and routine coagulation tests according to routine methods at the participating centers.
I123 thyroid nuclear imaging (Siemens Symbia® gamma camera, Germany) was performed in case of serum TSH levels at the lower limit of normal range to rule out a hyperfunctioning nodule.
HIFU procedure
The four centers performed HIFU treatment according to a similar protocol. After informing about the procedure and the possible need of further treatments in case of insufficient volume reduction, patients were placed in the supine position with extension of the neck. In one center (AHP), HIFU treatment was performed after local anesthesia with 2% lidocaine solution, while in two centers conscious sedation with intravenous midazolam (fl 2.5 mg) was used to improve compliance.
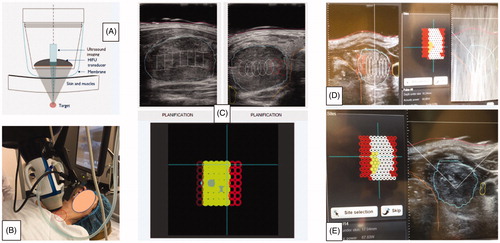
HIFU ablation was performed, according to the previously published technique [Citation21,Citation28], with an Echopulse system (Theraklion SA, Malakoff, France). The limits of the nodules and the margins of the surrounding cervical structures were delineated on the active screen with a pencil-like device. The Beamotion computer software automatically identified the areas suitable for safe treatment excluding the danger zones. The neck position was controlled by a laser mechanism that stopped treatment in case of patient movement. The first HIFU shot was delivered with an energy of 45 W/site and, successively, the dose was calibrated based on complaints and the efficacy of the pulse. The number of treated pixels resulted from the percentage of nodule volume eligible for treatment.
A pressure dressing and an ice bag were applied over the neck skin after HIFU treatment. Early complications were ruled out after one hour by US examination of the cervical region and patients were discharged with a non-opioid pain-killer prescription for the following 24 h.
The tolerability of the treatment was assessed with a visual-analog scale from 0 (no pain) to 10 (worst imaginable pain). Duration of treatment, mean delivered energy, peri-procedural complications, and protracted pain requiring parenteral analgesics were registered by the monitor.
Outcomes and follow-up
The volume reduction rate (VRR) of the treated nodules was calculated in percentage as follows: [(nodule final volume (mL) − nodule initial volume (mL))/nodule initial volume (mL)] × 100. Technical efficacy [Citation29] was defined as a VRR ≥50% at 6 months (intermediate follow-up). VRR was evaluated at 6 weeks, 3, 6, 12, 24, and 36 months. Improvement of local symptoms was evaluated at 6 and 12 months. In case of treatment failure, defined either as a VRR <50% at 6 months or as nodule regrowth during follow-up, a second treatment with TA (either HIFU, LTA, or RFA) or surgery was proposed. Early US changes, corresponding to an early nodular volume reduction, were evaluated at 1, 3 and 6 months.
During follow-up, major complications, minor complications, and side effects [Citation29] were recorded and classified, according to the criteria of the guidelines of the Society of Interventional Radiology (SIR) [Citation30].
Statistical analysis
Continuous variables were expressed as mean with standard deviation while categorical variables were expressed as numbers (percentage). The normality of any distribution was assessed using histograms and the Shapiro–Wilk test.
The consistency of results and the influence of the different operators performing the procedure (‘center effect’) on nodule volume reduction after HIFU treatment was evaluated using a longitudinal mixed model (center effect p value: 0.039; interaction time*center p value: <0.001). Pearson correlation was used to assess the relationship between continuous baseline parameters and nodule volume reduction at each time point. The relationship between the baseline nodule volume and the VRR at 6 months was evaluated using linear models. The possible influence of the baseline US features on VRR was evaluated with the comparison of the nodule structure groups using Student T-test. The evaluation of baseline risk factors of treatment failure requiring additional intervention was by logistic models.
The threshold of significance was 0.05. For analyses, R software version 3.4 (R Studio, Boston, MA, USA) was employed.
Ethics
All patients gave their written informed consent before the inclusion in the study protocol. Surgery was always proposed as a first-line treatment option and all enrolled patients declined surgery after a dialogue with the physician.
This study followed the tenants of the Declaration of Helsinki and was approved by the institutional review boards of the participating centers.
Results
Baseline features
Sixty-five consecutively treated adult patients [mean age: 51.1 ± 14.0 years; 56 females (86.2%) and 9 males] with a single or dominant TN were enrolled in the study (). All patients had initial cervical complaints [predominantly pressure symptoms: 45/65 (69.2%), predominantly cosmetic complaints: 20/65 (30.8%)]. A detailed description of the cohort is given in .
Table 1. Description of patients’ and nodules’ characteristics.
Baseline mean nodule volume was 9.8 ± 10.3 ml (range: 1.1 to 68.7 ml). All nodules were solid with ≤10% fluid component or spongiform (lesion containing multiple small cysts smaller than 5 mm interspersed within solid tissue for nearly all the volume) at US examination. Sixty-four of 65 nodules (98.5%) were classified as probably benign (TI-RADS category 3) while 1/65 (1.5%) was classified as a TI-RADS category 4. Central vascularization was registered in 40/65 cases (61.5%) and a scanty or peripheral vascularization in 25/65 cases (38.5%). The mean delivered energy was 7.1 ± 3.1 kJ (range: 2 to 15.5 kJ). The mean treatment duration was 45.8 ± 20.7 min (range: 17 to 103 min) ().
Table 2. HIFU ablation parameters.
The planned follow-up duration was 36 months but the actual follow-up time was 20.9 ± 14.0 months. Only 29/65 patients (44.6%) completed the 3-year follow-up while, due to technical failure of the treatment, 26/65 patients (40.0%) underwent a further therapeutic action before the conclusion of the planned follow-up period. Finally, 10/65 patients (15.4%) were lost to follow-up after the 12-month control. The number of nodules (N) controlled at each follow-up visit is given in .
Clinical efficacy and volume reduction
The median nodule VRR was: −16.7% (IQR: −30.4% to −4.4%) at 6 weeks, −19.5% (IQR: −33.3% to −6.0%) at 3 months, −30.3% (IQR: −39.9% to −22.3%) at 6 months, −31.5% (IQR: −38.6% to −23.1%) at 12 months, −33.3% (IQR: −39.0% to −27.3%) at 24 months, and −31.9% (IQR: −36.4% to −16.1%) at 36 months.
Technical efficacy was obtained in 17.2% of cases at 6 months, 17.8% at 12 months, 3.4% at 24 months, and 7.4% at 36 months. The early US changes registered at the 1-, 3- and 6-month US controls were predictive of the final nodule volume reduction assessed at the 12-, 24- and 36-month US evaluation (p<.05) (Supplemental Table 1).
The median nodule volume reduction at M12 was significantly different between the four participating centers (VRR: −12.5% (IQR: −28.1% to 0.0%) in center 1, −28.3% (IQR: −34.2% to −13.3%) in center 2, −33.3% (IQR: −35.6% to −30.2%) in center 3, and −59.6% (IQR: −67.2% to −33.7%) in center 4; p = 0.002). At M36, VRR was +20.4% (IQR: 9.5% to 42.7%) in center 1, −33.3% (IQR: −35.7% to −30.8%) in center 3, and −54.1% (−65.7% to −43.6%) in center 4 (p < 0.001). No data at 36 months were available for center 2 (Supplemental Tables 2 and 3).
The 12-month nodule volume reduction obtained in the first 33 cases was compared to the volume decrease registered in the last 32 cases of the present series. The VRR was not significantly greater in the last treated cases (VRR in cases #1–33: −14.3% vs. VRR in cases #34–65: −20.0%, p = 0.73). The VRR in the center with the shortest experience with HIFU treatment was significantly lower than in the centers with the more protracted experience (Supplemental Table 2).
No significant difference between solid and spongiform nodules, was observed concerning volume reduction rate at M1 (p = 0.34), M3 (p = 0.067), M6 (p = 0.33), M12 (p = 0.64) and M24 (p = 0.33). No significant correlation (data not shown) was observed between the initial size of the nodule, the nodule vascularization and the 12-month VRR.
Twenty-six out of 65 patients (40.0%) reported improvement of their cervical symptoms and disappearance of their cosmetic symptoms at the 12- and 36-month clinical control.
During follow-up, 26 out of 65 patients (40.0%) requested further therapy due to inadequate efficacy of the HIFU treatment. The additional therapy was given with a different modality of thermal ablation (LTA: 27.7%, RFA: 0%) or by surgical resection (12.3%).
HIFU treatment outcomes are summarized in and .
Figure 2. Nodule volume reduction from baseline. In brackets (): number of patients at each time of follow-up.
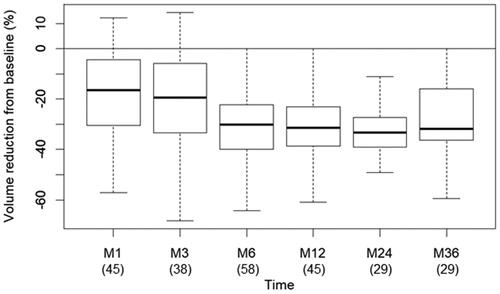
Table 3. Evaluation of volume reduction at each time of follow-up.
Tolerability and safety
The peri-procedural pain level, as evaluated on a visual-analog scale (0–10), was 6.3 ± 0.8. 48/65 patients (73.8%) requested conscious sedation or temporary suspension of the procedure for alleviating their neck and back pain before completion of the treatment. 75.4% of patients needed analgesics, as category 1 or 2 painkillers, for 48 h.
Adverse events are detailed in . One major and three minor adverse events were seen. One treatment was complicated by severe shoulder pain and immediate occurrence of Horner’s syndrome, that disappeared 6 months following treatment. Subcutaneous edema, not associated with pain and lasting from 2 weeks to 3 months, was observed in three patients.
Table 4. Evaluation of the safety of the HIFU ablation.
No recurrent laryngeal nerve palsy, hematoma, local blistering, sub-cutaneous abscess, nodule rupture or thyroid dysfunction was encountered.
Correlation between HIFU ablation parameters and nodule volume reduction at each time point
The number of treated pixels and the mean energy delivered were significantly positively correlated to VRR at M1, M6 and M12. However, the treated volume of the lesions, expressed in mL, was not significantly correlated with the percent volume reduction (Supplemental Table 4). The risk of treatment failure decreased by 4.3% for each additional unit of energy delivered. The procedure duration was inversely correlated with treatment failure (OR 1.043; 95% CI: 1.011–1.083; p = 0.014).
No baseline clinical or biochemical parameters were significantly associated with VRR or changes in local symptoms or signs.
Discussion
HIFU is the only completely noninvasive technique currently available for non-surgical management of symptomatic thyroid nodules [Citation20,Citation31] (). The procedure does not require expertise in interventional radiology and its computer-assisted modality of ablation may potentially prevent the occurrence of major complications. Notwithstanding these advantages, HIFU remains a promising but not yet thoroughly assessed, nor widely employed, procedure for the management of thyroid lesions that cause local symptoms [Citation19]. The reported HIFU outcomes appear favorable, even if widely variable, with a mean VRR at 12 months that ranges from 43% to 70% [Citation20,Citation21,Citation23,Citation25]. Presently, the outcomes of RFA and LTA appear as more satisfactory, with a VRR that ranges from 60 to 85% at 12 months and with a low rate of peri-procedural complications. In the published HIFU studies [Citation32,Citation33], inclusion and exclusion criteria, nodule structure, size, and function are frequently not clearly defined. In addition, most single center series do not accurately describe the modalities of treatment and whether repeat ablation sessions are given. For these reasons, the present multicenter study was addressed at establishing the safety and efficacy of a single HIFU ablation in a controlled series of solid, non-hyper functioning, well-characterized benign thyroid nodules.
Figure 3. HIFU procedure. (A) Diagram of the active head: focalization of ultrasound on a target zone guided by ultrasound imaging. (B) The treatment head is positioned in contact with the skin in front of the nodule. A laser beam (red circle) detects any movement of the patient and can stop the procedure (repositioning of the system). (C) Planning. The operator draws on the screen, the boundaries of the nodule, the skin the trachea and the carotid artery. The computer-controlled device will treat the target tissue, while preserving the surrounding tissues. (D) Software screen during treatment: on the left: Concentrated Interference Pattern. (E) Hyperechoic marks (whitish spots) correspond to a treated area.
Nodule volume changes
The mean VRR was about 30% at 12 months. The lower efficacy of HIFU in our study, in comparison with previous reports, is most likely due to the following reasons:
Our mean baseline nodule volume was significantly higher (9.8 ± 10.3 mL) than in previous studies, as summarized in a recent review (mean: 3 mL, range: from 0.6 to 24.5 mL) [Citation25]. The attainment of a similar ablation zone results in a lower percentage reduction in larger nodules. (Supplemental Table 5).
Conscious sedation and local anesthesia were not routinely used in our trial. The most successful reported data were obtained with the use of conscious sedation and or local anesthesia. In our experience, pain was a main limiting factor for completing the procedure with a high output. In our series, the procedure was interrupted and then resumed with a lower delivered energy in a large part of the cases. HIFU appears to be a more painful technique than radiofrequency or LTA [Citation34]. For this reason, the use of peri-thyroidal lignocaine infusion (PLI) was proposed during HIFU sessions, to provide additional analgesia in patients already receiving intravenous pain-killers [Citation35].
In large volume nodules, the peripheral areas are frequently excluded from treatment, because they are close to vital structures, resulting in a less complete ablation [Citation36]. Due to the unsatisfactory VRR in large nodules, 40% of our patients needed a second treatment with TA or surgery. This finding is in accordance with the results of recent studies, that registered the need of at least one repeat HIFU session to obtain a satisfactory volume reduction in large nodules [Citation37,Citation38].
HIFU outcomes were at least in part dependent on the centers’ expertise. The center with limited (3-month) HIFU experience obtained less satisfying results than those with longer practice. Independent hereof, also the three centers with protracted expertise obtained a lower VRR than that generally reached with LTA, RFA and MWA thermal therapy [Citation16,Citation39,Citation40].
Local symptom changes
Before the HIFU ablation, 70.7% (45/65) of our patients reported cervical problems, as pressure or cosmetic complaints. The improvement of cervical complaints after TA is generally reported as positively correlated to a clinically significant nodule volume decrease. According to the rather low rate of technical efficacy attained in our study, only 15% (7/45) of our patients had a significant improvement of their symptoms at 6, 12 and 24 months.
Safety
Complications and side-effects were rare in our study even if peri-procedural cervical pain with irradiation to the ipsilateral shoulder were registered in 75.4% of cases. HIFU seems a less tolerable technique than RFA or LTA because with these two techniques mild to severe pain is generally reported in about 50% of the treatments [Citation12–17].
HIFU is a safe procedure as we registered only one major complication, a Horner’s syndrome spontaneously resolving within 6 months. A similar case was described in only one previous prospective study. This complication was probably due to an overzealous treatment and to the nodule location, close to the sympathetic chain. This finding suggests the need of precisely identifying a further ‘sympathetic danger zone’ that is at risk to be involved by the ultrasound beam. Importantly, no cases of transient recurrent laryngeal nerve palsy, compressive hematoma, sub-cutaneous abscess or thyroid dysfunction were registered in our series of treatments. Three minor complications (4.6%) due to the development of subcutaneous cervical edema spontaneously resolving within three months were observed.
Cost and duration of the procedure
The cost of the HIFU device is around 250,000 euros (€) (vs. 30,000 €for laser, 17,000–25,000 €for RFA and 20,000–25,000 €for MWA) and the cost of disposables is about 500 €(vs. one fiber 300–500 €for laser, one electrode 700–900 €for RFA and one antenna 1000–1250 €for MWA). For a medical department it represents a significant financial investment, appropriate only if the apparatus is also utilized by other specialists, such as urologists and gynecologists. Besides, the duration of the procedure is longer than with RFA, LTA or MWA (as a mean, 15–30 min for RFA, LTA and MWA vs. 45–60 min). Again, the main limiting factor of the procedure is pain, that may induce involuntary movements and the interruption of the procedure (laser ‘lookout’), followed by a time-consuming repositioning of the patient.
Comparison with the other thermal ablation techniques
Very few studies have compared in prospective randomized controlled trials HIFU, versus RFA, LTA or MWA. In a recent cohort study, RFA showed a slightly better mean volume reduction (50%) than MWA (44%) and HIFU (49%) but the differences between RFA, MWA and HIFU were not significant [Citation40]. Notably, these nearly similar outcomes were biased by the different baseline size of the nodules, significantly smaller in the HIFU group. Besides the poor evidence provided by head-to-head studies, robust data from the literature demonstrate a greater efficacy of LTA and RFA versus HIFU for both VRR and symptom improvement in patients with nodular thyroid disease. Among the available well-controlled studies, a multicenter trial compared 138 patients treated by LTA and 138 patients treated by RFA after propensity score matching [Citation41]. Mean VRR at 6 and 12 months were 57% and 62% in the RFA group, and 67% and 70% in the LTA group, respectively. A systematic review demonstrated that a single RFA induced, at 6 months of follow-up, a 77% and LTA a 50% mean volume decrease in cold solid nodules [Citation16]. Similarly, a recent meta-analysis, showed that RFA and LTA induced, at 12 months, a 75% and a 52% VRR, respectively [Citation17]. Finally, a head-to-head evaluation of RFA and LTA outcomes demonstrated a 75% and 83% nodule volume decrease at 18 months in the RFA and in the LTA groups, respectively. Accordingly, the clinical symptoms improved significantly in both groups (cervical discomfort: −83%, cosmetic complaints: −84%, and dysphagia: −86%) [Citation13]. Thus, based on the greater efficacy, lower cost, and shorter time of RFA and LTA procedures, HIFU treatment should mainly be considered for selected small size nodules and for patients who decline other TA procedures because of the risk of cervical skin damage.
Limitations
Our study presents some major limitations: the small number of patients treated in each center and the limited experience of some operators in HIFU treatment. However, the HIFU outcomes in the initial part of the study did not differ significantly from those in the final part of the study, after a more protracted learning curve. For these reasons, a prospective trial on a large number of patients is required for a conclusive definition of the actual role of HIFU in clinical practice. Notably, 29 patients out of 45 (44.6%) achieved the complete follow-up (3 years). Besides the 26/65 patients (40.0%) who underwent a further therapeutic intervention because of technical failure, 10/65 patients (15.4%) were lost to follow-up. The dropout rate could theoretically have influenced the outcomes of the study if those lost during the study if those lost during the study were good responders to the HIFU treatment.
Conclusions and perspectives
HIFU is a promising procedure because of its completely noninvasive nature and its low risk of complications. In our study, a single treatment resulted in a decrease of the volume of symptomatic thyroid nodules that ranged between 30 and 35% and remained stable for the subsequent two years.
HIFU remains, despite the limited volume reduction rate, an interesting technique due to the absence of any injury to the neck during treatment and because it may be used in centers without specific expertise in interventional procedures. Improvements in technology, better control of pain, combined use of ethanol injection for small fluid areas [Citation42], adequate training, and appropriate selection of patients may in the future provide efficacy comparable to the other currently available thermal ablation procedures.
Ethical approval
The research was carried out in accordance with the World Medical Association Declaration of Helsinki. The Institutional Bioethics Committees of each center approved the study.
Authors’ contributions
HM, ABH and EP wrote the first version of the manuscript. ABH, AP, GB, AB, RG, BR, DT, DB and EP collected the data. ABH and FM performed and validated the statistical analysis. LH and all authors critically reviewed the paper and approved the final version of the manuscript.
Supplemental Material
Download PDF (307.1 KB)Supplemental Material
Download PDF (10.9 KB)Supplemental Material
Download PDF (6.9 KB)Supplemental Material
Download PDF (298.9 KB)Supplemental Material
Download PDF (180.6 KB)Acknowledgements
We are indebted to Mr. François MACHURON for helping with the statistical analysis.
Disclosure statement
Hervé Monpeyssen has previously consulted for Theraclion and Starmed (oral conference communication). Édouard Ghanassia has previously consulted for Ablatech. Laszlo Hegedüs is a former consultant and scientific board member for Theraclion. The other authors have nothing to disclose regarding this paper.
None of the above companies have had any influence on any aspect of this manuscript.
Any grants or fellowships supporting the writing of the paper: None.
References
- Al Dawish MA, Alwin Robert A, Thabet MA, et al. Thyroid nodule management: thyroid-stimulating hormone, ultrasound, and cytological classification system for predicting malignancy. Cancer Inform. 2018;17:117693511876513.
- Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926–935.
- Mathonnet M, Cuerq A, Tresallet C, et al. What is the care pathway of patients who undergo thyroid surgery in France and its potential pitfalls? A national cohort. BMJ Open. 2017;7:e013589.
- Sun GH, DeMonner S, Davis MM. Epidemiological and economic trends in inpatient and outpatient thyroidectomy in the United States, 1996-2006. Thyroid. 2013;23:727–733.
- Haugen BR, Sawka AM, Alexander EK, et al. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma Without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid. 2017;27:481–483.
- Sajid T, Qamar Naqvi SR, Qamar Naqvi SS, et al. Recurrent laryngeal nerve injury in total versus subtotal thyroidectomy. J Ayub Med Coll Abbottabad. 2016;28:559–561.
- Borel F, Christou N, Marret O, et al. Long-term voice quality outcomes after total thyroidectomy: a prospective multicenter study. Surgery. 2018;163:796–800.
- Lee DY, Seok J, Jeong W-J, et al. Prediction of thyroid hormone supplementation after thyroid lobectomy. J Surg Res. 2015;193:273–278.
- Kim JH, Baek JH, Lim HK, et al.; Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19:632–655.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules – 2016 update: appendix. Endocrine Practice. 2016;22:1–60.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36:376–382.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19:167–174.
- Ben Hamou A, Ghanassia E, Espiard S, et al. Safety and efficacy of thermal ablation (radiofrequency and laser): should we treat all types of thyroid nodules?. Int J Hyperthermia. 2019;36:666–676.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100:3903–3910.
- Deandrea M, Trimboli P, Garino F, et al. Long-term efficacy of a single session of RFA for benign thyroid nodules: a longitudinal 5-year observational study. J Clin Endocrinol Metab. 2019;104:3751–3756.
- Ha EJ, Baek JH, Kim KW, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J Clin Endocrinol Metab. 2015;100:1903–1911.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. 2020;67:35–43.
- Papini E, Monpeyssen H, Frasoldati A, et al. European Thyroid Association Clinical Practice Guideline for the use of image-guided ablation in benign thyroid nodules. ETJ. 2020;9:169–182.
- Hegedüs L, Frasoldati A, Negro R, et al. European Thyroid Association survey on use of minimally invasive techniques for thyroid nodules. Eur Thyroid J. 2020;9:194–204.
- Chung SR, Baek JH, Suh CH, et al. Efficacy and safety of high-intensity focused ultrasound (HIFU) for treating benign thyroid nodules: a systematic review and meta-analysis. Acta Radiol. 2020.
- Lang BHH, Woo Y-C, Chiu KW-H. Two-year efficacy of single-session high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Eur Radiol. 2019; 29:93–101.
- Lang BH-H, Wu ALH. High intensity focused ultrasound (HIFU) ablation of benign thyroid nodules – a systematic review. J Ther Ultrasound. 2017;5:11.
- Trimboli P, Pelloni F, Bini F, et al. High-intensity focused ultrasound (HIFU) for benign thyroid nodules: 2-year follow-up results. Endocrine. 2019;65:312–317.
- Zimmermann MB, Hess SY, Adou P, et al. Thyroid size and goiter prevalence after introduction of iodized salt: a 5-y prospective study in schoolchildren in Côte d’Ivoire. Am J Clin Nutr. 2003;77:663–667.
- Pałyga I, Pałyga R, Młynarczyk J, et al. The current state and future perspectives of high intensity focused ultrasound (HIFU) ablation for benign thyroid nodules. Gland Surg. 2020;9:S95–S104.
- Russ G, Bigorgne C, Royer B, et al. [The Thyroid Imaging Reporting and Data System (TIRADS) for ultrasound of the thyroid]. J Radiol. 2011;92:701–713.
- Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017;27:1341–1346.
- Korkusuz H, Fehre N, Sennert M, et al. Early assessment of high-intensity focused ultrasound treatment of benign thyroid nodules by scintigraphic means. J Ther Ultrasound. 2014;2:18.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29:611–618.
- Cardella JF, Kundu S, Miller DL, et al.; Society of Interventional Radiology. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189–S191.
- Kovatcheva RD, Vlahov JD, Stoinov JI, et al. Benign solid thyroid nodules: US-guided high-intensity focused ultrasound ablation-initial clinical outcomes. Radiology. 2015;276:597–605.
- Lang BH-H, Woo Y-C, Chiu KW-H. Single-session high-intensity focused ultrasound treatment in large-sized benign thyroid nodules. Thyroid. 2017;27:714–721.
- Korkusuz H, Sennert M, Fehre N, et al. Localized thyroid tissue ablation by high intensity focused ultrasound: volume reduction, effects on thyroid function and immune response. Fortschr Röntgenstr. 2015;187:1011–1015.
- Lang BHH, Woo Y-C, Chiu KW-H. Evaluation of pain during high-intensity focused ultrasound ablation of benign thyroid nodules. Eur Radiol. 2018; 28:2620–2627.
- Lang BHH, Woo Y-C, Chiu KW-H. Effect of perithyroidal lignocaine infusion (PLI) to pain experienced during high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Eur Radiol. 2019;29:5280–5287.
- Bernardi S, Giudici F, Cesareo R, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the Italian minimally-invasive treatments of the thyroid group. Thyroid. 2020.
- Lang BHH, Woo YC, Chiu KWH. Role of second high-intensity focused ultrasound (HIFU) treatment for unsatisfactory benign thyroid nodules after first treatment. Eur Radiol. 2019;29:1469–1478.
- Lang BHH, Woo YC, Chiu KWH. Two sequential applications of high-intensity focused ultrasound (HIFU) ablation for large benign thyroid nodules. Eur Radiol. 2019;29:3626–3634.
- Hu K, Wu J, Dong Y, et al. Comparison between ultrasound-guided percutaneous radiofrequency and microwave ablation in benign thyroid nodules. J Cancer Res Ther. 2019;15:1535–1540.
- Korkusuz Y, Gröner D, Raczynski N, et al. Thermal ablation of thyroid nodules: are radiofrequency ablation, microwave ablation and high intensity focused ultrasound equally safe and effective methods? Eur Radiol. 2018;28:929–935.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33:1–919.
- Lang BHH, Woo Y-C, Chiu KW-H. Combining high-intensity focused ultrasound (HIFU) ablation with percutaneous ethanol injection (PEI) in the treatment of benign thyroid nodules. Eur Radiol. 2020.