 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
To assess the safety and efficacy of a two-step single-session procedure, combining transarterial embolization (TAE) and percutaneous microwave ablation (MWA), in the treatment of > 3 cm unresectable liver metastases. We also compared the final volume obtained by the two techniques (VE-T) and the expected ablation volume of the stand-alone MWA (VT).
Methods
From January 2015 to December 2017, 22 consecutive patients, with a total of 24 unresectable hepatic metastases >3 cm in diameter underwent a two-step single-session combined treatment of TAE and MWA. Follow-up computed tomography scans were performed at 1-, 3-, 6-, 12, and 24 months post-procedure. VE-T as final ablation volume induced by the combined treatment (TAE-MWA), VN as initial nodule volume, VT as expected ablation volume of MWA treatment alone were evaluated and compared.
Results
Tumor dimensions ranged from 32 to 73 mm. Technical success was achieved in all treated tumors with no local tumor recurrence. Final ablation volumes ranged from 50 to 450 cm3 and the short-axis diameter of the ablation zone ranged from 12 to 48 mm. The mean ΔV increment in the final ablation volume with respect to the stand-alone MWA was 196% (ranging from 25 cm3 − 210 cm3) (p < 0.05). The VE-T mean was four times the VN mean, while the VT mean was about twice the VN mean. No recurrence and only one case of post-embolization bleeding were observed.
Conclusions
This study demonstrated the safety and efficacy of a combined two-step single-session TAE-MWA treatment of unresectable hepatic metastases > 3 cm in diameter.
Introduction
Liver metastases are a frequent finding in patients with solid tumors and are more common than primary liver cancers. Currently, although hepatic resection is considered the reference standard treatment, only about 20% of patients with hepatic metastases are suitable candidates for surgery, due to the number of tumoural foci, location and extension of the tumors, impaired liver function, or other comorbidities [Citation1].
Hence, ‘non-surgical’ minimally invasive treatments, such as thermal ablation and trans-arterial embolization (TAE), are emerging, with promising results, as alternative approaches.
Ablation therapy has been proven to be a safe and effective treatment for liver metastases up to 3 cm [Citation1].
Compared to radiofrequency ablation (RFA), microwave ablation (MWA) has the advantages of a shorter procedure time, wider ablation area, higher ablation rate, and lower susceptibility to a heat sink effect [Citation2]. Therefore, MWA potentially provides wider applicability compared to RFA, especially in the treatment of large tumors in a variety of target organs [Citation3,Citation4].
Tumor size, minimal margin size, and histology are significant in predicting local tumor progression (LTP). A minimum ablation margin of at least 5 mm is desired to obtain the best oncologic outcomes [Citation5].
TAE and trans-arterial chemoembolisation (TACE) are the most widely used primary treatments for unresectable large-sized hepatocellular carcinoma (HCC) and are influenced by many factors, such as blood supply, tumor size, and the ultraselectivity of the catheterization. Lately, TACE has also been investigated as a treatment for liver metastases from solid tumors [Citation6–8].
In the last decade, the combination of TAE/TACE and ablative therapies has been assessed to improve local control. The efficacy and safety of combined TAE and thermal ablation procedures, in particular RFA, have already been shown for the treatment of HCC [Citation9–12]. Nevertheless, evidence regarding the use of a combined treatment for liver metastases remains poor, and fewer studies have evaluated the effectiveness of combining chemoembolisation with MWA [Citation11].
This article aims to evaluate the safety and efficacy of a two-step single-session procedure, combining TAE and MWA in the treatment of > 3 cm unresectable liver metastases.
Materials and methods
Patients
All methods and procedures were carried out following the ethical standards of the institutional research committee (https://www.unicampus.it/documents/UCBM_Codice_Etico.pdf) and complied with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
From January 2015 to December 2017, 22 consecutive patients, including a total of 24 unresectable hepatic metastatic lesions, underwent a two-step single-session combined treatment of TAE and MWA.
Inclusion criteria were: (1) less than five liver metastatic nodules; (2) each nodule greater than 30 mm in diameter; (3) safety margins from major vascular structures of at least of 20 mm; (4) absence of any tumoural infiltration in adjacent organs; (5) no evidence of portal and/or venous thrombosis or uncontrollable ascites; (6) adequate hematologic function (platelet count >50 × 109 cells/L, hemoglobin >8.0 g/dL and prothrombin time <80%); (7) adequate hepatic function (albumin >2.5 g/dL, total bilirubin < 3 mg/dL, and alanine aminotransferase and aspartate aminotransferase levels < 5 mU/mL over the upper limit of the normal range); (8) adequate renal function (serum creatinine concentration < 1.5 mg/dL).
Exclusion criteria were: (1) patients with one to three small (< 3 cm) tumors accessible to percutaneous ablation were treated by ablation alone and (2) presence of other uncontrollable extrahepatic malignancies.
No patient had previously undergone liver surgery.
Procedure
Informed consent was obtained from all patients.
All procedures were performed using local anesthesia (1% Lidocaine Hydrochloride) and conscious sedation (Midazolam: 0.5–1 mg and Fentanyl 25–50 µg).
Antiemetic therapy (Plasil or Granisetron) and analgesics were prescribed as necessary.
All patients received antibiotic therapy during the procedure (Cefazolin 0.5–1 g ev) and in the following five days (Levofolxacin 500 mg once a day per os).
Transarterial embolization
A sheathed 4- or 5-French catheter was introduced through the femoral or brachial artery, depending on the anatomy of the celiac axis and the superior mesenteric artery, per previous CT scans; an angiographic examination of the hepatic vessels was performed to evaluate blood flow and to localize the tumor.
To confirm the tumor feeding vessel in hypovascular tumors, a contrast-enhanced US (Sonovue, Bracco Imaging, Italy) was used [Citation12].
Embozene Microspheres 75 µm and 100 µm spheres (Embozene, Color-Advanced Microspheres; Celonova BioSciences, Peachtree City, GA) were used to embolize the lesions.
Embolization was considered completed in the absence of blood flow in the feeding arterial vessels [Citation12].
Microwave ablation
After embolization, patients were transferred to the adjacent CT room, where MWA was performed under ultrasonographic/CT control.
The antenna (AMICA PROBE HS) positioning into the target was performed under ultrasound guidance and verified through CT scans.
Energy was delivered to target tumors through a 14- or 16-Gauge, coaxial antenna, internally cooled by a built-in peristaltic pump and equipped with a miniaturized quarterwave choke for the suppression of back-propagating waves, powered by a 2450 MHz generator. The type of ablation, probe number, ablation time, and wattage setting were chosen based on the tumor size and location of the initial lesion, following the manufacturer’s parameters.
Imaging and clinical follow-up
A follow-up CT scan was performed immediately after the procedure to evaluate the treatment area and immediate complications, and at 1-, 3-, 6-, 12- and 24-months to assess the local tumor control.
CT examinations were carried out on a multidetector Siemens 64 slice CT scanner, performing acquisitions before and after intravenous injection of 2 mg/kg of iodized contrast agent (Omnipaque, Ioherol, GE Healthcare), with a flow of 4.5 ml/sec, at the arterial phase (30 s), portal phase (60 s), and late phase (more than 180 s).
Complications and side effects were assessed and classified in accordance with the Society of Interventional Radiology clinical practice guidelines [Citation13].
The technical success of the overall loco-regional therapy was defined as having achieved complete target devascularisation, per immediate post-procedural CT scanning.
Any uptake of contrast medium in the area of treatment at post-procedural CT or follow-up CT was considered a residual unablated tumor or local tumor progression/recurrence, respectively.
Variable and statistical analysis
The geometrical appearance of both target nodules and ablated zones approximately resembles that of an ellipsoid, whose volume V may be calculated as:
where a, b and c are the three semi-axes of the ellipsoid.
The following features were analyzed for all the tumors and treatments included in this study: 1) VE-T, the final ablation volume induced by the combined TAE and MWA treatment (measured at post-procedural CT images); 2) VN, the initial nodule volume (measured from pre-procedural CT images); 3) VT, the expected ablation volume of MWA treatment alone, calculated based on AMICA PROBE manufacturer guidelines [Citation9,Citation14].
We compared the liver ablation area obtained at the end of the combined treatment with the expected stand-alone MWA ablation zone [Citation9].
To assess the ablation margin obtained by the combined TAE-MWA treatment around the target nodule, the following parameter was also calculated:
providing a normalized, volumetric treatment margin.
To assess the overall ablative volume obtained by the combined treatment compared to the volume obtained by MWA alone, the following parameter was calculated:
providing the percentile variation in ablated tissue volume with respect to a stand-alone theoretical thermal coagulation therapy.
A two-tailed t-test was performed to compare the pretreatment tumor volumes (i.e., VN) with the volumes attained using the combined treatment (i.e., VE-T) and with MWA alone (i.e., VT).
To compare the impact on ablation volume obtained by the combined treatment with stand-alone MWA, the following variables were assessed: 1) pretreatment tumor size; 2) embolizing particles’ diameter; 3) MWA treatment power and 4) MWA treatment time.
The patient population was divided into two groups, according to different criteria; respectively: 1) treatment power ≤60 W or >100 W; 2) pretreatment tumor volume ≤25cc or >25cc; 3) embolizing particles’ diameter of 100 µm or 75 µm; 4) MWA treatment time ≤10min or >10 min; 5) tumor location in upper segments (S8, S7, S2) or lower segments (S5, S6, S3).
A univariate correlation was used to assess the influence of technical and anatomical parameters on the necrotic area dimensions in these groups.
Statistical analyses were performed using SPSS software (Version 20, IBM).
Results
Twenty-two consecutive patients, 16 females and six males, of a mean age of 58.5 years (ranging from 43 to 81) were included in the study.
A total of 24 liver metastases, of a mean size of 37 mm (ranging from 32 to 73 mm, STD = 11.1) were treated.
The histological survey revealed 10 metastases from breast cancer, 10 from colorectal cancers, two from neuroendocrine tumors, and two from leiomyosarcomas.
The power at the probe tip ranged from 60 to 100 W and the ablation lasted from 5 to 12 consecutive minutes.
Imaging and clinical follow-up
Technical success was achieved in all treatments (24/24), with no residue/recurrence detected at the CT scan follow-up carried out until the last follow-up.
In 2 of 24 treated nodules (7%), a peripheral enhancement was observed on the 3-month CT scan and resolved at the following 6-month CT control, consistent with an altered perfusion post-treatment area.
The major complication rate was 4% with only one case (1/24) of bleeding detected as an intralesion leak of contrast medium in a post-embolization angiographic control, in a patient with a leiomyosarcoma metastasis. This case was successfully treated using microcoils ( and ).
Figure 1. (a) Axial pretreatment post-contrast CT-image of a 72-year-old woman, showing a metastasis in the VI hepatic segment originating from a leiomyosarcoma. (b) Selective diagnostic hepatic angiography showing the right hepatic artery and the feeding vessels to the liver metastasis. (c) US image performed during the MWA procedure showing the antenna correctly inserted into the liver tumor. (d) The post-embolization angiography shows a contrast leak, successfully treated using microcoils. (e) The selective diagnostic hepatic angiography shows the embolization with coils of the bleeding vessel. (f) 1-month axial CT-image control showing no residual tumor.
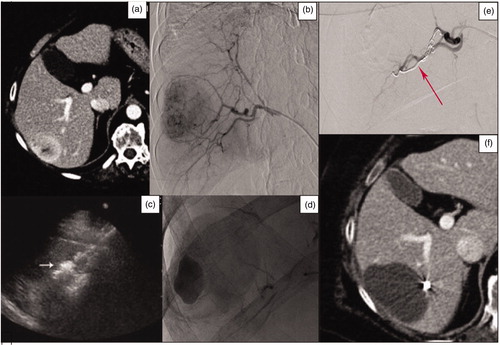
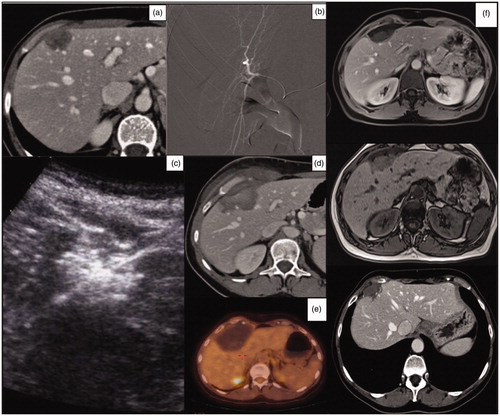
Figure 2. (a) Axial pretreatment post-contrast CT exam of a 54-year-old woman, showing a polilobated hypoenhancing colon cancer metastasis in segment IV. (b) Superselective angiography through the right femoral artery. A subsequent post-contrast ultrasound scan confirmed the artery network; embolization was performed by administering 75 μm Embozene particles. (c) Ultrasonographic monitoring of the subsequent MWA treatment. (d) Post-treatment axial CT-image control, showing a large area of necrosis. (e) PET-CT performed at 1 year showing no pathologic uptake in the treated area. (f) MRI-image control at 1-year and post-contrast CT scan at 2 years showing a decrease in the necrotic area.
Only one patient developed an enlarged necrotic area associated with local soft tissue swelling () and pain, treated only with analgesic therapy (Perfalgan 500 mg on demand), with a minor complication rate of 4%.
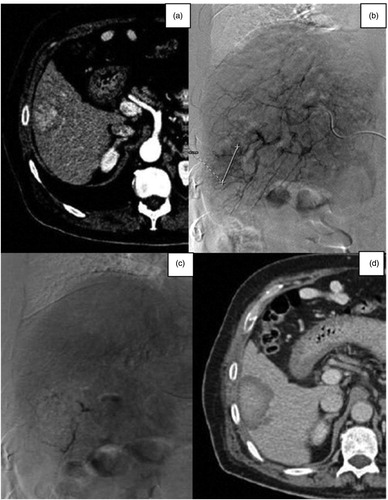
Figure 3. (a) Axial venous phase contrast-enhanced CT scan image of a 76-year-old woman, showing a gastric neuroendocrine metastasis in the V-VI hepatic segment. (b) Selective diagnostic angiography of the hepatic artery showing the tumor. (c) Post-TAE selective diagnostic angiography showing the complete devascularisation of the tumor. (d) Axial venous phase post-contrast CT-image showing the embolized tumor with a necrotic enlarged area.
Side effects were observed after three procedures (12%): one patient experienced intra-procedural pain, one patient had a fever and asymptomatic pleural effusion was observed in one case.
Both minor complications and side effects were managed by observation only.
No significant hepatic dysfunction or delayed discharge of the patient was observed after the combined single-session TAE-MWA.
Variable analysis
VE-T ranged between 50 cm3 and 450 cm3 (mean value: 180 cm3, STD = 130.7) with a maximal diameter ranged between 60 and 104 mm (mean value: 67 mm, STD = 10.5).
The mean ΔV increment in the final ablation volume with respect to the stand-alone MWA was calculated to be 196% (range: 25 cm3 − 210 cm3): this difference in performance between the combined therapy and expected stand-alone MWA was found to be statistically significant (p < 0.05). The average VE-T was four times larger than the average VN. The average VT was twice the initial nodule volume. ()
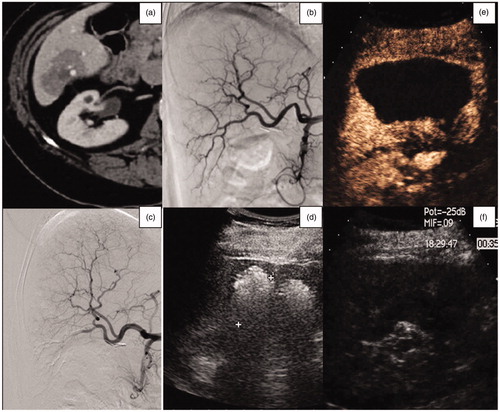
Figure 4. (a) Axial CT-image of a 74-year-old woman, showing a breast metastasis in the VI hepatic segment. (b, c) Selective diagnostic hepatic angiography, showing the hepatic vascularization of the right lobe and the embolization of the vessels feeding the liver metastasis. (d) US-monitoring of the subsequent MWA procedure. (e, f) US contrast-image performed 6 months after the TAE-RFA procedure, showing a large area of necrosis and no residual tumor.
VE-T attained in the 24 combined treatments compared to VT performed using identical working parameters (Hoffmann et. Al [Citation15]) were summarized in .
Figure 5. Comparison of the ablation volumes obtained using the combined treatment (red bars), ablation volumes which would have been obtained using stand-alone MWA (blue bars), and the pretreatment volumes of the nodules (yellow bars).
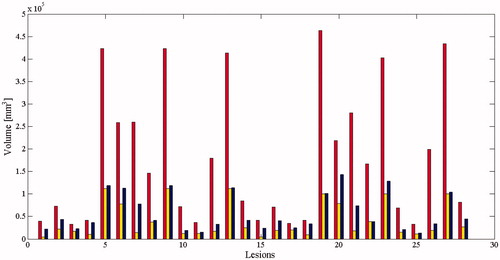
The mean short-axis diameter of the area obtained after the combined treatment was 51 mm (ranging from 34 to 71 mm, STD = 12.1), while the mean short axis of the pretreatment nodule and of the ablation area attained by MWA alone was 19 mm (ranging from 15 to 30 mm, STD = 3.9) and 35 mm (ranging from 20 to 48 mm, STD = 7.6), respectively.
The average of safety margin was 16 mm (ranging from 14 to 27 mm, STD = 3.2).
The sphericity index S of the quasi-ellipsoidal ablation areas induced by the combined treatments (i.e., S = D/L, where D is the shortest and the L is the longest diameter of the coagulated region; S = 1 for a perfectly spherical ablation) was 0.7. Compared to the commercially available MWA probes, which yielded values of S typically ranging from 0.5 to 0.8 [Citation15], the combined treatments in this study provided a very good performance in terms of ablation sphericity.
During the TAE procedure, 75 µm spheres were used in six patients with breast cancer metastases, 4 patients with rectal cancer metastases, and two patients with neuroendocrine metastases. One-hundred µm spheres were used in four patients with breast cancer metastases, 6 with colon metastases, and two with leiomyosarcoma.
Univariate analysis of technical factors
Splitting patients into two groups, based on different factors, as reported in , a statistically significant ΔV increment was observed in patients treated using wattage PT > 40 W, timing tT ≤10 min, for tumors >25cc, located in the lower segments, and when 75 µm particles were used for embolization.
Table 1. Influence of technical and anatomic parameters on the necrotic area dimensions.
Discussion
The purpose of the study was to evaluate the safety and efficacy of a two-step single-session combined treatment with TAE and MWA on large (> 3 cm) liver metastatic tumors performed with a curative intent. The main problem associated with the percutaneous ablation of liver metastases is local recurrence [Citation2,Citation16–20]. Therefore, the aim of the combined treatment strategy was to obtain a more regular morphology and an extended necrotic area on the target tumors, to achieve more adequate safety margins, without determining a higher complication rate [Citation18–22].
The RFA treatment of liver tumors showed similar benefits and limitations compared to surgery, though with adequate local efficacy only for nodules smaller than 2 cm [Citation23]. However, the presence of blood vessels or bile ducts adjacent to the target nodule was demonstrated to be a further predictor of higher local recurrence or incomplete ablation, due to the convective heat subtraction operated by the circulating fluids [Citation24].
In contrast, MWA can yield remarkably higher intra-tumoural temperatures and is less sensitive to the heat sink effects and the variability in physical properties of target tissues, providing a broader zone of active heating and a more uniform ablation area in a shorter treatment time, even in the proximity of large blood vessels. Many authors confirm the feasibility and safety of MWA for the treatment of liver tumors, which is associated with no mortality and a very low rate of major complications [Citation5,Citation17,Citation25,Citation26,Citation27,Citation28]
The combination of TAE with RFA, in either sequence, has widely been found to produce remarkably larger coagulation zones compared to stand-alone embolization or RFA procedures in the treatment of primary and secondary liver tumors, both in humans [Citation1,Citation12,Citation29] and in animals. IJ Lee et al. compared RFA alone, RFA combined with subsequent TAE, and RFA preceded by TAE in rabbit livers, demonstrating the clear superiority of the combined therapies in terms of ablation volumes [Citation2,Citation6,Citation22]. Motoki et al. performed similar experiments on porcine models demonstrating that RFA following TAE with iodized oil and gelatin sponge induces a larger coagulation necrosis throughout a shorter ablation time compared to RFA alone [Citation20,Citation24].
In this study, we decided to perform TAE immediately followed by percutaneous MWA on liver metastases larger than 30 mm, although few articles in the literature support this specific combination pathway [Citation23].
The execution of TAE as a first treatment, obtaining the devascularisation of target tumors [Citation24,Citation30] for the treatment of large liver malignancies was meant to ensure a favorable background before carrying out the subsequent MWA procedure. In fact, it reduces the heat sink effect and hence amplifies the MWA thermocoagulative effect [Citation27,Citation33,Citation34].
Moreover, based on the same rationale used by Wang et al [Citation34], we also decided to perform MWA immediately after TAE in order to prevent the possible opening of new collateral tumor feeding arteries after the embolization procedure [Citation27,Citation33,Citation34].
Our data yielded very good results after TAE procedures performed using small embolizing particles of 75 µm which reflected a selective hypoxia status. Hypoxia caused by arterial particle obstruction reduces the temperature at which coagulative necrosis occurs, enabling the formation of a larger necrotic area [Citation1,Citation27]. The combination of these procedures in a single session may lead to hepatic dysfunction and hepatic infarction.
However, no significant hepatic dysfunction or delayed discharge of the patient was observed after the combined single-session TAE-MWA in our study population [Citation27].
Our results, in terms of complication and recurrence rates, compare favorably to the one reported by Alexander et al. (20% vs 45% and 0% vs 19.5%, respectively) [Citation35].
Factors influencing the combined treatment were analyzed.
The percentage increase in terms of diameter of the treated tumors with respect to stand-alone MWA procedures (as per previous ex-vivo experiments conducted with the same MWA equipment in the same working conditions) was higher when delivering a higher MW power for a shorter time. Larger tumors yielded better results (i.e., exhibited a higher percentile increase in ablation volume with respect to stand-alone MWA treatments), especially those localized in the lower hepatic segments. These results may be explained by considering the different vascular distributions between the upper and lower hepatic segments. In particular, the blood is supplied to the lower segments mainly by the right hepatic artery, while the upper segments are fed by the right and left hepatic arteries and phrenic arteries. This may result in a less clearly defined vascularization for tumors located in the upper segments, which in turn may reduce the local efficacy of TAE procedures.
To the best of our knowledge, there are no studies on the effect on the dimension of the ablative treated area and its location in upper and lower hepatic segments. In contrast, the ablative areas are influenced by vessel size, the distance between the vessels, and the antenna’s tip and distance from the hilar plain.
The influence of arterial and venous vascularization on the effectiveness of HCC treatments in cirrhotic patients has been investigated by several studies; the LTP rates seems to be related only to arterial patency [Citation36].
Economic and logistic aspects were also considered in the combined treatment planning. In fact, a combined treatment enables a shorter hospital stay and/or the number of hospital admissions per patient, thereby improving patients’ compliance.
While a final consensus has not yet been reached regarding the ideal interval between the two steps of a combined treatment [Citation27], it is a common clinical practice to perform both steps within the same session in order to achieve better results [Citation10].
The combination of the two procedures, the TAE and MWA, allowed us to obtain large and regular oval-shaped ablation areas, perfectly comprising the target tumors [Citation11,Citation37]. The increment in the overall coagulation volume obtained from the combined treatment confirms the synergistic effect between thermal ablation and embolization treatments, which was already known, though not yet thoroughly described in the literature.
Our study is limited in that it is monocentric, retrospective, and based on a limited number of cases.
The combination of TAE and MWA in the same session represents an original proposal in the current scientific panorama for the treatment of moderate to large lesions in oligometastatic patients, and, to the best of our knowledge, this is the first such study in the literature.
Future studies with randomized controlled protocols are recommended in order to obtain more comparable real data regarding, in particular, the short and midterm clinical outcomes of this combined approach and non-combined approaches.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kagawa T, Koizumi J, Kojima S, et al. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma comparison with surgical resection. Cancer. 2010;116:3638–3644.
- Chiang J, Hynes K, Brace CL. Flow-dependent vascular heat transfer during microwave thermal ablation. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2012 Aug 28–Sep 1; San Diego, CA. 2012.
- Flanders VL, Gervais DA. Ablation of liver metastases: current status. J Vasc Interv Radiol. 2010;21:S214–S222.
- Ryan MJ, Willatt J, Majdalany BS, et al. Ablation techniques for primary and metastatic liver tumors. World J Hepatol. 2016;8:191–199.
- Sotirchos VS, Petrovic LM, Gönen M, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280:949–959.
- Vogl TJ, Zangos S, Eichler K, et al. Colorectal liver metastases: regional chemotherapy via transarterial chemoembolization (TACE) and hepatic chemoperfusion: an update. Eur Radiol. 2007;17:1025–1034.
- Liu C, Liang P, Liu F, et al. MWA combined with TACE as a combined therapy for unresectable large-sized hepotocellular carcinoma. Int J Hyperthermia. 2011;27:654–662.
- Yan S, Xu D, Sun B. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2013;58:2107–2113.
- Lloyd DM, Lau KN, Welsh F, et al.; International Microwave Tumour Ablation Group (IMTAG). International multicentre prospective study on microwave ablation of liver tumours: preliminary results. HPB (Oxford). 2011;13:579–585.
- Morimoto M, Numata K, Kondou M, et al. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–5460.
- Knavel EM, Green CM, Gendron-Fitzpatrick A, et al. Combination therapies: quantifying the effects of transarterial embolization on microwave ablation zones. J Vasc Interv Radiol. 2018;29:1050–1056.
- Fong ZV, Palazzo F, Needleman L, et al. Combined hepatic arterial embolization and hepatic ablation for unresectable colorectal metastases to the liver. Am Surg. 2012;78:1243–1248.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–S202.
- AMICA. Aparatus for Microwawe Ablation.pdf [Internet]. [cited 2020 May 29]. Available from: http://www.mermaidmedical.dk/PDF/Norway/9_Thermoablation/AMICA%20Aparatus%20for%20Microwawe%20Ablation.pdf
- Hoffmann R, Rempp H, Erhard L, et al. Comparison of four microwave ablation devices: an experimental study in ex vivo bovine liver. Radiology. 2013;268:89–97.
- Shibata T, Isoda H, Hirokawa Y, et al. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–913.
- Murakami T, Ishimaru H, Sakamoto I, et al. Percutaneous radiofrequency ablation and transcatheter arterial chemoembolization for hypervascular hepatocellular carcinoma: rate and risk factors for local recurrence. Cardiovasc Intervent Radiol. 2007;30:696–704.
- Bonomo G, Della Vigna P, Monfardini L, et al. Combined therapies for the treatment of technically unresectable liver malignancies: bland embolization and radiofrequency thermal ablation within the same session. Cardiovasc Intervent Radiol. 2012;35:1372–1379.
- Peng Z-W, Chen M-S. Transcatheter arterial chemoembolization combined with radiofrequency ablation for the treatment of hepatocellular carcinoma. Oncology. 2013;84 Suppl 1:40–43.
- Kim JH, Won HJ, Shin YM, et al. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011;18:1624–1629.
- Seki T, Tamai T, Nakagawa T, et al. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer. 2000;89:1245–1251.
- Lee IJ, Kim YI, Kim KW, et al. Radiofrequency ablation combined with transcatheter arterial embolisation in rabbit liver: investigation of the ablation zone according to the time interval between the two therapies. Br J Radiol. 2012;85:e987–e994.
- Konopke R, Roth J, Volk A, et al. Colorectal liver metastases: an update on palliative treatment options. J Gastrointestin Liver Dis. 2012; Mar;21:83–91.
- Nakai M, Sato M, Sahara S, et al. Radiofrequency ablation in a porcine liver model: effects of transcatheter arterial embolization with iodized oil on ablation time, maximum output, and coagulation diameter as well as angiographic characteristics. World J Gastroenterol. 2007;13:2841–2845.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175.
- Strickland AD, Clegg PJ, Cronin NJ, et al. Experimental study of large-volume microwave ablation in the liver. Br J Surg. 2002;89:1003–1007.
- Livraghi T, Meloni F, Solbiati L, et al.; Collaborative Italian Group using AMICA system. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868–874.
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007;72 Suppl 1:124–131.
- Miyayama S, Yamashiro M, Okuda M, et al. Chemoembolization for the treatment of large hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:1226–1234.
- Huppert P. Current concepts in transarterial chemoembolization of hepatocellular carcinoma. Abdom Imaging. 2011;36:677–683.
- Liu Y, Zheng Y, Li S, et al. Percutaneous microwave ablation of larger hepatocellular carcinoma. Clin Radiol. 2013;68:21–26.
- Lang EK, Brown CL. Colorectal metastases to the liver: selective chemoembolization. Radiology. 1993;189:417–422.
- Kettenbach J, Stadler A, Katzler IV, et al. Drug-loaded microspheres for the treatment of liver cancer: review of current results. Cardiovasc Intervent Radiol. 2008;31:468–476.
- Wang Z-J, Wang M-Q, Duan F, et al. Transcatheter arterial chemoembolization followed by immediate radiofrequency ablation for large solitary hepatocellular carcinomas. WJG. 2013;19:4192–4199.
- Alexander ES, Mick R, Nadolski GJ, et al. Combined chemoembolization and thermal ablation for the treatment of metastases to the liver. Abdom Radiol (NY). 2018;43:2859–2867.
- Chiang J, Cristescu M, Lee MH, et al. Effects of microwave ablation on arterial and venous vasculature after treatment of hepatocellular carcinoma. Radiology. 2016;281:617–624.
- Veltri A, Moretto P, Doriguzzi A, et al. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol. 2006;16:661–669.
