Abstract
Background
Microwave ablation (MWA) is a safe and effective locoregional ablation modality, but it is not clear whether the curative effect of MWA as to hepatocellular carcinoma (HCC) is comparable to that of surgical resection (SR). We aimed to compare the outcomes of MWA and SR for patients with HCC ranging from 3 to 5 cm.
Methods
197 patients treated for HCC between 3 and 5 cm by MWA or SR were included from 2010 to 2017. Overall survival (OS), progression-free survival (PFS), complication and hospital stay of those patients were compared by using propensity score matching. The registration number of this clinical trial was ChiCTR2000033983.
Results
For patients with HCC between 3 and 5 cm, the 1-, 3-, and 5- years OS rates were 90.3%, 79.7%, and 65.5% in the MWA group, and 96.7%, 88.6%, and 71%% in the SR group, respectively (p = 0.457). The 1-, 3- and 5- years PFS rates were 63.6%, 36.8% and 32.7% in the MWA group, and 74.2%, 41.9% and 35.5% in the SR group, respectively (p = 0.397). The MWA group showed fewer complications (55% versus 78.8%, p = 0.041) and shorter hospital stays (8 versus 15 days, p < 0.001) compared with the SR group.
Conclusion
MWA showed similar survival outcomes compared with SR for HCCs ranging from 3 to 5 cm. However, it showed favorable results in terms of hospital stay and complication rate compared to SR.
Introduction
Hepatocellular carcinoma (HCC) is now the fifth most common cancer and the second most frequent cause of cancer-related mortality in the world [Citation1]. Curative resection and thermal ablation represent two treatment options for HCC. For thermal ablation, radiofrequency ablation (RFA) and microwave ablation (MWA) are the two most commonly used modalities. In recent years, microwave ablation (MWA) has attracted a lot of attention. Compared with RFA, MWA applies a higher temperature in a short period of time leading to excellent local tumor control and fewer concerns for heat sink effect [Citation2–4]. The American Association for the Study of Liver Diseases (AASLD) considered that MWA may treat the tumor larger than 3 cm in diameter more effectively than RFA [Citation5].
The treatment of HCC is mostly based on the characteristics of the tumors. Although previous studies reported that local ablation therapy had comparable therapeutic efficacy for HCC smaller than 3 cm with SR [Citation6–8], there has been no prospective study comparing the efficacy between WMA and SR for HCC ranging from 3 to 5 cm. According to the interventional radiology and previous articles, the European Association for the Study of the Liver considered that RFA is inferior to SR for local tumor control and long-term patient survival in HCC above the 3 cm threshold [Citation1]. Previous studies have confirmed this conclusion when the tumor is up to 5 cm [Citation9–11].
However, several studies showed that MWA has a promising performance for local tumor control, tumor inactivation, and long-term survival compared to RFA in larger neoplasm [Citation12–15]. Since SR requires a well-preserved liver function, MWA may represent a better alternative to SR for HCC ranging from 3 to 5 cm.
To our knowledge, no study has been published to compare MWA with SR for HCC patients less than 3 nodules and between 3 and 5 cm in size. In this study, we aimed to investigate the therapeutic outcomes of MWA and SR for these patients, using propensity score matching (PSM) to minimize potential confounding bias at patient backgrounds.
Methods
Patients
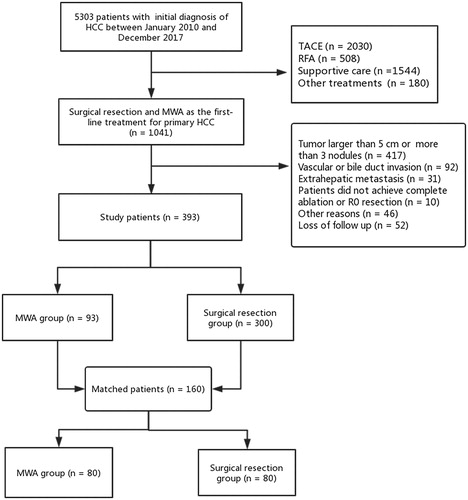
Our study was a retrospective study. From January 2010 to December 2017, 1041 HCC patients underwent initial SR or MWA at Shandong Provincial Hospital Affiliated to Shandong First Medical University. Among them, 393 patients were identified by the following inclusion criteria: (1) age older than 18 years; (2) up to 3 tumor nodules with each nodule ≤5 cm; (3) no evidence of vascular or bile duct invasion; (4) no extrahepatic metastasis and (5) Child-Pugh class A or B. Patients were excluded based on the following exclusion criteria: (1) patients who hospitalized for rupture of HCC; (2) patients did not achieve R0 resection for SR (R0 resection was defined as a negative surgical margin observed microscopically and macroscopically); (3) patients did not achieve complete ablation after MWA (complete ablation was defined as no nodular or irregular enhancement within or adjacent to the ablation zone during the arterial phase on the first contrast-enhanced dynamic computed tomography (CT) or magnetic resonance imaging (MRI) scan performed approximately 1 month after ablation); or (4) other significant comorbidities such as cardiopulmonary compromise or renal failure.
The diagnosis of HCC was based on criteria in practice guidelines of AASLD [Citation5]. In the SR group, 6 patients did not achieve R0 resection and received transcatheter arterial chemoembolization (TACE) and other treatments thereafter were excluded. In term of the MWA group, 7 patients achieved complete ablation after a secondary MWA (1 months after the primary technical failure) were included, and 4 patients received SR or TACE due to failures to get a complete ablation after MWA were excluded. The flow chart of study patient selection was detailed in . This clinical trial has been prospectively registered in Chinese Clinical Trial Registry. The registration number was ChiCTR2000033983. The present study was approved by the institutional review board and the informed consent requirement was waived.
Treatment and patient follow up
In this study, HCC patients received SR or MWA. In the SR group, the type and extent of resection were based on per patient’s hepatic functional reserve, tumor extent, and the preference of the operators. During the parenchymal dissection, operators attempt to achieve a resection margin of at least 1 cm.
In the MWA group, the procedures were performed with patients under the guidance of CT or ultrasound. Microwave ablation therapeutic instrument (MTC-3C, Nanjing Qinghai Research Institute of Microwave Electric, China) we used can produce 0 ∼ 100 W of power at a frequency of 2450 ± 50 MHz. One microwave antenna is used for tumors ≤3 cm in maximum diameter, and 2 antennae for tumors >3 cm. Antennae were placed sequentially at different numbers of sites in the tumor depended on the tumor size and shape. Each site performed MWA with a power of 60–80 W for 4–8 min. Antenna track ablation was routinely performed during needle removal. In this group, performers also tried to achieve a complete tumor ablation with at least a 1 cm margin.
Patients generally underwent CT or MRI 1–2 months postoperatively of both groups. Then patients were followed every 3 months in the first year and 3–6months thereafter. Each follow up consisted of serum chemistry evaluations including alpha-fetoprotein and at least one imagining examination (contrast-enhanced dynamic CT or MRI). Once the tumor recurred, the therapy was based on preference of patients and clinical practice of surgeons and clinicians. Repeated ablation or resection was the first choice for patients with recurrent tumors whenever possible, while percutaneous ethanol injection, TACE and other nonradical treatments were the treatment options for patients unsuitable for SR or ablation. All patients were followed up until death, 30 April 2020, or lost to follow up, whichever came first.
Complications and hospital stay
We used the Clavien-Dindo Classification System to grade complications after treatment [Citation16]. The complications of Grade 1 and 2 were defined as minor complications, while the Grade ≥3 were considered as major complications [Citation17]. Hospital stay was defined as the time from the date of admission to the date of discharge or death.
Survival outcome
The primary endpoint of the study was overall survival (OS), and the secondary endpoint was progression-free survival (PFS). OS was defined as the time from date of operation to the death or the last follow-up before 30 April 2020. PFS was defined as the interval from date of operation to the date of the onset of progression, death, or last follow-up visit. Tumor progression was identified after the first treatment, when recurrence, invasion or metastasis occurred. The cumulative rates of local tumor progression in the MWA group were also investigated. The local tumor progression was defined as the appearance of tumor at the edge of the ablation zone [Citation18].
Subgroup analyses of OS and PFS were conducted according to tumor number (single or 2–3). Since few patients with Child-Pugh B class disease received SR (n = 7) in the present study, we considered comparing the prognosis of patients in different albumin–bilirubin (ALBI) grades (grade 1 and grade 2) based on prior literature [Citation19].
Propensity score matching
To decrease the selection bias between the two study groups, we performed a 1:1 propensity score matching (PSM) to create a comparable control cohort, including age, sex, Child-Pugh class, presence of liver cirrhosis, model for end-stage liver disease (MELD) score, antiviral treatment, serum AFP, alanine aminotransferase level and aspartate aminotransferase level, presence of portal hypertension, primary tumor number and tumor size.
Statistical analysis
Categorical variables were presented as counts and percentages, continuous variables were expressed as median and interquartile range. Categorical Variables were assessed by Pearson’s χ2 test or Fisher’s exact test in two groups. Continuous variables were analyzed by using the Mann-Whitney test in two groups. OS and PFS curves were constructed by using the Kaplan-Meier method and compared by the log-rank test. Univariate and multivariate Cox proportional hazards regression models were used to analyze prognostic factors related to OS and PFS. Covariate balance was measured using the standardized mean difference (SMD). An SMD smaller than 0.1 indicated a well-matching balance. All statistical analyses were performed using SPSS 24.0 for Windows (SPSS Inc., Chicago, IL, USA). All tests were two-tailed, and differences of p < 0.05 were considered statistically significant.
Results
Patient characteristics
Among the 393 patients enrolled in this study, 93 patients were initially received MWA, and 300 patients were initially received SR. In this study, most of the study population had HBV infection (n = 361, 91.9%). In total cohort, there are 81 (87.1%) patients with Child-Pugh class A in the MWA group, and 293 (97.7%) in the SR group (p < 0.001). As for tumor number, there are 64 (68.8%) patients with single tumor nodules in the MWA group, and 255 (85%) in the SR group (p < 0.001). The MELD score for the MWA group was 3.74 (1.80–6.07) compared to 5.23 (2.76–6.93) for the SR group (p = 0.024). Regarding tumor size, the median of maximum tumor diameter was 2.8 cm (2.0–3.6 cm) in the MWA group and 3.3 cm (2.5–4.0 cm) in the SR group (p < 0.001). After the PSM, a new cohort comprising 80 patients in the MWA group and 80 patients in the SR group was generated. All the relevant background characteristics were balanced ().
Table 1. baseline characteristics of the total cohort and PSM cohort for HCC up to 5 cm.
MWA provided similar efficacy compared with SR for HCC ranging from 3 to 5 cm
We were interested in the comparison of MWA and SR for HCC ranging from 3 to 5 cm. Thus, we performed the analysis for patients with HCC between 3 and 5 cm. Among these patients, 35 patients initially received MWA, and 162 patients initially received SR. The median follow-up period was 59 months (range, 1–119 months). In the total cohort of HCC ranging from 3 to 5 cm, the mean OS time for patients with MWA was 5.36 years, compared to 7.38 years for those with SR. The OS rates at 1, 3, and 5 years were 91.3%, 78.5%, and 64.6% in the MWA group, compared to 95.4%, 82.5%, and 65.8% in the SR group, respectively (p = 0.548) (). The mean PFS time for patients with MWA was 2.70 years compared to 3.62 years for those with SR. The 1-, 3-, and 5-years PFS rates were 61%, 33.5%, and 29.8% in the MWA group, and 67.6%, 43.9%, and 31.4% in the SR group, respectively (p = 0.306) ().
Figure 2. Overall survival and progression-free survival curves of patients with hepatocellular carcinoma ≥ 3 cm who underwent microwave ablation or resection. There were no significant differences in overall survival curves (A) and progression free survival curves (B) between MWA group and SR group in total cohort for hepatocellular carcinoma ≥ 3 cm (p = 0.548, p = 0.306). And there were no significant differences in overall survival curves (C) and progression-free survival curves (D) between MWA group and SR group in PSM cohort for hepatocellular carcinoma ≥ 3 cm (p = 0.457, p = 0.397).
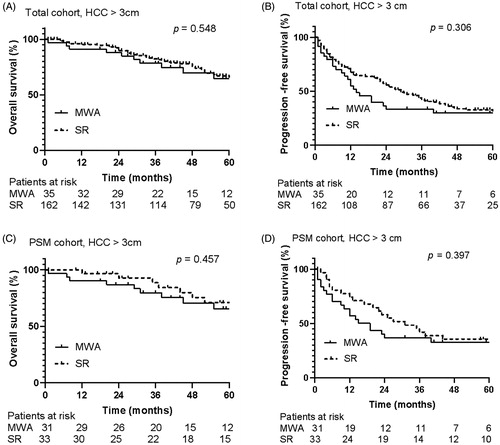
In the PSM cohort, 31 patients received MWA, and 33 patients received SR. The mean OS time was 5.40 years versus 7.65 years in the MWA and SR group, respectively. The 1-, 3-, and 5-years OS rates were 90.3%, 79.7%, and 65.5% in the MWA group, and 96.7%, 88.6%, and 71%% in the SR group, respectively (p = 0.457) (). The mean PFS time was 2.88 years in the MWA group compared to 4.14 years in the SR group. The 1-, 3-, and 5-years PFS rates were 63.6%, 36.8%, and 32.7% in the MWA group, and 74.2%, 41.9%, and 35.5% in the SR group, respectively (p = 0.397) ().
For the total cohort of HCC up to 5 cm, the median follow-up period was 59 months (range, 1–121months). The mean OS time for patients with WMA was 7.47 years, compared to 7.44 years for those with SR. The OS rates at 1, 3, and 5 years were 90.1%, 82.9% and 71.9% in the MWA group, compared to 95.5%, 86.1%and 67.6% in the SR group, respectively (p = 0.879) (). The mean PFS time for patients with MWA was 4.19 years, compared to 3.72 years for those with SR. The 1-, 3-, and 5-years PFS rates were 66.3%, 46.1%, and 33.7% in the MWA group, and 71.8%, 44.3%, and 31.8% in the SR group, respectively (p = 0.932) ().
Figure 3. Overall survival and progression-free survival curves of patients with hepatocellular carcinoma ≤ 5 cm who underwent microwave ablation or resection. There were no significant differences in overall survival curves (A) and progression free survival curves (B) between MWA group and SR group in total cohort for hepatocellular carcinoma ≤ 5 cm (p = 0.879, p = 0.932). And there were no significant differences in overall survival curves (C) and progression-free survival curves (D) between MWA group and SR group in PSM cohort for hepatocellular carcinoma ≤ 5 cm (p = 0.965, p = 0.801).
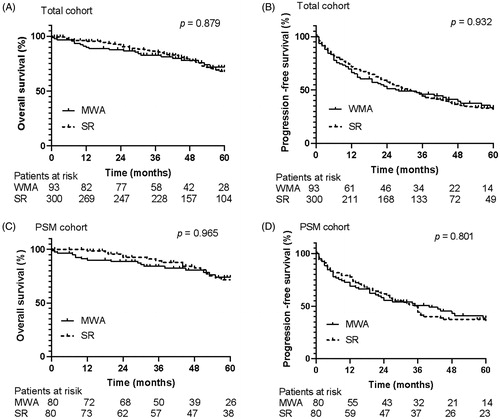
In the PSM cohort of HCC up to 5 cm, the mean OS time was 7.75 years versus 7.79 years in the MWA and SR group, respectively. The 1-, 3-, and 5-years OS rates were 89.9%, 88.6%, and 74.0% in the MWA group, and 98.6%, 89.4%, and 71.6% in the SR group (p = 0.965) (). The mean PFS time was 4.59 years for patients with MWA compared to 3.62 years for those with SR. The 1-, 3-, and 5-years PFS rates were 68.8%, 50.7%, and 38.7% in the MWA group, and 77.7%, 49.6%, and 35.7% in the SR group (p = 0.801) ().
The cumulative rates of local recurrence in the MWA group were reproduced in Supplementary Figure 1. The cumulative rates of local tumor progression rates were higher after MWA compared with SR (6.3% versus 1.3% at 1 year, 9.3% versus 3.0% at 4 years) for HCC ranging from 3 to 5 cm in the total cohort, but the difference did not reach statistical significance (p = 0.072). In the MWA group, the cumulative local tumor progression rates of HCCs between 3 and 5 cm were similar to those of HCCs up to 3 cm (6.3% versus 7.1% at 1 years, 9.7% versus 9.2% at 2 years, p = 0.954).
There were no differences in efficacy between MWA and SR for subgroup analyses
The results of subgroup analyses based on tumor number (single or 2–3) and ALBI grade (grade 1 or grade 2) in the total and PSM cohort are summarized in . Kaplan-Meier analyses showed that there were no differences in OS and PFS between the MWA and SR groups for these subgroup analyses (all p ≥ 0.05).
Table 2. Subgroup analysis for the total cohort and the PSM cohort.
Factors associated with OS and PFS
In the total cohort patients, multivariate analyses showed that age (hazard ratio (HR), 1.029; 95% confidence interval (CI), 1.007–1.051; p = 0.013) and Child-Pugh class (HR, 3.129; 95% CI, 1.489–6.579; p = 0.003), were significant factors for OS. After PSM, Child-Pugh class (HR, 3.710; 95% CI, 1.269–10.841; p = 0.017) and MELD score (HR, 1.136; 95% CI, 1.006–1.283; p = 0.040) were significant factors for OS ().
Table 3. Factors associated with OS of the total cohort and the PSM cohort for HCC up to 5 cm.
On the multivariate analysis for PFS in total cohort patients, multiple tumor nodules (HR 1.618; 95% CI, 1.174–2.230; p = 0.003) were significant factor. In terms of PSM cohort, multiple tumor nodules (HR 1.623; 95% CI, 1.004–2.265; p = 0.048) and MELD score (HR 1.071; 95% CI 1.006–1.139; p = 0.03) were significant factors ().
Table 4. Factors associated with PFS of the total cohort and the PSM cohort for HCC up to 5 cm.
MWA performed fewer complications and shorter hospital stays compared with SR
In the whole study population, a total of 3 postoperative deaths wereobserved, 1 in the MWA group, and 2 in the SR group. The patient in the MWA group with a medical history of HBV infection, cirrhosis, and portal hypertension died of massive hemorrhage of the upper alimentary tract. The first treatment-related death in the SR group had a similar medical history to the above patient and died of sudden abdominal bleeding. Another death in the SR group developed postoperative infection and finally died of septic shock.
For HCC ranging from 3 to 5 cm, complications of patients in the two groups were shown in Supplementary Table 1. There was a higher rate of overall procedure-related complications in the SR group compared to the MWA group (74.7% vs 57.1% p = 0.037), but the rate of major complications did not show a significant difference (8.0% vs 14.2% p = 0.244). The complications of PSM cohort showed similar result to those in the total cohort. Moreover, compared with the SR group, the MWA group had shorter median hospital stays both in the total cohort (8 versus 14 days, p < 0.001) and PSM cohort (8 versus 15 days, p < 0.001). In terms of HCC up to 5 cm, the comparison of complications and hospital stays between the MWA and SR group was consistent with the analysis of HCC between 3 and 5 cm (Supplementary Table 2).
Discussion
With a PSM, the present study showed that MWA had no difference in efficacy compared to SR for primary HCC patients with tumor nodules between 3 and 5 cm or nodules up to 5 cm, but with fewer complications and shorter hospital stays. Similar results were shown in subgroup analysis, including the number of tumors (single or 2–3) and ALBI grade (grade 1 or 2).
Currently, SR and RFA are the mainstays of treatments for early HCC. SR has advantages over thermal ablation as it can provide substantial safety margins. On the contrary, thermal ablation represents a less invasive procedure with a lower risk of treatment-related complications. Prior literature showed a comparable efficiency between both treatments for patients with HCC up to 3 cm [Citation6,Citation10,Citation20]. Therefore, RFA is nowadays considered as an acceptable option for small HCC [Citation1,Citation5]. However, several studies have suggested that SR was superior to RFA for patients with HCC up to 5 cm [Citation9,Citation11,Citation21]. This could be linked to the limited capacity of RFA to produce sufficiently large and homogeneous destruction, particularly near large vessels due to heat-sink effect [Citation17].
Prior retrospective cohort studies also reported that patients with MWA had a similar OS curve compared to SR for primary HCC within 3 cm and 3 nodules [Citation7,Citation22]. Compared with RFA, MWA can generate a higher temperature in a shorter period of time leading to a larger ablation area and less affected by the heat-sink effect. Several studies showed that MWA had better performance for local tumor control, tumor inactivation and long-term survival compared RFA in larger neoplasm [Citation12–15]. Former comparative studies had compared the result of MWA with SR for HCC conforming to Milan criteria and showed a similar OS benefit for both groups [Citation23,Citation24]. Likewise, the meta-analysis by Zhang et al. of nine retrospective studies revealed no differences between MWA and SR regarding OS and PFS [Citation25]. As a supplement, the current study showed that MWA was as efficient as SR both in OS and PFS for HCC nodules between 3 and 5 cm in diameter and up to 3 nodules.
Indeed, the Child-Pugh class and MELD score were indicators of hepatic reserve, which were logically closely related to hepatocellular carcinoma prognosis. It had been shown to have a reliable prediction value for patient’s survival in prior literature [Citation26–28]. The present study found that Child-Pugh class B (compared to class A) and a higher MELD score were significantly associated with a poorer OS.
Moreover, some authors had indicated that MELD score was an independent factor for recurrence [Citation26,Citation29]. Umeda et al. suggested that poor hepatic reserve was an independent factor for HCC recurrence [Citation30]. This was consistent with the analysis of the current study. Another observation in this study was that multiple tumors were significantly associated with PFS. This was consistent with the result from prior studies [Citation31–33].
This study had several limitations. First, due to its retrospective nature, this study might have suffered from potential bias. Although the current study attempted to balance the group and minimize the bias by using the propensity score matching method, some uncontrolled baseline confounding factors might remain and influence the outcomes. Second, this study was a single-center review. Thus, the results might not be generalizable to other centers. Third, the number of tumors between 3 and 5 cm was not large in this study population. Therefore, further research is warranted to validate the results of the current study. Lastly, this study did not analyze the detail of recurrence patterns and retreatment strategies of each recurrent case. It might have an influence on long-term survival.
In conclusion, MWA showed similar survival outcomes compared with SR for HCCs up to 5 cm. However, it showed favorable results in terms of hospital stay and complication rate compared to SR. For HCC ranging from 3 to 5 cm, MWA was a promising treatment for patients to obtain a similar survival benefit compared to SR. However, multi-center, prospective, and large randomized controlled trials are required to verify the conclusions of the current study.
Registration
This clinical trial has been prospectively registered in Chinese Clinical Trial Registry. The registration number was ChiCTR2000033983.
Supplemental Material
Download PDF (273.5 KB)Supplemental Material
Download TIFF Image (392.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Vogl TJ, Farshid P, Naguib NN, et al. Ablation therapy of hepatocellular carcinoma: a comparative study between radiofrequency and microwave ablation. Abdom Imaging. 2015;40(6):1829–1837.
- Andreano A, Huang Y, Meloni MF, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37(6 Part 1):2967–2973.
- Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018;68(4):783–797.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
- Shiina S, Sato K, Tateishi R, et al. Percutaneous ablation for hepatocellular carcinoma: comparison of various ablation techniques and surgery. Can J Gastroenterol Hepatol. 2018;2018:4756147.
- Li W, Zhou X, Huang Z, et al. Short-term and long-term outcomes of laparoscopic hepatectomy, microwave ablation, and open hepatectomy for small hepatocellular carcinoma: a 5-year experience in a single center. Hepatol Res. 2017;47(7):650–657.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223(2):331–337.
- Liu H, Wang ZG, Fu SY, et al. Randomized clinical trial of chemoembolization plus radiofrequency ablation versus partial hepatectomy for hepatocellular carcinoma within the Milan criteria. Br J Surg. 2016;103(4):348–356.
- Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–307.
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–912.
- Lee KF, Wong J, Hui JW, et al. Long-term outcomes of microwave versus radiofrequency ablation for hepatocellular carcinoma by surgical approach: a retrospective comparative study. Asian J Surg. 2017;40(4):301–308.
- Glassberg MB, Ghosh S, Clymer JW, et al. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Onco Targets Ther. 2019;12:6407–6438.
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2016;32(3):339–344.
- Yu J, Yu XL, Han ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66(6):1172–1173.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Mohkam K, Dumont PN, Manichon AF, et al. Touch multibipolar radiofrequency ablation vs. surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5cm. J Hepatol. 2018;68(6):1172–1180. No
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Toyoda H, Lai PB, O’Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer. 2016;114(7):744–750.
- Hung HH, Chiou YY, Hsia CY, et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin Gastroenterol Hepatol. 2011;9(1):79–86.
- Lee YH, Hsu CY, Chu CW, et al. Radiofrequency ablation is better than surgical resection in patients with hepatocellular carcinoma within the Milan criteria and preserved liver function: a retrospective study using propensity score analyses. J Clin Gastroenterol. 2015;49(3):242–249.
- Zhang EL, Yang F, Wu ZB, et al. Therapeutic efficacy of percutaneous microwave coagulation versus liver resection for single hepatocellular carcinoma ≤3 cm with Child-Pugh A cirrhosis. Eur J Surg Oncol. 2016;42(5):690–697.
- Shi J, Sun Q, Wang Y, et al. Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. J Gastroenterol Hepatol. 2014;29(7):1500–1507.
- Liu W, Zou R, Wang C, et al. Microwave ablation versus resection for hepatocellular carcinoma within the Milan criteria: a propensity-score analysis. Ther Adv Med Oncol. 2019;11:1758835919874652.
- Zhang M, Ma H, Zhang J, et al. Comparison of microwave ablation and hepatic resection for hepatocellular carcinoma: a meta-analysis. Onco Targets Ther. 2017;10:4829–4839.
- Lee S, Rhim H, Kim YS, et al. Post-ablation des-gamma-carboxy prothrombin level predicts prognosis in hepatitis B-related hepatocellular carcinoma. Liver Int. 2016;36(4):580–587.
- Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41(4):707–716.
- Martins A, Cortez-Pinto H, Marques-Vidal P, et al. Treatment and prognostic factors in patients with hepatocellular carcinoma. Liver Int. 2006;26(6):680–687.
- Elalfy H, Besheer T, El-Maksoud MA, et al. Monocyte/granulocyte to lymphocyte ratio and the MELD score as predictors for early recurrence of hepatocellular carcinoma after trans-arterial chemoembolization. Br J Biomed Sci. 2018;75(4):187–191.
- Umeda Y, Matsuda H, Sadamori H, et al. A prognostic model and treatment strategy for intrahepatic recurrence of hepatocellular carcinoma after curative resection. World J Surg. 2011;35(1):170–177.
- Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107(4):569–577. quiz 578.
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31(4):426–432.
- Ding J, Jing X, Wang Y, et al. Thermal ablation for hepatocellular carcinoma: a large-scale analysis of long-term outcome and prognostic factors. Clin Radiol. 2016;71(12):1270–1276.