Abstract
Objective
To explore correlations between the therapeutic effect of high intensity focused ultrasound (HIFU) and histopathological characteristics of uterine fibroids with different Shear Wave Velocity (SWV) values.
Methods
A retrospective study was conducted on 36 women (43 fibroids) who had undergone high intensity focused ultrasound (HIFU) uterine fibroids ablation between January 2019 and January 2020. Preoperative fibroids tissue sections were obtained for histopathological examination. The pathological sections were stained with Masson-trichrome, and were observed and imaged under a Low-power microscope (4 × 10), while the smooth muscle cell (SMC) and collagen fiber content were semi-quantitatively measured. Preoperative fibroid SWV was measured using the Virtual Touch tissue quantification (VTQ) technique. Within one month after HIFU ablation, all patients had undergone a pelvic cavity MRI examination, which measured the size, volume, and non-perfused volume (NPV) of the fibroids. The formula: the ablation rate = NPV/target fibroid volume × 100% was used to calculate the ablation rate of the uterine fibroids. Correlation analysis of SWV values, HIFU ablation rate, along with the smooth muscle cell (SMC) and collagen fiber content, were conducted using the Spearman’s correlation test.
Results
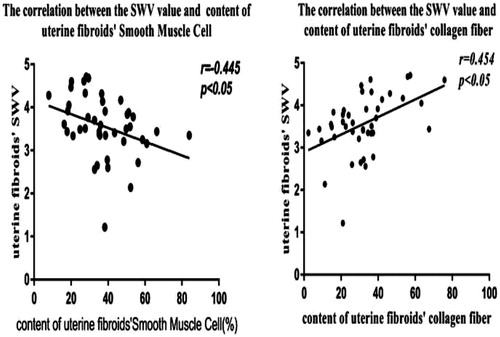
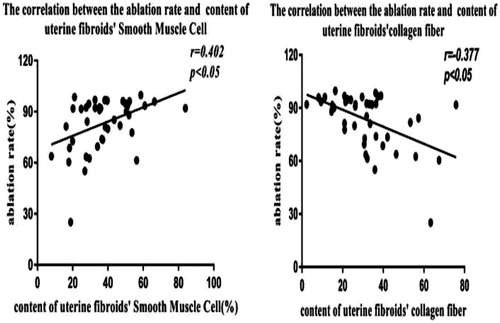
The collagen fiber and SMC content of the preoperative fibroids were 32.09 ± 15.90%/view and 37.61 ± 15.32%/view, respectively. Preoperative fibroid SWV value was 3.56 ± 0.71 m/s. Preoperative fibroid SWV was negatively correlated with SMC content (r = −0.445, p = 0.003), but positively correlated with collagen fiber content (r = 0.454, p = 0.002). The ablation rate was negatively correlated with collagen fiber content (r = −0.377, p = 0.013), but positively correlated with SMC content (r = 0.402, p = 0.007).
Conclusion
Differences in histopathological characteristics may be important factors that induce differences in the therapeutic effects of HIFU ablation on uterine fibroids with different SWV values.
Introduction
Uterine fibroids are the most common benign tumors of the female reproductive system. Its incidence in middle-aged women is 20–40% [Citation1], and is often accompanied by abdominal pain, bleeding, and other clinical symptoms [Citation2,Citation3], which seriously affect the quality of life, as well as physical and mental health of the affected women. At present, the main method of treatment for uterine fibroids are surgical treatment, interventional treatment, and drug treatment. High-intensity focused ultrasound (HIFU) is a noninvasive local tumor treatment technology, which has been widely used for the treatment of uterine fibroids. Compared with traditional treatment methods, it shows high levels of efficacy, lower levels of pain, and quick postoperative recovery. A large number of clinical studies have proven that high intensity focused ultrasound can effectively ablate uterine fibroids and improve clinical symptoms of patients [Citation4,Citation5]. Along with the development of HIFU for the ablation of uterine fibroids, the study of noninvasive examination techniques for pretreatment efficacy estimation, real-time monitoring of the treatment process, and immediate evaluation have become a hot therapy-related topics. At present Contrast-enhanced MR is used to predict the efficacy of HIFU uterine fibroids ablation. Along with the development of ultrasound technology, elastography, which uses the mechanical characteristics (strain and elasticity modulus) of human soft tissues for clinical imaging [Citation6], has gradually been introduced into clinical practice. Acoustic Radiation Force Pulse Imaging Technology (ARFI) is a new elastography-based technology that integrates virtual touch tissue quantification (VTQ) with virtual touch tissue imaging (VTI), and has been found to effectively identify the properties of tissues by detecting the elastic stiffness of tissues [Citation7–9]. Previous studies [Citation10] have confirmed that the ARFI technique is a practical and feasible noninvasive ultrasound technique for the preoperative prediction of the efficacy of HIFU uterine fibroid ablation. Studies have shown that with the same single point of sound power, uterine fibroids with different Shear Wave Velocity (SWV) show differences in the efficacy of HIFU ablation. The preoperative uterine fibroid SWV was negatively correlated with the ablation rate. Uterine fibroids with higher SWV values were more difficult to ablate than uterine fibroids with lower SWV values. What are the main reasons for these differences in ablation rates? It is necessary for us to observe the histopathological characteristics of uterine fibroids with different SWV values and explore correlations between the therapeutic effect of HIFU and the histopathological characteristics of uterine fibroids. Therefore, the reasons behind differences in the therapeutic efficacies of HIFU ablation on uterine fibroids with different SWV can be studied using a histopathological characteristics perspective.
Materials and methods
Study population
Most leiomyomas are easily diagnosed through imaging. However, in clinical practice, sarcomas have been found to lack imaging specificity. To avoid unnecessary controversies, patients are informed of the associated risks during preoperative discussion. Although the probability of sarcoma is extremely low, some patients still have concerns about the associated risk. HIFU is a noninvasive method of treatment, in which no tissue samples are left for pathological examination after surgery. Therefore, some patients actively request puncture biopsy for pathological verification. Specimens obtained from such patients, who have undergone voluntary biopsy for pathological examination, are the source of tissue specimens used in this study. This retrospective study was conducted on 36 women (43 fibroids) who voluntarily requested for pathological examination of uterine fibroids before HIFU uterine fibroid ablation between January 2019 and January 2020. The age range of the patients was 27–51 years, with an average age of 42.0 ± 5.6 years. The maximum diameter of uterine fibroids ranged from 32.7 − 87.1 mm, with an average diameter of 56.73 ± 16.03 mm. The inclusion criteria was as follows: (1) women of childbearing age; (2) refused surgical operation and had a strong desire to preserve the uterus; (3) fibroids can be displayed well using airborne ultrasound, and uterine fibroids treatment in a secure acoustic pathway; (4) no communication barriers. The exclusion criteria were as follows: (1) other gynecological diseases (such as vaginitis, pelvic inflammatory disease, cervical carcinoma and other tumors); (2) connective tissue disease; (3) received ≥45Gy of abdomen radiotherapy; (4) cerebral infarction and cerebral hemorrhage within half a year; (5) severe heart, brain, lung, kidney and other system diseases; (6) Body Mass Index (BMI) greater than 32; (7) uterine fibroids with a large number of necrotic areas; (8) pregnant and lactating status. All participants provided informed consent.
Imaging techniques
Shear wave velocity (SWV) values of the uterine fibroids were measured in all patients before HIFU ablation using a SIEMENS S3000 color Doppler ultrasonic diagnostic apparatus. During SWV measurement, the patient lay in the supine position, with the probe placed directly on the lower abdomen. After obtaining the maximum uterine fibroids longitudinal sections, the VTQ mode of ARFI was switched on. The area of interest was determined and was placed at the center, outer upper, outer lower, inner upper and inner lower areas of the fibroid (adjusted according to the size of the fibroid). The patient was instructed to hold their breath and a high-intensity low-frequency pulse wave was generated by pressing the update key, and the transverse shear wave velocity (SWV) was measured in m/s. A single SWV value can be automatically obtained after launching 1 propulsion pulse, which reflects the elasticity of the region. The shear wave velocity related to tissue stiffness in the region of interest was read and recorded. The measurement was repeated 3 times on the same area, and the average value was used.
Specimen disposal
Puncture biopsy of the uterine fibroid was performed using an 18 G automatic biopsy device (Bard Company, Murray Hill, NJ, USA) under ultrasonic guidance to obtain tissue strips of the uterine fibroids (2/fibroids). The specimens were fixed using 10% formalin and sent to the Department of pathology for pathological examination. After routine pathological examination, the remaining wax blocks of fibroid tissue were cut into 2 pieces for MASSON staining. Then, the sections were air-dried, observed and photographed using a Nikon ECLIPSE 80i optical microscope. The pathological image data were analyzed. Finally, the average of the two tissue strips of each fibroid was recorded.
Pathological image collection was performed as follows: under the guidance of pathologists, the exposure, delay time and brightness values of the picture obtained under low magnification (4 × 10 times) were adjusted until the images in the image acquisition system were completely consistent with those obtained under the light microscope. The images under the microscope were recorded and their parameters were observed. All sections were observed and photographed under the low power (4 × 10) field of vision and were also reviewed by a pathologist to ensure that the sections met test requirements.
Semi-quantitative analysis of the histopathological characteristics of the uterine fibroids
MASSON stains collagen fibers a strong blue color, and smooth muscle a red color. Semi-quantitative analysis of collagen fiber and smooth muscle cell content in Masson stained images were processed using Photoshop CS 5 image analysis software.
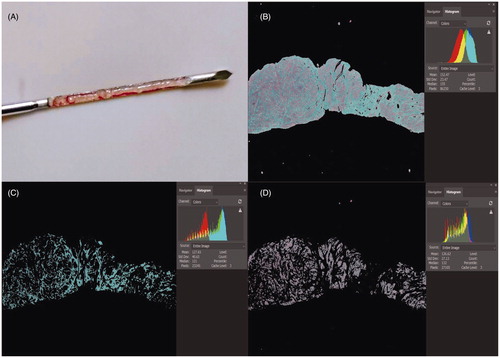
First, the non-fibroid tissues were removed by adjusting the parameters and the pixel value of the whole fibroid tissue was automatically calculated. Then, the non-collagen fiber components were removed by adjusting the parameters and the pixel value of blue collagen fibers was automatically calculated. The non-smooth muscle cells were removed by adjusting the parameters and the pixel value of red smooth muscle cell was automatically calculated. Finally, collagen fiber or smooth muscle cell content was evaluated by calculating the ratio of the area of blue collagen fibers or red smooth muscle cell to the total area of the whole fibroid tissue (). Image analyses were performed separately by two pathologists who were blinded to the results of the other. The two pathologists checked the results of the other after the analysis was completed. The results were considered valid and average results were evaluated only if the difference between SMC and collagen fiber content was within 5%. If results were invalid, then both pathologists repeated the measurements separately until the results were valid.
Figure 1. Collagen fiber and smooth muscle cell content in Masson staining images: microscopic magnification (4 × 10). Photoshop analysis software was used to analyze the image pixels. (A) Fibroid tissue obtained using needle biopsy (B) Pixel image of the whole fibroid tissue. The pixel value of this field is 86,250 (C) Pixels of the blue collagen fiber. The pixel value of this field is 23245.(D) Pixel image of the red smooth muscle cells. The pixel value of this field is 27105.
Hifu treatment
Uterine fibroid ablation was performed using a JC200 focused ultrasound tumor treatment system (Chongqing HIFU technology co, LTD). The therapeutic parameters included: (1) a therapeutic frequency of 0.97 MHz; (2) focus width of 3 mm; (3) focal length of 8 mm; (4) focal length of 162 mm; (5) a single therapeutic power of 400 W; (6) the irradiation and pause times of 1 s each.
Patients were carefully placed in the prone position on the HIFU table, ensuring that the skin overlying the target lesion was in contact with the water balloon and degassed water contained in the water tank, where the HIFU transducer was placed. The procedure was performed under sedation using intravenous administration of midazolam (1–10 mg) and fentanyl (25–100 mg) by an anesthetist. Intraoperative continuous intravenous infusion of oxytocin (80 U þ 500 ml normal saline) was administered at 40 drops/min to decrease the blood supply to the uterine fibroids.
The target lesion was first detected using the airborne combined therapeutic device, which consists of a diagnostic probe for locating and monitoring lesions and a therapeutic device for launching high-intensity focused ultrasound waves. A preliminary planning scan was then performed to identify a suitable safe ablation track, which was stored as a baseline in cine loop format. The entire fibroid volume was then divided into 5 mm interspersed axial US slices, which were recorded using software of the HIFU equipment for use as the treatment plan. The scanning mode adopted was point to line, line to surface, and surface to body layer, where layers were scanned from deep to shallow levels. This procedure was repeated on a slice-by-slice basis to achieve complete ablation of the entire volume of the fibroid. The single-point ablation power used was 400 w. During continuous therapy, the irradiation and pause times were 1 s each. The distance between points, the row spacing, and the ratio of irradiation to pause times were adjusted according to changes in tissue echogenicity and the response of the patient to acoustic irradiation. During ablation, real-time scans were obtained immediately before and after individual energy exposures (i.e., HIFU sonication) and were compared to assess changes in the echogenicity of the treated region, which reflects the ablation degree. The post-sonication appearance was expected to be similar to that of an ill-defined hyperechoic area. If the echogenicity of all uterine fibroids had changed, infusion of oxytocin was stopped for 10 min to remove the influence of oxytocin on the blood supply to uterine fibroids. SonoVue contrast ultrasound injection was used to immediately evaluate ablation results. Ablation was considered effective when the contrast agent could not be perfused into the tumor.
Therapeutic effect evaluation
Contrast-enhanced MRI examination was performed using a 3.0 T Philips Achieve Magnetic Resonance Imaging scanner system within one month (2–22 days with an average day of 8.17 ± 5.67 day), after HIFU ablation. All patients underwent a conventional Spin Echo (SE) sequence T1WI and Turbo Spin Echo (TSE) sequence T2WI cross-sectional, coronal, and sagittal scans, and inhibition lipid sequences were added. Before examination, patients were instructed to drink water and fill their bladder. The non-perfused volume (NPV) of ablation was measured. The sizes of the postoperative uterine fibroids were measured using enhanced MRI images, and included length diameter (D1), anteroposterior diameter (D2), left and right diameter (D3), and postoperative NPV. The uterine fibroid volume, the NPV, and the ablation rate were also calculated. The volume (V) and the ablation rate were calculated using the following formulas: V = 0.5233 × D1 × D2 × D3; ablation rate = NPV × 100%/target fibroid volume.
Statistical analysis
The data were analyzed using SPSS version 19.0 (IBM company, 2010) software. The correlations between ablation rate, SWV value, collagen fiber and smooth muscle cell content were analyzed using Spearman correlation analysis. The correlation coefficient was expressed as r. Correlation with a p value of <0.05 was considered statistically significant.
Results
Semi-quantitative analysis and comparison of uterine fibroid tissue components
The morphology and distribution of smooth muscle cells and collagen fibers obtained through the pathological examination of uterine fibroids are shown in and .
Figure 2. Pathological images of different types of uterine fibroids. (A) The content of smooth muscle cell was rich and the collagen fibers showed a thin, scattered distribution. (B) The content of smooth muscle cell was rare and the collagen fibers were thick, densely arranged and showed a bunched distribution. (C) The content of smooth muscle cell was lower, and the collagen fibers were of different thicknesses and were densely arranged.
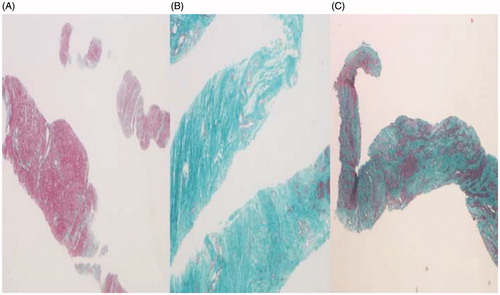
Table 1. The morphology and distribution of SMCs and CFs obtained through the pathological examination of uterine fibroids.
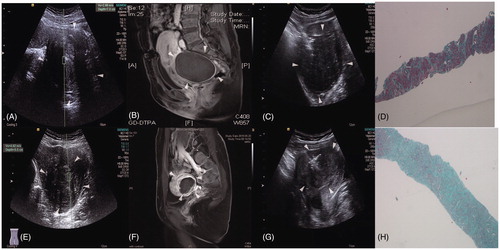
The SWV values, enhanced MRIs of the postoperative uterine fibroids, and images of the tissues at low power (4 × 10) are shown in .
Figure 3. The SWV values, enhanced MRIs of postoperative uterine fibroids, and images of tissues at low power (4 × 10). (A) Preoperative fibroids with SWV values of 2.89 m/s and an ablation rate of ≥70%. (B) Enhanced MRI showing no residual mass. (C) Preoperative biopsy images of uterine fibroids. (D) Fibroid tissue at low power (4 × 10) and an ablation rate of ≥70%. (E) Preoperative fibroids with a SWV value of 4.62 m/s and an ablation rate of <70%. (F) Enhanced MRI showing residual mass with an ablation rate of <70%. (G) Preoperative biopsy images of the uterine fibroids. (H) Fibroid tissue at low power (4 × 10) and an ablation rate of <70%.
Correlation between the SWV value and content of collagen fiber and smooth muscle cell in the preoperative uterine fibroids
The correlation analysis showed that preoperative uterine fibroid SWV was negatively correlated with the SMC content (r = −0.445, p = 0.003), but positively correlated with the collagen fiber content (r = 0.454, p = 0.002) ().
Correlation between the ablation rate and content of uterine fibroids collagen fiber and smooth muscle cell
The ablation rate was negatively correlated with collagen fiber content (r = −0.377, p = 0.013), but positively correlated with SMC content (r = 0.402, p = 0.007) ().
Discussion
Clinical practice shows that there can be differences in the efficacy of high-intensity focused ultrasound (HIFU) ablation of different uterine fibroids. Some uterine fibroids are difficult to be ablated, and an ideal ablation effect may be difficult to be achieved even when using a high therapeutic dose. The reasons for these variabilities are still unclear. It has been found [Citation10] that the preoperative shear wave velocity (SWV) value of uterine fibroids is negatively correlated with the ablation rate. Uterine fibroids with higher SWV values are more difficult to ablate than those with lower SWV values. Different histopathological characteristics of fibroids may influence the effectiveness of HIFU treatment. Therefore, it is necessary for us to analyze using a pathological perspective and explore the reasons for the differences in ablation efficacy of uterine fibroids with different SWV values.
Uterine fibroid, also known simply as a fibroid, is one of the most common benign tumors of the female reproductive system [Citation1]. It is mainly composed of proliferative uterine smooth muscle cells and fibrous connective tissue, with the outer surface being a thin layer of pseudocapsule formed by surrounding connective tissue bundles and muscle fiber bundles that are connected to a radial vascular branch that supplies the fibroid [Citation11]. This study began by analyzing the main pathological components of uterine fibroids, which are collagen fibers and smooth muscle cells, to explore the reasons behind the differences in ablation results of fibroids with different SWV values.
In this study, the correlation between preoperative uterine fibroid SWV and the content of smooth muscle cells and collagen fibers in fibroids were analyzed. The results showed that uterine fibroid SWV was negatively correlated with the content of smooth muscle cells but positively correlated with the content of collagen fibers. Therefore, the higher the SWV value of the uterine fibroid, the higher the collagen fiber content in uterine fibroid tissue and the lower the smooth muscle cell content, and the greater the elastic modulus, and the higher the stiffness of the uterine fibroid [Citation12,Citation13]. This result is similar to that reported by Chamming et al. [Citation14] on the relationship between Young’s modulus and collagen fiber content in breast cancer, which showed that tumor stiffness increases along with the increase in collagen fiber content. Therefore, the main pathological factor that determines the stiffness of uterine fibroids is the difference in smooth muscle cell and collagen fiber content.
In this experiment, the postoperative ablation rate of HIFU with the same single-point sound power was used as a quantitative index to evaluate the therapeutic effect. The correlation between the postoperative ablation rate of HIFU and the content of smooth muscle cells and collagen fibers in fibroids were analyzed. The results showed that the ablation rate was positively correlated with the content of smooth muscle cells, but negatively correlated with the content of collagen fibers. This may be because the higher the content of smooth muscle cells in fibroids, the lower the content of interstitial components, such as micro-vessels and collagen fibers, while when effective microcirculation in tumor tissues is lower, the better the heat transfer effects [Citation15], and in turn the better the ablation effect. In addition to directly causing tumor cell death, long-term indirect damages to tumor cells that do not appear immediately after thermal stimulation may also be exerted [Citation15–17]. Clinical and experimental data show that tissue damage will continue after thermal ablation has been completed [Citation18]. Nikfarjam et al. [Citation19,Citation20] found that the peak value of tumor cells damaged after hyperthermia was recorded 4–5 days later. Using Nada-diaphorase staining, they found that indirect damage was not related to the initial thermal effect. Therefore, direct and indirect damage produced by thermal energy on tumor cells is stronger. The higher the content of smooth muscle cells and the lower the content of collagen fibers was, the better the overall effect of HIFU ablation.
The correlations between the content of smooth muscle cells and collagen fibers in fibroids and the SWV value of uterine fibroids were obtained through statistical analysis. The SWV value of uterine fibroids was negatively correlated with the content of smooth muscle cells but positively correlated with the content of collagen fibers. The higher the content of smooth muscle cells and the lower the content of collagen fibers, the lower the SWV value of uterine fibroids. This result provided a pathological foundation for the prediction of the efficacy of HIFU uterine fibroids ablation based on the SWV value of uterine fibroids. Therefore, previous experimental results were further verified from a pathological perspective, and the SWV value of uterine fibroids was found to be negatively correlated with the HIFU ablation rate. The results showed that the content of collagen fibers and smooth muscle cells varied among uterine fibroids, along with their SWV values. Therefore, we hypothesized that this difference may be an important factor for differences in the efficacy of HIFU ablation of uterine fibroids of different SWV values.
This study explored factors that influence differences in the HIFU ablation efficacy of uterine fibroids of different SWV values based on the main pathological structures of uterine fibroids. However, there are certain limitations of the study.In addition to the number of smooth muscle cells and the content of collagen fibers, other factors, such as blood supply and water content, may also affect the elasticity and ablation rate of uterine fibroids, and there may be random sampling errors in core biopsy due to the heterogeneity of fibroids, In future, we will explore the impact of other factors on elasticity and the ablation rate of uterine fibroids to further explore factors that give rise to difference in ablation efficacy using a more comprehensive pathological perspective.
In conclusion, this study explored the relationship between ablation rate, shear wave velocity (SWV) value, and the content of smooth muscle cells and collagen fibers in the tissue structure of fibroids, and further confirmed that the SWV value of fibroids can reflect the soft and hard degree of fibroids. This provided a reliable and effective reference value for the preoperative prediction of high-Intensity focused ultrasound(HIFU) uterine fibroid ablation efficacy and a basis for patient selection and program development prior to HIFU.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48:312–324.
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95–114.
- Parker WH. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2005;105:216–217. author reply 217.
- Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermo ablative technique. Am J Obstet Gynecol. 2003;189:48–54.
- Zhang L, Zhang W, Orsi F, et al. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: A review of safety and efficacy. Int J Hyperthermia. 2015;31:280–284.
- Garra BS. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007;23:255–268.
- J Zhan JMJ, Xh Diao YC. Acoustic radiation force impulse imaging (ARFI) for differentiation of benign and malignant thyroid nodules—a meta-analysis. Eur J Radiol. 2015;84:2181–2186.
- Kim JE, Lee JY, Bae KS, et al. Acoustic radiation force impulse elastography for focal hepatic tumors: usefulness for differentiating hemangiomas from malignant tumors. Korean J Radiol. 2013;14:743–753.
- Bai M, Du L, Gu J, et al. Virtual touch tissue quantification using acoustic radiation force impulse technology: initial clinical experience with solid breast masses. J Ultrasound Med. 2012;31:289–294.
- Zhang DL, Liu XX, Tang JQ, et al. The value of acoustic radiation force impulse imaging in preoperative prediction for efficacy of high-Intensity focused ultrasound uterine fibroids ablation. Int J Hyperthermia. 2020;37:423–429.
- Yifu S, Zhu X. Progress in pathology of uterine fibroids. J Appl Oncol. 2004:5–8.
- Hall TJ. AAPM/RSNA physics tutorial for residents: topics in US: beyond the basics: elasticity imaging with US. Radiographics. 2003;23:1657–1671.
- Ophir J, Céspedes I, Ponnekanti H, et al. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–134.
- Chamming’s F, Latorre-Ossa H, Le Frère-Belda MA, et al. Shear wave elastography of tumour growth in a human breast cancer model with pathological correlation. Eur Radiol. 2013;23:2079–2086.
- Dudar TE, Jain RK. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res. 1984;44:605–612.
- Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56.
- White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15.
- Wiersinga WJ, Jansen MC, Straatsburg IH, et al. Lesion progression with time and the effect of vascular occlusion following radiofrequency ablation of the liver. Br J Surg. 2003;90:306–312.
- Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res. 2005;127:208–223.
- Nikfarjam M, Malcontenti-Wilson C, Christophi C. Focal hyperthermia produces progressive tumor necrosis independent of the initial thermal effects. J Gastrointest Surg. 2005;9:410–417.